Abstract
Background
Few studies have investigated the quantitative computed tomography (CT) features associated with the severity of bronchiectasis in COPD patients. The purpose of this study was to identify the quantitative CT features and clinical values to determine the extent of bronchiectasis in moderate-to-severe COPD patients.
Methods
A total of 127 moderate-to-severe COPD patients were selected from the cohort of COPD in Dusty Areas (CODA). The study subjects were classified into three groups according to the extent of bronchiectasis on CT: no bronchiectasis, mild bronchiectasis, and moderate-to-severe bronchiectasis. The three groups were compared with respect to demographic data, symptoms, medical history, serum inflammatory markers, pulmonary function, and quantitative CT values.
Results
Among 127 moderate-to-severe COPD subjects, 73 patients (57.5%) were detected to have bronchiectasis, 51 patients (40.2%) to have mild bronchiectasis, and 22 patients (17.3%) to have moderate-to-severe bronchiectasis. Compared with COPD patients without bronchiectasis, those with bronchiectasis were older and had higher frequency of prior tuberculosis, lower prevalence of bronchodilator reversibility (BDR), and more severe air trapping (P < 0.05). Moderate-to-severe bronchiectasis patients had lower body mass index (BMI), higher frequency of prior tuberculosis, lower prevalence of BDR, worse pulmonary function, and more severe air trapping (P < 0.05) than those in the mild bronchiectasis group.
Conclusion
Moderate-to-severe bronchiectasis was associated with a history of pulmonary tuberculosis, lower BMI, severe airflow obstruction, and lower BDR in moderate-to-severe COPD patients. Quantitative analysis of CT showed that severe air trapping was associated with the extent of bronchiectasis in these patients.
Introduction
COPD is a heterogeneous disease with a variable clinical course and is one of the leading causes of morbidity and mortality in the developed world.Citation1,Citation2 The widespread availability of computed tomography (CT) has resulted in an apparent increase in the prevalence of bronchiectasis in COPD patients.Citation3 The Global Initiative for Chronic Obstructive Lung Disease (GOLD) defined bronchiectasis as a comorbidity of COPD.Citation4 Bronchiectasis is characterized by the presence of permanent dilatation of one or more bronchi and mild-to-moderate airflow obstruction.Citation5,Citation6 Studies have reported the presence of bronchiectasis in up to 69% of COPD patients.Citation7–Citation13 Inflammation is important in the pathogenesis of COPD and bronchiectasis.Citation14,Citation15 In addition, COPD and bronchiectasis share clinical presentations and pathologic mechanisms, such as productive cough and susceptibility to recurrent exacerbations.Citation3,Citation16
The impact of bronchiectasis on the natural history of COPD has been emphasized; moreover, early diagnosis and management of bronchiectasis are important for COPD patients.Citation1,Citation9 In recent meta-analyses, the presence of bronchiectasis in COPD patients was shown to be associated with higher exacerbation frequency and mortality, more severe airflow obstruction, and higher rate of isolation of potentially pathogenic microorganisms, which adequately demonstrated that bronchiectasis is more than a radiological feature.Citation1,Citation3 In previous studies, the analysis of CT features associated with bronchiectasis was largely qualitative. Few studies have investigated the quantitative CT features associated with the severity of bronchiectasis in COPD patients. The aim of this study was to identify the quantitative CT features and clinical values to determine the extent of bronchiectasis in moderate-to-severe COPD patients.
