Abstract
COPD is a progressive condition that leads to a pathological degeneration of the respiratory system. It represents one of the most important causes of mortality and morbidity in the world, and it is characterized by the presence of many associated comorbidities. Recent studies emphasize the thoracic area as one of the areas of the body concerned by the presence of pain with percentages between 22% and 54% in patients with COPD. This article analyzes the possible causes of mediastinal pain, including those less frequently taken into consideration, which concern the role of the fascial system of the mediastinum. The latter can be a source of pain especially when a chronic pathology is altering the structure of the connective tissue. We conclude that to consider the fascia in daily clinical activity may improve the therapeutic approach toward the patient.
Introduction
COPD is a worsening/degenerative condition that leads to a pathological degeneration of the respiratory system.Citation1 It represents one of the most important causes of mortality and morbidity in the world, and it is characterized by the presence of many associated comorbidities. The World Health Organization (WHO) estimated a current mortality rate of ~64 million people per year, and in 2030 COPD will represent the third cause of mortality in the world.Citation2 Approximately 51% of patients with COPD reportedly have at least a comorbidity, although the precise relation between COPD and these comorbidities is not entirely clear.Citation1–Citation3 This pathological scenario is associated with a decrease in survival rates and with more frequent hospitalization.Citation3
There is a strong relation between the presence of pain perceived by the patient and the presence of comorbidities.Citation3 This clinical picture worsens where the patient suffers from anxiety or depression or from other preexisting pathologies.Citation3,Citation4 The pain experienced by patients with COPD is stronger than the one perceived by those without a respiratory pathology or by patients with other chronic diseases. The former is only comparable to the pain observed in oncologic patients at the terminal stage.Citation3,Citation5 The most common causes of pain are arthritis, osteoporosis, systemic myopathy and costochondritis. Pain is generally localized in the trunk, the neck, the lumbodorsal area and the inferior limbs.Citation3 Female patients allegedly report to experience a stronger pain.Citation5
Recent studies emphasize the thoracic area as one of the body areas concerned by the presence of pain, with percentages between 22% and 54% in patients with COPD.Citation5,Citation6
This article analyzes the potential causes of mediastinal pain, that is, gastroesophageal reflux disease (GERD), a loss of elasticity of the parietal pleura, the visceral pleural receptors (VPRs) and vagal receptors, bronchial spasm or edema or a pathological adaptation of the diaphragm and from its innervating system. Furthermore, this article considers the other possible sources of mediastinal pain that are related to the fascial system, which is an integral part of the somatic and visceral thoracic area: the muscular and fascial system of the rib cage, the movement and shape of the rib cage and the endothoracic and visceral fascia ().
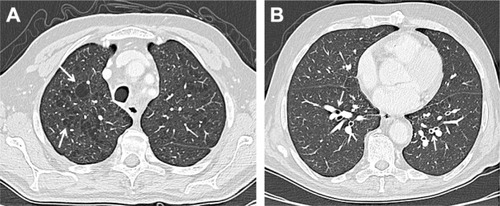
Figure 1 Chest CT on axial planes. Chest CT usually shows emphysema which can be centrilobular, panlobular or paraseptal; the first one is the most common type of emphysema (A), usually related to smoking status and more marked in the upper lobes; the parenchymal destruction is centered around the terminal bronchiole, representing the center of the secondary pulmonary lobule. Other findings include bronchial wall thickening (B), air trapping and narrowing of the trachea in the coronal plane.
Thoracic pain: non-fascial causes
GERD
Thoracic pain can have multiple, isolated or concomitant, causes. The presence of GERD and/or hiatal hernia is a comorbidity often found in patients with COPD. This can be due to the peripheral sensitization provoked by the constant stimulation of inflammatory mediators (pepsin, bile, ATP, cytokines, hydrochloric acid and histamine), which lower the vagal and nociceptive afferential threshold. This reduction increases the permeability to cations of pain receptors, with the appearance of primary hyperalgesia. With time, other nociceptors will activate, including acid-sensing ion channels, transient receptor potential V1 and P2X purinoceptors (ATP-gated ion channel).Citation7 This symptomatological scenario can lead to phenomena such as secondary hyperalgesia and allodynia, with central sensitization caused by the persistent involvement of medullary neurons and cerebral areas, and the subsequent involvement of the psychoneuroimmune system.Citation7 We invite the reader, if interested, to reread the article by Boeckxstaens and Rohof.Citation7
Loss of elasticity of the parietal pleura
Thoracic pain in patients with COPD can also derive from the loss of elasticity of the parietal pleura, which is innervated by somatic fibers (intercostal nerves), and sympathetic and vagal fibers and contains nociceptors.Citation8,Citation9 The parietal pleura can suffer from adhesions with the visceral pleura and from scarring processes caused by a chronic inflammatory environment. In patients with COPD, the presence of inflammatory cytokines, such as transforming growth factor-beta, weakens the immune system, favoring adhesions and scars in the pleurae (parietal and visceral).Citation10 In the animal model, pleural adhesions determine the creation ex novo of nervous and vascular structures, suggesting that these adhesions can be a source of pain.Citation11 The creation of pleural adhesions and scars further reduces the lung expansion in patients with COPD.Citation9
VPRs
Another source of thoracic pain can be the visceral pleura. The VPRs are myelinated sensitive nervous fibers that derive from the spinal roots of the upper dorsal tract, which could reach the lungs through the sympathetic trunks.Citation12 The VPRs are connected to the elastic fibers of the pulmonary parenchyma, and they are thought to be able to translate mechanical or pain stimuli in electric signals.Citation8 If the mechanical environment is altered or overstimulated, these receptors will activate rapidly.Citation13 The overstretching of the visceral pleura caused by a chronic overinflation of the lungs, for example, could stimulate these receptors.
