Abstract
Long-acting muscarinic antagonists (LAMAs), along with long-acting β2-agonists (LABAs), are the mainstay for treatment of patients with COPD. Glycopyrrolate, or glycopyrronium bromide, like other LAMAs, inhibits parasympathetic nerve impulses by selectively blocking the binding of acetylcholine to muscarinic receptors. Glycopyrrolate is unusual in that it preferentially binds to M3 over M2 muscarinic receptors, thereby specifically targeting the primary muscarinic receptor responsible for bronchoconstriction occurring in COPD. Inhaled glycopyrrolate is slowly absorbed from the lungs and rapidly eliminated from the bloodstream, most likely by renal excretion in its unmetabolized form, limiting the potential for systemic adverse events. Inhaled glycopyrrolate is a fast-acting, efficacious treatment option for patients with moderate–severe COPD. It improves lung function, reduces the risk of exacerbations, and alleviates the symptoms of breathlessness, which in turn may explain the improvement seen in patients’ quality of life. Inhaled formulations containing glycopyrrolate are well tolerated, and despite being an anticholinergic, few cardiovascular-related events have been reported. Inhaled glycopyrrolate is thus of value as both monotherapy and in combination with other classes of medication for maintenance treatment of COPD. This review covers the mechanism of action of inhaled glycopyrrolate, including its pharmacokinetic, pharmacodynamic, and safety profiles, and effects on mucus secretion. It also discusses the use of inhaled glycopyrrolate in the treatment of COPD, as monotherapy and in fixed-dose combinations with LABAs and inhaled corticosteroid–LABAs, including a triple therapy recently approved in Europe.
Plain language summary
Patients with COPD have narrowed airways and cannot fully empty their lungs, which together can make breathing uncomfortable. Doctors often prescribe an inhaler containing drugs that widen the airways or reduce inflammation in the lungs, making it easier for patients with COPD to breathe. Patients who do not show enough benefit from treatment with one drug alone may be given two or more drugs, which can be combined into one inhaler. One drug used to widen the airways in patients with COPD is glycopyrrolate (also referred to as glycopyrronium bromide). Glycopyrrolate can be used to treat COPD on its own, as well as in combination with other drugs. In this article, we discuss the evidence for how glycopyrrolate works in the body, how glycopyrrolate enters and leaves the body, and the effectiveness and side effects of glycopyrrolate when used to treat patients with COPD in clinical trials alone and combined in one inhaler with another airway-widening drug with or without a drug used to reduce inflammation in the lungs.
Introduction
Long-acting muscarinic antagonists (LAMAs) or long-acting β2-agonists (LABAs), alone or in combination, are the mainstay for the maintenance treatment of patients with COPD.Citation1,Citation2 In the 1980s, inhaled glycopyrrolate, also known as glycopyrronium bromide, was found to be a long-acting bronchodilatorCitation3,Citation4 and improved pulmonary function after exercise in patients with asthma,Citation5 although inhaled glycopyrrolate is not currently licensed for use in asthma.Citation6 Inhaled glycopyrrolate, a rapid-onset LAMA, is now US Food and Drug Administration (FDA)- and European Medicines Agency (EMA)-approved for maintenance treatment of patients with COPD.Citation1,Citation6,Citation7 This review covers the mechanism of action of inhaled glycopyrrolate, its pharmacokinetic (PK) and pharmacodynamic (PD) profiles, safety profile, effects on mucus secretion, and use in the treatment of COPD as monotherapy and in fixed-dose combinations (FDCs) with LABAs and inhaled corticosteroid (ICS)–LABAs.
Pharmacokinetics and pharmacodynamics of inhaled glycopyrrolate
Preganglionic parasympathetic nerves innervate the airways via the vagus nerve ().Citation8 At parasympathetic ganglia, preganglionic nerves synapse with postganglionic nerves.Citation8 Acetylcholine (ACh) is a neurotransmitter that is released during parasympathetic nerve impulses and acts by binding to and activating muscarinic receptors and nicotinic receptors.Citation8,Citation9 There are five muscarinic receptor subtypes, referred to as M1–M5. Citation9
Figure 1 Role of ACh and muscarinic receptors in the lung.
Abbreviations: ACh, acetylcholine; ASM, airway smooth muscle; CNS, central nervous system.