Patients and methods
Study population
A total of 159 patients diagnosed with moderate-to-severe COPD were initially selected from the cohort of COPD in Dusty Areas (CODA), a Korean study that was designed to observe the clinical outcomes of patients living near cement factories. The design of this cohort had been previously described in detail.Citation17,Citation18 All enrolled patients were evaluated by medical interview, physical examination, spirometry, laboratory tests, and CT scan. COPD was defined as a postbronchodilator ratio of forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) of <0.7.Citation4 Patients with GOLD grades 2–4 were included. The severity of airflow limitation was defined as described by the 2017 GOLD grading system as grade 2 (50% ≤ FEV1 < 80%), grade 3 (30% ≤ FEV1 < 50%), or grade 4 (FEV1 < 30%).Citation4 We excluded 32 patients with acute infection, previous pulmonary tuberculosis (TB) resulting in severe lung parenchymal distortion, pneumoconiosis with progressive massive fibrosis, a diagnosis of bronchiectasis before COPD was diagnosed, history of lobectomy, interstitial lung disease, pleural effusion, and lung cancer on chest CT. Of the 159 moderate-to-severe COPD patients who were initially considered, 127 were included in this study after exclusion. These 127 moderate-to-severe COPD patients were allocated to one of the three groups according to the extent of bronchiectasis: no bronchiectasis (n = 54), mild bronchiectasis (n = 51), and moderate-to-severe bronchiectasis (n = 22). The selection criteria for this study are shown in . This study was approved by the institutional review board (IRB) of Kangwon National University Hospital (IRB number 2012-06-007), and all participants provided written informed consent.
Clinical variables and pulmonary functions
The interview questionnaire included demographic characteristics, medical history, environmental exposure, respiratory symptoms, and history of exacerbations during the previous year. The main contents of the questionnaire are presented in Table S1. Exacerbations were defined as acute events of worsening respiratory symptoms requiring treatment with systemic steroids or antibiotics, the need to visit an emergency room, or the need for hospitalization. Dyspnea was evaluated using the modified Medical Research Council (MMRC) scoring system. Health-related quality of life was evaluated using the patient-reported COPD assessment test (CAT). The study subjects were questioned regarding the history of direct exposure to biomass using the following question: “for cooking and/or heating, have you ever been exposed to fuels of wood or charcoal?” Positive biomass exposure was defined as a history of more than 10 years of direct exposure.Citation19,Citation20 In addition, the peripheral levels of white blood cells (WBCs), neutrophils, eosinophils, interleukin (IL)-6, IL-8, C-reactive protein (CRP), and albumin were used as markers of systemic inflammation and nutritional status.
Pulmonary function tests were performed as described by the American Thoracic Society/European Respiratory Society using the Easy One kit (NDD Medizintechnik AG, Zurich, Switzerland) to evaluate lung function and bronchodilator reversibility (BDR).Citation21 Postbronchodilator FEV1 values were determined by spirometry before and 15 minutes after the administration of 400 μg salbutamol through a metered-dose inhaler with a spacer. BDR was defined as an increase of at least 12% or 200 mL in FEV1 or FVC after salbutamol administration.Citation21,Citation22
Image analysis
All patients underwent volumetric thin-section chest CT at full inspiration and expiration in a supine position. Scans were obtained using a first-generation dual-source CT system (Somatom Definition; Siemens Healthcare, Forchheim, Germany) in the caudocranial direction using the following parameters: 140 kVp, 100 mA, 0.9–1 beam pitch, and section thickness of 0.6 mm. CT data were reconstructed using soft kernel (B30f; Siemens Medical System, Forchheim, Germany). The CT images were obtained without injection of contrast medium. Lung window images (width, 1,500 Hounsfield unit [HU]; level, −700 HU) were used to assess bronchiectasis.