Vagal receptors
There are at least seven different vagal receptors in the pulmonary area, such as high-threshold A-delta receptors and bronchial and pulmonary C-fiber receptors, which are considered as nociceptors.Citation12 These receptors may send painful sensations because of an altered mechanometabolic environment. The inflammation of bronchial pathways causes the production of free radicals (reactive oxygen species) (H2O2, isoprostanes and nitrotyrosine residues), which can be found in patients with COPD and which may activate these receptors.Citation13
Bronchial spasm
When there is a constriction, a spasm or an edema, the bronchial pathways can be a source of thoracic pain. This happens because of the activation of the abovementioned vagal receptors.Citation13 It is important to remember that, in the presence of COPD, the mechanical bronchial tension can radically change, because of the remodeling of the inner bronchial structure caused by constant inflammation. The epithelium can generate fibroblasts, creating a pathological bronchial fibrosis.Citation14
The intrapulmonary epithelium is innervated by a group of pulmonary neuroendocrine cells (PNECs), which can be activated by mechanical stimuli such as stretching or metabolic changes.Citation12 We still know little about PNECs, but they could probably play a role in the sensation of pain.Citation12
A pathological adaptation of the diaphragm and from its innervating system
The limitation of the progressive air flux in patients affected by COPD determines a pathological adaptation of the diaphragm, even though these changes are still not entirely understood. The respiratory district changes position, influencing negatively patients’ tolerance for exercise. The diaphragmatic dome is lower in inhaling attitude.Citation4 Contractile strength diminishes, with metabolic and electric alterations. The muscle thickness increases, particularly in the left side, with diminished mechanical excursion.Citation4 This increase is probably due to the shortening of the fibers. There is a decrease in the presence of fibers of the anaerobic type (type II) and an increase in aerobic fibers (type I), a circumstance which becomes increasingly relevant as the pathology advances. The increase in the oxidative capacity, though, does not coincide with the improvement of the diaphragmatic function. The percentage of myosin drops, with an altered sarcomeric organization, further decreasing the expression of contractile strength.Citation4 Phrenic activity is altered, probably because of the strain caused by the chronic lowering of the diaphragm, producing neuropathy.Citation4 The diaphragm influences the perception of pain and the emotional state of the individual. This event seems to mirror the intervention of the baroreceptors.Citation4
The baroreceptors located in the carotid body and in the area of the aortic arch, in the adventitia of the vessels, are structures that activate particularly, thanks to mechanical stimuli, for instance, when the vessels are stretched during inhalation or the flowing of blood.Citation15 The baroreceptorial afferences are gathered by the nucleus of the solitary tract (NTS), which modulates the efferential intervention to the vagal system and the sympathetic inhibitory efference on a spinal level in the proximity of the nucleus ambiguus, of the dorsal motor nucleus and of the ventrolateral rostral area of the medulla oblongata.Citation16 The afferences influence different areas of the central nervous system, with a general inhibitory effect. The NTS is interconnected with the reticular formation, sending information to the anterior, lateromedial and prefrontal insulae and to the anterior cingulate cortex; the thalamus, the hypothalamus and the periaqueductal gray area receive baroreceptorial signals from the NTS.Citation16 In a healthy context, the diaphragm stimulates the baroreceptors to raise the pain threshold, favoring, with its movements, the upward venous and lymphatic return.Citation4,Citation15 This modulation of pressure influences the redistribution of blood, which is probably the action that determines the response of the baroreceptors and the perception of pain.Citation4,Citation15 Chronic and acute pain can alter the functions of the baroreceptors, just like an altered function and position of the diaphragm can negatively influence the baroreceptorial system.Citation4,Citation15 This creates a vicious circle, and it becomes difficult to understand the cause and the effect.