Chest CT images were independently interpreted by two radiologists who were unaware of clinical data for the presence and extent of bronchiectasis. Different interpretations were resolved by consensus. The presence of bronchiectasis was determined according to Naidich et alCitation23 as follows: 1) lack of tapering of the bronchial lumen toward the periphery; 2) dilatation of the bronchi when the internal diameter of the bronchi was greater than that of the accompanying pulmonary artery; or 3) the presence of peripheral bronchi within 1 cm of the pleura. The extent of bronchiectasis was assessed based on the number of pulmonary segments affected with the lingula considered as an independent lobe; the scores were 0= absence of bronchiectasis, 1= mild (1–5 segments), 2= moderate (6–9 segments), and 3= severe (more than 10 segments).Citation24 Small cylindrical bronchiectatic lesions that were visible in only one pulmonary segment were ignored because these are present in a significant portion of a healthy population.Citation25
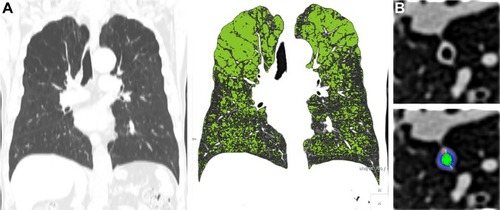
To quantitatively assess emphysema, air trapping, and bronchial wall thickness, whole-lung images were automatically extracted from the chest wall, mediastinum, and large airway; the attenuation coefficients of pixels in these images were then measured using in-house software. Emphysema index (EI) was defined as the percentage of low attenuated area below −950 HU at full inspiration (). The expiratory to inspiratory ratio of mean lung density (E/I-ratioMLD) was used to evaluate air trapping.Citation26,Citation27 Using in-house software, the airway dimensions, including wall area (WA), lumen area, and WA% (defined as WA/[WA + lumen area] × 100), were measured near the origin of the right apical and left apicoposterior segmental bronchi (). In the current study, WA% was used to assess airway thicknesses; the mean values of the segmental bronchi were used in the statistical analyses.
Figure 2 Quantitative CT analysis of emphysema and bronchial wall thickness.
Abbreviations: CT, computed tomography; HU, Hounsfield unit.
Determination of previous TB infection or pneumoconiosis
History of pulmonary TB was defined as the presence of pleural thickening, traction bronchiectasis, parenchymal bands, cicatrization atelectasis, or sharply defined nodules with the upper lung predominance on CT images and/or a previous diagnosis of pulmonary TB.Citation28 All patients had parenchymal or pleural changes due to previous TB on CT. Pneumoconiosis was defined by the following criteria: 1) history of diagnosed pneumoconiosis or 2) history of direct exposure to biomass plus visualization of perilymphatic nodules, reticular opacities, progressive massive fibrosis with upper lobe predominance, or lymph node enlargement with egg shell calcification.Citation29
Statistical analyses
Parametric data were expressed as mean ± SD, whereas nonparametric data were expressed as numbers and percentages. The Student’s t-test or the Chi-square test was used to determine the significance of differences between patients with bronchiectasis and those without bronchiectasis. Analysis of variance or the Chi-square test was used to determine the significance of differences among the three groups of the extent of bronchiectasis. Multivariate logistic regression analysis, which was adjusted for sex, smoking, and body mass index (BMI), was used to identify the factors independently associated with bronchiectasis. Statistical significance was accepted for P-values < 0.05. Data analysis was performed using SAS version 9.3 (SAS Institute Inc., Cary, NC, USA).
Results
Patient characteristics
The characteristics of the patients are summarized in Table S2. All 127 study subjects had moderate-to-severe COPD and had a mean age of 71.5 ± 7.6 years (range, 48–96 years); 107 (84.3%) were men. GOLD severity was grade 2 in 107 (84.2%), grade 3 in 18 (14.2%), and grade 4 in 2 (1.6%). A total of 40 (31.5%) patients had a history of pulmonary TB, and eight (6.3%) patients had a history of pneumoconiosis.
COPD with and without bronchiectasis
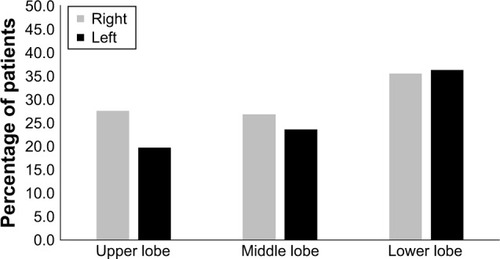
Of the 127 study subjects, 73 (57.5%) presented with bronchiectasis, which was most frequently detected in the lower lobes (47.2%; ); the mean score for the extent of bronchiectasis in these patients was 1.34. Bronchiectasis was not associated with sex, smoking status, GOLD grade, exacerbation, BMI, MMRC dyspnea scale, CAT score, history of pneumoconiosis, inflammatory markers, bronchial wall thickness, or emphysema severity (P > 0.05). COPD patients with comorbid bronchiectasis were older (72.9 ± 7.0 vs 69.8 ± 8.1, P = 0.024), had higher prevalence of previous pulmonary TB (43.8% vs 14.8%, P = 0.001), had lower prevalence of positive BDR (16.4% vs 35.2%, P = 0.015), and exhibited more severe air trapping than patients without bronchiectasis (1.0 ± 0.0 vs 0.9 ± 0.0. P = 0.016; ). Multivariate logistic regression analysis showed that a history of pulmonary TB independently predicted the presence of bronchiectasis in COPD patients (P = 0.002).
Table 1 Factors associated with bronchiectasis in a logistic regression model (adjusted for sex, smoking, and BMI)
Factors associated with the extent of bronchiectasis
A summary of the comparisons among the three groups according to the extent of bronchiectasis is provided in . No significant intergroup differences were observed for age, sex, smoking status, MMRC dyspnea scale, CAT score, exacerbations, history of pneumoconiosis, bronchial wall thickness, and emphysema extent (P > 0.05). As the extent of bronchiectasis increased, the BMIs decreased (P = 0.015) and the number of patients with previous TB increased (P = 0.002). Moreover, COPD patients with moderate-to-severe bronchiectasis had more severe airflow obstruction (FEV1%, P = 0.010), lower prevalence of positive BDR (P = 0.049), and more severe air trapping (P = 0.042). Inflammatory markers, including IL-6 and CRP, tended to increase in moderate-to-severe bronchiectasis patients, but this was not significant. Multivariate logistic regression analysis showed that a history of pulmonary TB independently predicted the mild bronchiectasis in COPD patients (P = 0.002; ).
Table 2 Characteristics of 127 patients in association with the extent of bronchiectasis
Table 3 Factors associated with mild bronchiectasis in a logistic regression model (adjusted for sex, smoking, and BMI)
Discussion
The current study evaluated the quantitative CT features and clinical manifestations associated with the extent of bronchiectasis in moderate-to-severe COPD patients. We found that the history of TB, lower BMI, more severe airflow obstruction, lower prevalence of positive BDR, and more severe air trapping were associated with the extent of bronchiectasis in moderate-to-severe COPD patients. Furthermore, a history of pulmonary TB was found to independently predict the presence of bronchiectasis.
Previous studies have reported a prevalence of bronchiectasis ranging from 50.0% to 57.6% in moderate-to-severe COPD patients.Citation8,Citation10,Citation11,Citation30 In the current study, the prevalence of bronchiectasis in these patients was 57.5%. The prevalence of bronchiectasis in Korea health screening examinations was 9.1%.Citation31 Based on our results, the proportion of bronchiectasis in moderate-to-severe COPD patients was significantly higher compared with the healthy subjects in Korea. Most of the patients in our study were male (84.3%). The prevalence of COPD in 2015 using the data from Korea National Health and Nutrition Examination Survey (KNHANES) was 21.6% in male and 5.8% in female.Citation32 Considering the sex ratio of COPD patients in Korea, the proportion of subjects in our study seems to be similar. Similar to previous studies,Citation11,Citation30 the current study showed that bronchiectasis was most frequently detected in the lower lobes (47.2%). Patel et alCitation30 reported bronchiectasis in the upper lobes in 11.3% of patients; however, in the current study, the predilection for upper lobe bronchiectasis was substantially higher at 34.6%.Citation33 Bronchiectasis has been reported to show upper lobe predominance in patients with a history of pulmonary TB;Citation14 notably, the relatively high rate (31.5%) of history of pulmonary TB in our study population may explain the higher predilection for upper lobe bronchiectasis. Jin et alCitation8 found that previous TB was an independent risk factor for coexistent bronchiectasis in COPD patients in China. Because of the high prevalence of TB, post-TB bronchiectasis in Asian was more common than that in Europe.Citation33 Previous TB infection was an important risk factor for the development of bronchiectasis in subjects who underwent a health screening examinations in Korea.Citation31 Severely destructed lungs with TB may lead to severe bronchiectasis, and airflow limitation can occur due to post-TB bronchiectasis rather than COPD. To minimize the effect of airflow limitation mainly caused by bronchiectasis, we excluded 20 patients who had severely destructed lungs with TB and were diagnosed as having bronchiectasis before the diagnosis of COPD. In the current study, multivariate logistic analysis showed that previous TB was independently associated with the presence of bronchiectasis in COPD patients even after the exclusion of patients with prior pulmonary TB with destroyed lungs (P = 0.002). These results suggested that COPD patients with a history of pulmonary TB were more likely to have more extensive and severe bronchiectasis and other clinical manifestations compared with COPD patients without prior pulmonary TB.