The vagus nerve and the perception of pain
The afferences of the vagus nerve are generally able to inhibit the activity of the second order of medullary neurons in the spinothalamic and spinoreticular tracts and in the trigeminal nuclei.Citation4,Citation15 Recent scientific evidence, however, highlights the ability to transport painful afferences from the vagus nerve, particularly visceral pain to supraspinal centers.Citation4,Citation15 This happens also because of the retrograde transport of inflammatory biochemical substances that travel through the nerve. The nerve itself cooperates in the creation and the maintenance of pain memory at a central level, also modulating the descending inhibitory pathway that leads back to the nociceptive medullary areas.Citation4,Citation15 We have no precise information on these and descending mechanisms (probably involving the NTS, the parabrachial nuclei, the periaqueductal gray area, the hypothalamus, the limbic area, the magnus raphe nucleus and the locus coeruleus), but we can state that the vagal tone has an important influence on the perception of pain.Citation15 We know that the compression of the vagus nerve can alter its function and ability to transport, just like the dysfunction of a peripheral nerve, mimicking the nerve entrapment syndrome.Citation17 We can suppose that an anomalous tension of the diaphragm in the area of the vagal passage could induce the compression of the nerve, negatively affecting its anti-nociceptive and anti-inflammatory ability.Citation17
The phrenic nerve and the perception of pain
The phrenic nerve is a mixed nerve, able to send efferences to the diaphragm and to receive a lot of visceral information, as well as information from the lungs, the pericardium, the vena cava, the Glisson’s capsule and the peritoneal subdiaphragmatic area.Citation18 Considering the pathological condition of the phrenic nerve in patients with COPD (neuropathy and constant nerve stretching), its anastomoses with the vagus nerve at the level of the ansa cervicalis and the relation with the NTS, we could suppose that there are phrenic, somatic and visceral nociceptive afferences.Citation17–Citation19 Through the spinothalamic tract and near the medullary segment C2–C3, the phrenic afferences overlap with visceral and trigeminal vagal afferences, precisely in the dorsal areas of the laminae I–IV.Citation20,Citation21 Phrenic afferences transport mechanical (proprioceptive and mechanoreceptors) and nociceptive information, not only from the diaphragm but also from the viscera that they meet on their path.Citation21,Citation22
The difficulty of the inspiratory muscle to bear protracted solicitations puts the respiration in a context of fatigue.Citation5,Citation23 Diaphragmatic fatigue in patients with COPD is more probably related to the central nervous system.Citation24 Regardless of the cause that leads to diaphragmatic fatigue, in the presence of contractile difficulty, the phrenic afferences of type IV transport nociceptive and somatic information.Citation25
Thoracic pain in patients with COPD can also originate from cardiac pathologies, often connected to the presence of chronic respiratory disorders.Citation5
Thoracic pain: possible fascial somatic causes
The muscular system of the rib cage is an integral part of the fascial continuum.Citation26 At present, there is no univocal definition of fascia, probably because of the scientific imprint of each professional who tries to create a single point of view.Citation27 From a macro-anatomic perspective, the fascial tissue is equally distributed throughout the entire body, creating various layers at different depths and forming a three-dimensional metabolic and mechanical matrix.Citation28 The fascial organization that covers the contractile part of the muscle can be defined as the myofascial system.Citation26 The fascia allows the muscles to act in synergy, thanks to the fact that the fascial tissues connect all the muscular districts.Citation26
The movement and shape of the rib cage
The movement and shape of the rib cage are altered in patients with COPD. The anteroposterior axis of the chest is some centimeters longer than in healthy patients, particularly in the lower area, taking a rounder form.Citation29 During forced breathing, however, patients have less rib mobility than people who are not affected by chronic respiratory disorders.Citation29 Sternocostal and costovertebral joints become stiffer, with a reduced ability to move; we can often notice a hyperkyphosis tendency of the thoracic spine in patients who are in an advanced stage of the disease.Citation30 Costal biomechanics changes, altering the physiologic movement during inhalation. Lower lateral ribs paradoxically tend to move inwards (Hoover’s sign) during inhalation.Citation31 A paradox movement of the lower sternum during inhalation can also occur.Citation32 Joint stiffness and altered costal dynamics could be related to the somatic fascial system (thoracic rib muscles) ().
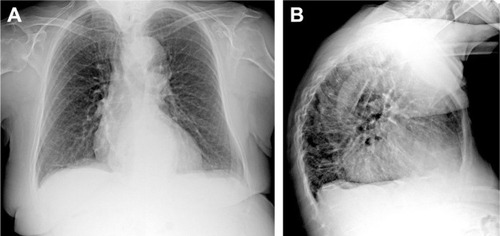
Figure 2 Chest X-ray in PA (A) and LL (B) projections. Chest X-ray has poor sensitivity to detect COPD; possible findings include prominence of the hilar vessels and decreased peripheral bronchovascular markings, flattened diaphragm due to hyperexpansion and hyperkyphosis and increased lung lucency (especially seen in the retrosternal region in LL projection) and bullae (round focal lucency over 1 cm).
Somatic fascial system
One of the causes of thoracic pain in patients with COPD can be related to costal muscles, which face an alteration of their physiologic length (shortening or lengthening) caused by the change of costal biomechanics, influenced in turn by lung movements.Citation9,Citation29,Citation33
There are different muscles that operate on the ribs and that take part in the respiratory acts, whose behavior is not always easy to determine. Externally, the intercostal muscles are covered by the deep fascia originating from the deep cervical fascia, while, internally, they are covered by the endothoracic fascia. Laterally, these muscles form two thin layers between the intercostal spaces: one is located more externally, forming the external intercostal contractile districts, while the other is located internally, forming the internal intercostal muscles.Citation34 Behind these muscles and near the vertebrae, the external intercostal muscles form the levator doral muscles, while the external intercostal muscles form the subcostal muscles.Citation34,Citation35 In the abdomen, between the sternum and the costochondral joint, the external intercostal muscles form a fibrous aponeurosis or anterior intercostal membrane, while the internal intercostal muscles form the intercostal parasternal muscles.Citation34 The latter are covered by the triangularis sterni muscle or transversus thoracis muscle.Citation36 The intercostal muscles that we described are innervated by the respective intercostal nerves.Citation34
The description of the respiratory footprint of these muscles lies outside the context of this article (on this point, we refer the reader to the article by De Troyer et al).Citation34
To understand how costal muscular districts act, it is important to remember the multiple vectors of the contractile fibers and the disposition of each rib: the muscular moment changes depending on the costal disposition (Hamberger mechanism).Citation34 We know that the costal position changes in patients with COPD, so we can deduce that the costal contractile apparatus is in an unfavorable and non-physiologic biomechanical situation.Citation34,Citation37
The muscular respiratory disadvantage increases the risk of hospitalization.Citation37 Costal muscles work harder, and weakness, fatigue and inflammation appear.Citation37–Citation39 The level of inflammatory cytokines found in costal muscles is linked to the seriousness of COPD.Citation40 Muscles suffer from hypotrophy with an increase in the amount of fat inside contractile fibers. This sarcopenia correlates with the severity of COPD.Citation41 During intense exercise, costal muscles suffer from a reduction of blood perfusion, mirroring the inability of the circulatory system to satisfy the needs of the peripheral muscles.Citation42
Costal muscles can be a source of chronic pain. The contractile and connective tissues have nervous terminals (group II and IV, respectively, myelinated and non-myelinated) that gather nociceptive information by mechanical, chemical and thermal stimuli. These somatic afferences travel inside intercostal nerves, bringing information to the lamina I in the medullary dorsal area, from where they will travel through the spinothalamic tract to the central nervous system, the nucleus submedius, the anterior paraventricular nucleus, the posterolateral ventral nuclei of the thalamus, the anterior cingulate cortex, the primary and secondary somatosensory cortex, the prefrontal cortex and the insular cortex.Citation43–Citation45
A constant stimulation of the nociceptive system will cause a plastic change in the peripheral and central nervous structures, creating the mechanism known as sensitization.Citation16 The latter manifests itself as a lowering of the threshold of activation of spinal neurons (allodynia). In chronic conditions, the pathways of nociceptive excitation activate even when the original stimulus has ceased (hyperalgesia), often causing a similar response also by the neighboring tissues which have not been necessarily damaged (secondary hyperalgesia).Citation16
The deep fascia
The deep fascia that covers externally the intercostal muscles and the endothoracic fascia that covers the inside of the rib cage could be sources of chronic pain. The deep fascia originates from the deep cervical fascia.Citation46,Citation47 Thanks to studies on other fascial areas of the human body (lower limbs, abdomen, trunk and cervical tract), we know that the fascial tissue can be innervated by spinal nerves and/or by the sympathetic system. These fibers are able to transport nociceptive afferences.Citation48–Citation50 In the fascia that covers intercostal muscles and in the pectoral fascia, there are nervous fibers that respond to the mechanical and nociceptive stimuli (polymodal receptors with fibers of type C), which are activated by the stretching of the rib cage.Citation51 We know that the rib cage suffers from a biomechanical limitation and that costal muscles tend to become fibrotic. We know that the fascial tissue is in close proximity to the muscles (deep fascia and epimysium) and that it can suffer from the same non-physiologic adaptation of the contractile districts (and vice versa).Citation28,Citation50,Citation52 We know that the nociceptors that are in the fascia have a lower threshold of activation if the fascial tissue is less compliant (greater stiffness).Citation52 We can suppose that the deep fascia covering externally the ribs is one of the causes of thoracic pain.