Morphologic changes, such as emphysema, air trapping, and bronchial wall thickening are CT characteristics of COPD; in fact, quantitative analysis of these morphologic changes has been performed in COPD patients.Citation34 On the other hand, only few studies have performed quantitative CT analysis of the morphologic changes in COPD patients with bronchiectasis. In this study, we quantitatively assessed emphysema, air trapping, and bronchial wall thickening, as well as the association between the extent of bronchiectasis and quantitative CT features, in moderate-to-severe COPD patients and found that air trapping was associated with the extent of bronchiectasis in COPD patients. Air trapping, which reflects small airway disease, is common in patients with bronchiectasis even when it is mild.Citation35,Citation36 The pathologic status of small airway disease was confirmed in lobectomy specimens of patients with bronchiectasis.Citation36–Citation38 Air trapping was detected in 17% of the lobes without bronchiectasis on CT images, which suggested that small airway inflammation may precede the development of overt bronchiectasis.Citation35,Citation36 A previous study reported that the severity of bronchiectasis was correlated with the severity of bronchial wall thickening.Citation7 However, we found no relation between bronchial wall thickening and the extent of bronchiectasis in this study. Bronchiectasis is usually encountered in the lower lobes due to the gravity-dependent retention of infectious secretions.Citation6 In COPD patients, the extent of bronchiectasis in the upper airways tends to be less than that observed in other bronchiectasis patients.Citation6 In the current study, the airway dimensions were measured near the origins of the right apical and left apicoposterior segmental bronchi. Therefore, our measurements may have not reflected the overall bronchial wall thickness.
A recent study suggested that greater reversibility of airflow limitation at baseline was associated with better long-term outcomes in COPD patients without significant comorbidities.Citation39 Chronic inflammation in bronchiectasis leads to bronchomalacia or excessive collapsibility, resulting in obstructive airway physiology.Citation40,Citation41 However, few studies have investigated the association between bronchiectasis and BDR in moderate-to-severe COPD patients. In one study, BDR and the radiological manifestations of bronchiectasis were unrelated,Citation42 whereas in the current study, moderate-to-severe bronchiectasis patients showed lower prevalence of positive BDR. As the extent of bronchiectasis increased, more irreversible bronchial changes were considered to result in lower BDR in moderate-to-severe COPD patients. The significance of BDR, its association with the clinical parameters, and the prognostic implications require further investigation in COPD patients with bronchiectasis.
Previous analyses revealed that COPD patients with bronchiectasis presented with more symptoms and more severe exacerbations.Citation1,Citation3 However, we found no relationship between the presence of bronchiectasis and symptoms or exacerbations. In this study, 84.2% of the patients were in GOLD grade 2 and exacerbations occurred in few subjects compared with the findings in other studies. In the current study, we first attempted to identify the association between bronchiectasis and CAT scores. However, no relationship between the two was found in moderate-to-severe COPD patients, presumably because of few patients with GOLD grades 3 and 4. However, moderate-to-severe bronchiectasis patients had a higher CAT score than those without bronchiectasis, although this was not statistically significant.