The endothoracic fascia
The endothoracic fascia derives from the deep cervical fascia and delimits the internal costal area, covering the intercostal muscles and touching the parietal pleura.Citation53–Citation55 It contains lymphatic vessels, particularly in the parasternal and para-vertebral areas. Especially in the latter, the endothoracic fascia comes into contact with spinal nerves and sympathetic ganglia. Vessels of the internal mammary artery can be found in the parasternal area of the fascia.Citation55,Citation56 The fibrosis of the different tissues that can be found in patients with COPD (muscles and pulmonary system) could be noticed in the endothoracic fascia as well, for reasons linked to anatomic and functional continuity. The fascia influences the function of the viscera it wraps around and vice versa.Citation28,Citation57 The fibrosis of the muscles, the fascia or the visceral tissues is similar to the wound-healing mechanism, which modifies the mechanical abilities, without necessarily altering their shape.Citation58 The deep fascia, like the endothoracic fascia, is made up of several layers that slide over one another (a layer defined as subserous layer of connective tissue is located between the endothoracic fascia and the parietal pleura); it is made up of structures which render it elastic, for example, elastin, proteoglycans, glycosaminoglycans and hyaluronic acid.Citation58,Citation59 The different layers will lose the anisotropic ability to slide if they lose water and hyaluronic acid (like in fibroses), creating greater stiffness and density of the tissue. This happens in the fascial tissue of many anatomic areas where there is chronic pain (back, neck) or in pathologies (diabetes, traumata and surgery and hormonal variations) and aging.Citation58 The fascial tissue that loses part of its ability to slide could create adhesions to the tissues it comes into contact with, further stimulating the nociceptive fascial afferences.Citation49 We have no data on the adaptation of the endothoracic fascia in patients with COPD, but we can suppose, considering scientific elements coming from other body areas, that this deep structure could be one of the sources of the chronic pain perceived by patients.
The visceral fascia
The different organs of the mediastinum communicate with one another, thanks to visceral fascial relations. The visceral fascia that covers the organs and connects them with other organs is able to stretch and to adapt to the shape and movements of the viscera. In the presence of traumas, infections or inflammations, the visceral fascia loses its compliance and adaptability to the viscera, causing reduced movement capability and pain.Citation60 The fascia that envelopes the lung is rich in elastic and sympathetic fibers.Citation60 The visceral fascia that covers the lungs is closely connected to the fascial layer that envelopes the pericardium (parietal pericardium) and to the endothoracic fascia.Citation61 In the superior mediastinum, the pulmonary visceral fascia connects the two lungs, passing behind the subclavian and the brachiocephalic arteries. This connection is called interpleural ligament or ligament of Morosow.Citation62 In the inferior mediastinum, the esophagus (right side) and the aorta connect with a fascial structure, whose fascial boundaries attach bilaterally to the pulmonary fascia.Citation62 The fascia between the esophagus and the descending aorta is called meso-esophageal fascia, with the presence of vagal terminals. This fascia is connected to the bronchi, to the pericardial sac (area of the left atrium) and to the trachea.Citation63,Citation64
Among the intrathoracic fasciae, we also have the broncho-pericardial or tracheobroncho-pericardial ligament.Citation65 It connects the terminal part of the trachea and the bronchi with the pericardium (area of the left atrium), anteriorly.Citation65 The pretracheal fascia, deriving from the deep fascia of the neck, originates from the upper boundary of the thyroid cartilage, then covers the trachea anteriorly, touches the posterior part of the parietal pericardium (the fascia that envelopes the heart) and joins the endothoracic fascia covering the diaphragm.Citation66 It is probably innervated by the recurrent laryngeal nerve.Citation66 The parietal pericardium is a connective structure; it is the fascial system that covers the pericardial sac, connecting the heart and the abovementioned structures with the diaphragm, the sternum, some ribs (from the fourth to the sixth one on the left) and the thoracic vertebrae (D10–D11).Citation67 The parietal pleura is innervated by phrenic nerves, the sympathetic system and the vagus nerve.Citation67
We have no data on how the fascial web adapts inside the mediastinum in patients with COPD. We know that the fascia can lose its elasticity and, probably, negatively influence the viscera that it covers or with which it is in contact, altering visceral elasticity. This could lead to a change in the movement and shape of the viscera themselves, an event that, as we know, can occur in the respiratory and cardiac systems in the presence of chronic pathologies of the airways.Citation68,Citation69 An anomalous distention of the viscera could activate nociceptive pathways connected to the cerebellum through mossy and climbing fibers and diffuse monoaminergic and cholinergic afferents.Citation70 A-delta fibers and C-fibers, afferential nociceptive pathways, activate the previously mentioned nervous fibers through postsynaptic connections in the dorsal medullary area. Through the spino-olivo-cerebellar pathway, the nociceptive information reaches the Purkinje cells of the cerebellum.Citation70 The cerebellum processes this nociceptive information through motor efferences.Citation70 This scenario could be one of the reasons for the lack of motor coordination found in patients with COPD.Citation71
The pain coming from the viscera, caused by the stiffness of the visceral fascia, could not only be one of the causes of perceived pain but also contribute to the patients’ lack of neuromotor coordination.
The visceral fascial system could be considered as a proprioceptive organ.Citation72 The fascia that envelopes and links the different mediastinal organs is probably a tool that the central nervous system uses to perceive the functional status of the organs, just like its position.Citation27 If an alteration of the fascial mechanical tension (stiffness) occurs, the proprioceptive terminals will send nociceptive information, determining another cause of the mediastinal pain perceived by the patients.Citation27
The inflammation itself could cause nociceptive fascial afferences.Citation73 The fascia has endocannabinoid receptors (CB1 and CB2), which, once activated, are able to eliminate pro-inflammatory cytokines. The presence of an inflammation could alter the endocannabinoid receptor response.Citation73 Local (pulmonary) and systemic (circulatory) inflammatory environments produce multiple substances (neutrophils, macrophages, T-lymphocytes, leukotrienes B4, different interleukins and tumor necrosis factor-alpha) that are able to perpetuate this non-physiologic situation.Citation74 Some of these substances, like macrophages and neutrophils, can synthesize neuropeptides (corticotropin-releasing factor, urocortins), which increase the sensitization of the sympathetic terminals, further lowering the threshold of the pain coming from the visceral fascia.Citation75,Citation76
This mechanism of inflammation that makes the fascial tissue fibrotic is always bidirectional. The lungs increase the fascial stiffness by releasing pro-inflammatory substances, just as the fascial tissue releases inflammatory substances toward the lung, probably because of a diffusion mechanism.Citation74,Citation77 If these scenarios should ever be confirmed by more extensive research, the visceral fascial inflammation could be proved to be another cause of thoracic pain in patients with COPD.
Several mechanisms that are important to understand the thoracic pain perceived by patients are still not entirely understood.Citation6
Conclusion
Recent studies emphasized that between 22% and 54% of patients with COPD may suffer from thoracic pain. This pain can have different causes, for example, the presence of GERD, and nociceptive afferences by pleurae, from fibrotic or bronchial branches in spasm and from intrapulmonary epithelium. Another source of painful sensations could derive from the fascial system that covers the rib cage and/or from the mediastinal visceral fascial system. The fascial continuum remains a largely underexplored area in pathologies such as COPD, even though it would deserve more attention and more extensive research. The focus of this article was to hypothesize the possible nociceptive scenarios coming from the fascial tissue, such as the external thoracic fascia and the costal muscular complex, the endothoracic fascia and the fascial connections between the viscera of the thorax. In a local and systemic inflammatory environment such as that existing in the presence of COPD or of other mechanical and morphological alterations of the ribs and sternum, it makes sense to take into consideration the fascial system as a potential source of pain.
Disclosure
The authors report no conflicts of interest in this work.
References
- SmithMCWrobelJPEpidemiology and clinical impact of major comorbidities in patients with COPDInt J Chron Obstruct Pulmon Dis2014987188825210449
- SmithSMSonegoSKetchesonLLarsonJLA review of the effectiveness of psychological interventions used for anxiety and depression in chronic obstructive pulmonary diseaseBMJ Open Respir Res201411e000042
- ChenYWCampPGCoxsonHOComorbidities that cause pain and the contributors to pain in individuals with chronic obstructive pulmonary diseaseArch Phys Med Rehabil20179881535154327866992
- BordoniBMarelliFMorabitoBSacconiBDepression, anxiety and chronic pain in patients with chronic obstructive pulmonary disease: the influence of breathMonaldi Arch Chest Dis201787181128635197
- ChristensenVLHolmAMKongerudJOccurrence, characteristics, and predictors of pain in patients with chronic obstructive pulmonary diseasePain Manag Nurs201617210711827095390
- JanssenDJWoutersEFParraYLStakenborgKFranssenFMPrevalence of thoracic pain in patients with chronic obstructive pulmonary disease and relationship with patient characteristics: a cross-sectional observational studyBMC Pulm Med2016164727052199
- BoeckxstaensGERohofWOPathophysiology of gastroesophageal reflux diseaseGastroenterol Clin North Am2014431152524503356
- FinleyDJRuschVWAnatomy of the pleuraThorac Surg Clin201121215716321477764
- BrimsFJDaviesHELeeYCRespiratory chest pain: diagnosis and treatmentMed Clin North Am201094221723220380952
- ThomasBJKan-OKLovelandKLEliasJABardinPGIn the shadow of fibrosis: innate immune suppression mediated by transforming growth factor-βAm J Respir Cell Mol Biol201655675976627603223
- MontesJFGarcía-ValeroJFerrerJEvidence of innervation in talc-induced pleural adhesionsChest2006130370270916963666