This study had several limitations. First, 70% of moderate-to-severe COPD patients with bronchiectasis showed mild bronchiectasis in our study. The results of our study did not fully reflect what may be seen in moderate-to-severe bronchiectasis. Second, the fact that we did not control for patient’s treatment as a variable could have caused bias. Third, we did not evaluate the severity of bronchiectasis using the Bhalla et alCitation24 score system, which is used to quantify the severity of bronchiectasis on CT. Subsequent studies are needed to evaluate the association between the severity of bronchiectasis using the Bhalla score and quantitative CT features. Finally, in this study, the potentially pathogenic microorganisms that are known to be associated with bronchiectasis and their effects were not evaluated.
Conclusion
We found that moderate-to-severe bronchiectasis was associated with lower BMI, more frequent history of TB, severe airflow obstruction, and lower BDR in moderate-to-severe COPD patients. In addition, quantitative analysis of CT showed that severe air trapping was associated with the extent of bronchiectasis in moderate-to-severe COPD patients.
Author contributions
All of the authors contributed to the conception and design of the study, acquisition or analysis of data, interpretation of data, and drafting the manuscript, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Acknowledgments
The authors would like to thank the CT technologists at the Department of Radiology, Kangwon National University Hospital. This work was supported by a grant from the Ministry of Environment (Environmental Health Center), Republic of Korea.
Supplementary materials
Table S1 The interview questionnaire
Table S2 Demographics of moderate-to-severe COPD patients with or without bronchiectasis
Disclosure
The authors report no conflicts of interest in this work.
References
- DuQJinJLiuXSunYBronchiectasis as a comorbidity of chronic obstructive pulmonary disease: a systematic review and meta-analysisPLoS One2016113e015053226978269
- KimWJLeeCYEnvironmental exposures and chronic obstructive pulmonary diseaseMol Cell Toxicol201713251255
- NiYShiGYuYHaoJChenTSongHClinical characteristics of patients with chronic obstructive pulmonary disease with comorbid bronchiectasis: a systemic review and meta-analysisInt J Chron Obstruct Pulmon Dis2015101465147526251586
- VogelmeierCFCrinerGJMartinezFJGlobal strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summaryAm J Respir Crit Care Med2017195555758228128970
- AmalakuhanBMaselliDJMartinez-GarciaMAUpdate in bronchiectasis 2014Am J Respir Crit Care Med2015192101155116126568240
- KingPTThe pathophysiology of bronchiectasisInt J Chron Obstruct Pulmon Dis2009441141920037680
- GatheralTKumarNSansomBCOPD-related bronchiectasis; independent impact on disease course and outcomesCOPD201411660561424983298
- JinJYuWLiSLuLLiuXSunYFactors associated with bronchiectasis in patients with moderate-severe chronic obstructive pulmonary diseaseMedicine20169529e421927442646
- MaoBLuHWLiMHThe existence of bronchiectasis predicts worse prognosis in patients with COPDSci Rep201551096126077673
- Martinez-GarciaMAde la Rosa CarrilloDSoler-CatalunaJJPrognostic value of bronchiectasis in patients with moderate-to-severe chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2013187882383123392438
- Martinez-GarciaMASoler-CatalunaJJDonat SanzYFactors associated with bronchiectasis in patients with COPDChest201114051130113721546440
- O’BrienCGuestPJHillSLStockleyRAPhysiological and radiological characterisation of patients diagnosed with chronic obstructive pulmonary disease in primary careThorax200055863564210899238
- RocheNKouassiBRabbatAMounedjiALorutCHuchonGYield of sputum microbiological examination in patients hospitalized for exacerbations of chronic obstructive pulmonary disease with purulent sputumRespiration2007741192516675894
- MillironBHenryTSVeeraraghavanSLittleBPBronchiectasis: mechanisms and imaging clues of associated common and uncommon diseasesRadiographics20153541011103026024063