- AdriaensenDBrounsITimmermansJPSensory input to the central nervous system from the lungs and airways: a prominent role for purinergic signalling via P2X2/3 receptorsAuton Neurosci2015191394725953244
- Taylor-ClarkTEUndemBJSensing pulmonary oxidative stress by lung vagal afferentsRespir Physiol Neurobiol2011178340641321600314
- PainMBermudezOLacostePTissue remodelling in chronic bronchial diseases: from the epithelial to mesenchymal phenotypeEur Respir Rev20142313111813024591669
- BordoniBMarelliFBordoniGA review of analgesic and emotive breathing: a multidisciplinary approachJ Multidiscip Healthc201699710227013884
- BordoniBMarelliFFailed back surgery syndrome: review and new hypothesesJ Pain Res20169172226834497
- BordoniBBordoniGReflections on osteopathic fascia treatment in the peripheral nervous systemJ Pain Res2015873574026586962
- BordoniBZanierEAnatomic connections of the diaphragm: influence of respiration on the body systemJ Multidiscip Healthc2013628129123940419
- El-TantawiGAImamMHMorsiTSPhrenic nerve conduction abnormalities correlate with diaphragmatic descent in chronic obstructive pulmonary diseaseCOPD201512551652425774441
- ChandlerMJQinCYuanYForemanRDConvergence of trigeminal input with visceral and phrenic inputs on primate C1-C2 spinothalamic tract neuronsBrain Res19998291–220420810350551
- GoshgarianHGRoubalPJOrigin and distribution of phrenic primary afferent nerve fibers in the spinal cord of the adult ratExp Neurol19869236246383709737
- BałkowiecAKukułaKSzulczykPFunctional classification of afferent phrenic nerve fibres and diaphragmatic receptors in catsJ Physiol1995483Pt 37597687776256
- BachassonDWuyamBPepinJLTamisierRLevyPVergesSQuadriceps and respiratory muscle fatigue following high-intensity cycling in COPD patientsPLoS One2013812e8343224324843
- JolleyCLuoYSteierJNeural respiratory drive and symptoms that limit exercise in chronic obstructive pulmonary diseaseLancet2015385Suppl 1S51
- HillJMDischarge of group IV phrenic afferent fibers increases during diaphragmatic fatigueBrain Res20008561–224024410677632
- BordoniBMarelliFThe fascial system and exercise intolerance in patients with chronic heart failure: hypothesis of osteopathic treatmentJ Multidiscip Healthc2015848949426586951
- BordoniBMarelliFEmotions in motion: myofascial interoceptionComplement Med Res2017242110113 German28278494
- BordoniBMarelliFMorabitoBSacconiBThe indeterminable resilience of the fascial systemJ Integr Med201715533734328844209
- CassartMGevenoisPAEstenneMRib cage dimensions in hyper-inflated patients with severe chronic obstructive pulmonary diseaseAm J Respir Crit Care Med19961543 Pt 18008058810622
- WangJSEffect of joint mobilization and stretching on respiratory function and spinal movement in very severe COPD with thoracic kyphosisJ Phys Ther Sci201527103329333126644703
- Garcia-PachonEPadilla-NavasIFrequency of Hoover’s sign in stable patients with chronic obstructive pulmonary diseaseInt J Clin Pract200660551451716700846
- GilmartinJJGibsonGJMechanisms of paradoxical rib cage motion in patients with chronic obstructive pulmonary diseaseAm Rev Respir Dis198613446836872945502
- CharalampidisCYouroukouALazaridisGPleura space anatomyJ Thorac Dis20157Suppl 1S27S3225774304
- De TroyerAKirkwoodPAWilsonTARespiratory action of the intercostal musclesPhysiol Rev200585271775615788709
- KimJHWonHSChungIHKimIBThe enigmatic subcostal muscle: anatomical study with application to spine and chest pain syndromes and avoidance of confusion on imagingClin Anat20152881017102126384842
- JelevLHristovSOvtscharoffWVariety of transversus thoracis muscle in relation to the internal thoracic artery: an autopsy study of 120 subjectsJ Cardiothorac Surg201161121272314
- BarreiroEGeaJRespiratory and limb muscle dysfunction in COPDCOPD201512441342625438125
- GeaJCasadevallCPascualSOrozco-LeviMBarreiroEClinical management of chronic obstructive pulmonary disease patients with muscle dysfunctionJ Thorac Dis20168113379340028066619
- BarreiroEFerrerDSanchezFInflammatory cells and apoptosis in respiratory and limb muscles of patients with COPDJ Appl Physiol (1985)2011111380881721636562
- CasadevallCCoronellCRamírez-SarmientoALUpregulation of pro-inflammatory cytokines in the intercostal muscles of COPD patientsEur Respir J200730470170717626109
- ParkMJChoJMJeonKNMass and fat infiltration of intercostal muscles measured by CT histogram analysis and their correlations with COPD severityAcad Radiol201421671171724809313
- VogiatzisIAthanasopoulosDHabazettlHIntercostal muscle blood flow limitation during exercise in chronic obstructive pulmonary diseaseAm J Respir Crit Care Med201018291105111320622032
- ConacherIDSudarshanGSoniAKPain disaggregation theory – statistical nonsense or a pointer to a paradigm for quantum nociception?Br J Anaesth200391227928112878629