- NurwidyaFDamayantiTYunusFThe role of innate and adaptive immune cells in the immunopathogenesis of chronic obstructive pulmonary diseaseTuber Respir Dis2016791513
- HurstJRElbornJSDe SoyzaACOPD-bronchiectasis overlap syndromeEur Respir J201545231031325653262
- HahmCRLimMNKimHYImplications of the pulmonary artery to ascending aortic ratio in patients with relatively mild chronic obstructive pulmonary diseaseJ Thorac Dis2016871524153127499939
- KooHKHongYLimMNYimJJKimWJRelationship between plasma matrix metalloproteinase levels, pulmonary function, bronchodilator response, and emphysema severityInt J Chron Obstruct Pulmon Dis2016111129113727313452
- HongYJiWAnSHanSSLeeSJKimWJSex differences of COPD phenotypes in nonsmoking patientsInt J Chron Obstruct Pulmon Dis2016111657166227524891
- JiWLimMNBakSHDifferences in chronic obstructive pulmonary disease phenotypes between non-smokers and smokersClin Respir J201812266667327805311
- MillerMRHankinsonJBrusascoVStandardisation of spirometryEur Respir J200526231933816055882
- PellegrinoRViegiGBrusascoVInterpretative strategies for lung function testsEur Respir J200526594896816264058
- NaidichDPMcCauleyDIKhouriNFStitikFPSiegelmanSSComputed tomography of bronchiectasisJ Comput Assist Tomogr1982634374447096687
- BhallaMTurciosNAponteVCystic fibrosis: scoring system with thin-section CTRadiology199117937837882027992
- LynchDANewellJDTschomperBACinkTMNewmanLSBethelRUncomplicated asthma in adults: comparison of CT appearance of the lungs in asthmatic and healthy subjectsRadiology199318838298338351357
- LeeYKOhYMLeeJHQuantitative assessment of emphysema, air trapping, and airway thickening on computed tomographyLung2008186315716518351420
- MetsOMZanenPLammersJWEarly identification of small airways disease on lung cancer screening CT: comparison of current air trapping measuresLung2012190662963323064488
- KimHYSongKSGooJMLeeJSLeeKSLimTHThoracic sequelae and complications of tuberculosisRadiographics2001214839858 discussion 859–86011452057
- ChongSLeeKSChungMJHanJKwonOJKimTSPneumoconiosis: comparison of imaging and pathologic findingsRadiographics2006261597716418244
- PatelISVlahosIWilkinsonTMBronchiectasis, exacerbation indices, and inflammation in chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2004170440040715130905
- KwakHJMoonJYChoiYWHigh prevalence of bronchiectasis in adults: analysis of CT findings in a health screening programTohoku J Exp Med2010222423724221127394
- HwangYIParkYBYooKHRecent trends in the prevalence of chronic obstructive pulmonary disease in KoreaTuberc Respir Dis2017803226229
- GaoYHGuanWJLiuSXAetiology of bronchiectasis in adults: a systematic literature reviewRespirology20162181376138327321896
- MatsuokaSYamashiroTWashkoGRKuriharaYNakajimaYHatabuHQuantitative CT assessment of chronic obstructive pulmonary diseaseRadiographics2010301556620083585
- BonavitaJNaidichDPImaging of bronchiectasisClin Chest Med201233223324822640843
- HansellDMWellsAURubensMBColePJBronchiectasis: functional significance of areas of decreased attenuation at expiratory CTRadiology199419323693747972745
- ChurchillEDThe segmental and lobular physiology and pathology of the lungJ Thorac Surg194918327929318131320
- CulinerMMObliterative bronchitis and bronchiolitis with bronchiectasisDis Chest19634435136014072736
- MarinJMCiudadMMoyaVAirflow reversibility and long-term outcomes in patients with COPD without comorbiditiesRespir Med201410881180118824933205
- NishinoMSiewertBRobertsDHExcessive collapsibility of bronchi in bronchiectasis: evaluation on volumetric expiratory high-resolution CTJ Comput Assist Tomogr200630347447816778624
- NishinoMWashkoGRHatabuHVolumetric expiratory HRCT of the lung: clinical applicationsRadiol Clin North Am201048117718319995635
- GuanWJGaoYHXuGBronchodilator response in adults with bronchiectasis: correlation with clinical parameters and prognostic implicationsJ Thorac Dis201681142326904207