- LuzLLFernandesECSivadoMKokaiESzucsPSafronovBVMonosynaptic convergence of somatic and visceral C-fiber afferents on projection and local circuit neurons in lamina I: a substrate for referred painPain2015156102042205126098437
- GregoryNSSlukaKAAnatomical and physiological factors contributing to chronic muscle painCurr Top Behav Neurosci20142032734824633937
- SteccoAMacchiVMasieroSPectoral and femoral fasciae: common aspects and regional specializationsSurg Radiol Anat2009311354218663404
- LepageDTatuLLoiselFReyPBObertLParratteBAnatomical and computed tomography study of the eighth costochondral junction: topography for costochondral graft harvestingSurg Radiol Anat201638780981526846136
- TesarzJHoheiselUWiedenhöferBMenseSSensory innervation of the thoracolumbar fascia in rats and humansNeuroscience201119430230821839150
- WilkeJSchleipRKlinglerWSteccoCThe lumbodorsal fascia as a potential source of low back pain: a narrative reviewBiomed Res Int20172017534962028584816
- BordoniBZanierEClinical and symptomatological reflections: the fascial systemJ Multidiscip Healthc2014740141125258540
- SteccoCMacchiVPorzionatoADuparcFDe CaroRThe fascia: the forgotten structureItal J Anat Embryol2011116312713822852442
- FedeCAlbertinGPetrelliLHormone receptor expression in human fascial tissueEur J Histochem2016604271028076930
- MiyakeNTakeuchiHChoBHMurakamiGFujimiyaMKitanoHFetal anatomy of the lower cervical and upper thoracic fasciae with special reference to the prevertebral fascial structures including the suprapleural membraneClin Anat201124560761821647961
- NataleGCondinoSSteccoASoldaniPBelmonteMMGesiMIs the cervical fascia an anatomical proteus?Surg Radiol Anat20153791119112725946970
- BrogliaBBiseroESclavoLAndreozziPTuberculosis of the endothoracic fasciaPediatr Pulmonol200641544144416566030
- Stopar PintaricTVeranicPHadzicAKarmakarMCvetkoEElectron-microscopic imaging of endothoracic fascia in the thoracic para-vertebral space in ratsReg Anesth Pain Med201237221521822286520
- BordoniBZanierESkin, fascias, and scars: symptoms and systemic connectionsJ Multidiscip Healthc20137112424403836
- PavanPGSteccoASternRSteccoCPainful connections: densification versus fibrosis of fasciaCurr Pain Headache Rep201418844125063495
- KarmakarMKThoracic paravertebral blockAnesthesiology200195377178011575553
- SteccoCSfrisoMMPorzionatoAMicroscopic anatomy of the visceral fasciaeJ Anat2017231112112828466969
- YalcinNGChoongCKEizenbergNAnatomy and pathophysiology of the pleura and pleural spaceThorac Surg Clin201323111023206712
- KatoTAkitaKAbstracts presented at the 20th Japanese Research Society of Clinical Anatomy on November 12th 2016 at National Cancer Center HospitalSurg Radiol Anat201739910291043
- CuestaMAWeijsTJBleysRLA new concept of the anatomy of the thoracic oesophagus: the meso-oesophagus. Observational study during thoracoscopic esophagectomySurg Endosc20152992576258225480608
- WeijsTJGoenseLvan RossumPSThe peri-esophageal connective tissue layers and related compartments: visualization by histology and magnetic resonance imagingJ Anat2017230226227127659172
- NakanishiKGotoHItoTFascial reinforcement fixing the bronchi to the heart: its anatomy and clinical significanceSurg Radiol Anat201739121301130828577160
- CavdarSKrauseFDalçikHArifoğluYThe anatomy of lamina pretrachealis fasciae cervicalisOkajimas Folia Anat Jpn1996732–31051088870472
- RodriguezERTanCDStructure and anatomy of the human pericardiumProg Cardiovasc Dis201759432734028062264
- NeilanTGBakkerJPSharmaBT1 measurements for detection of expansion of the myocardial extracellular volume in chronic obstructive pulmonary diseaseCan J Cardiol201430121668167525442461
- BodduluriSBhattSPHoffmanEACOPDGene InvestigatorsBiomechanical CT metrics are associated with patient outcomes in COPDThorax201772540941428044005
- MoultonEASchmahmannJDBecerraLBorsookDThe cerebellum and pain: passive integrator or active participator?Brain Res Rev2010651142720553761
- OliveiraCCLeeALMcGinleyJBalance and falls in acute exacerbation of chronic obstructive pulmonary disease: a prospective studyCOPD201714551852528745525
- SteccoCSternRPorzionatoAHyaluronan within fascia in the etiology of myofascial painSurg Radiol Anat2011331089189621964857
- FedeCAlbertinGPetrelliLExpression of the endocannabinoid receptors in human fascial tissueEur J Histochem2016602264327349320
- SindenNJStockleyRASystemic inflammation and comorbidity in COPD: a result of ‘overspill’ of inflammatory mediators from the lungs? Review of the evidenceThorax2010651093093620627907
- MargiorisANDermitzakiEVenihakiMTsatsaniCGravanisAAvgoustinakiPLiapakisGThe multi-faceted profile of Corticotropin-Releasing Factor (CRF) family of neuropeptides and of their receptors on the paracrine/local regulation of the inflammatory responseCurr Mol Pharmacol Epub201719
- JänigWSympathetic nervous system and inflammation: a conceptual viewAuton Neurosci201418241424525016
- LangevinHMKeelyPMaoJConnecting (T)issues: how research in fascia biology can impact integrative oncologyCancer Res201676216159616227729327