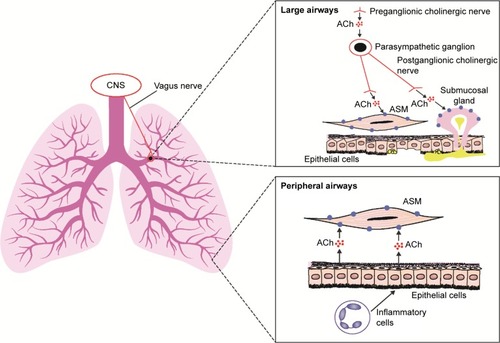
M1 receptors are highly expressed in the peripheral airways, whereas M2 and M3 receptors predominate in the larger airways.Citation8 M3 receptors are the muscarinic receptors primarily responsible for ACh-induced bronchoconstriction,Citation8 as they activate phospholipase C, which produces inositol 1,4,5-triphosphate and diacylglycerol, leading to intracellular calcium release.Citation9 Anticholinergics block parasympathetic nerve impulses by selectively preventing ACh from binding to muscarinic receptors.Citation8,Citation10 These drugs inhibit bronchoconstriction in peripheral airways by antagonizing the effects on airway smooth muscle cells of ACh released by epithelial cells; this release is stimulated by inflammatory cells.Citation11
The anticholinergic effects of inhaled glycopyrrolate are primarily limited to the airways, thereby reducing the likelihood of systemic adverse events (AEs).Citation12 Inhaled glycopyrrolate has a bioavailability of 57%, with 53% absorbed via the lungs.Citation12 Based on its ability to inhibit methacholine-induced calcium release (half-life [t½] 6.1±2.1 minutes), inhaled glycopyrrolate has a rapid onset of action.Citation13 Inhaled glycopyrrolate is long lasting in the body, with a terminal elimination-phase t½ of 52.5 hours following inhalation (vs t½ of 6.2 hours following intravenous administration).Citation12 Population PK modeling has shown that inhaled glycopyrrolate is absorbed slowly, predominantly unchanged, from the lungs.Citation12 It has a slow-phase absorption t½ of 3.5 days, accounting for 79% of drug absorption.Citation12 Furthermore, inhaled glycopyrrolate is eliminated rapidly from the bloodstream.Citation12 Intravenous glycopyrrolate is excreted, mainly in its unmetabolized form, by the kidney.Citation14,Citation15 Metabolism is less important for the elimination of this drug from the body.Citation14,Citation15 Renal excretion is also likely to be important for the elimination of glycopyrrolate systematically absorbed from the lung.Citation15 However, no inhaled glycopyrrolate dose adjustments are required for patients with mild–moderate renal impairment, and those with severe renal impairment may be given inhaled glycopyrrolate if the benefits are judged to outweigh the risks.Citation6
Pharmacokinetic and pharmacodynamic profiles of inhaled glycopyrrolate vs other LAMAs
Available anticholinergic drugs bind to all muscarinic ACh receptors present in the airways (ie, M1 – M3). Citation8,Citation16 Importantly, M3 receptors are the primary therapeutic target for bronchodilation, with antagonism at M2 autoreceptors tending to attenuate bronchodilator effects.Citation17 The PK and PD profiles of glycopyrrolate have been compared with other LAMAs in various preclinical studies, although not all of these studies tested the inhaled delivery of glycopyrrolate ().Citation13,Citation16,Citation18,Citation19 Glycopyrrolate is the only anticholinergic to date to show higher relative affinity for M3 than M2 receptors, although its absolute binding affinity for these receptors is lower than that of aclidinium and tiotropium.Citation13,Citation16 Glycopyrrolate, aclidinium, tiotropium, and ipratropium (a short-acting muscarinic antagonist) all dissociate more rapidly from M2 than M3 receptors, with all four drugs showing similar ratios of dissociation t½.Citation13,Citation16 In addition, glycopyrrolate has a shorter absolute dissociation time at both M2 and M3 receptors than at aclidinium and tiotropium.Citation16 An ex vivo study examining muscarinic receptor binding in the rat lung showed receptor binding lasted 24 hours for glycopyrrolate and tiotropium, whereas ipratropium binding was observed at 2 hours, but not at 12 hours.Citation18
Table 1 Preclinical studies comparing the pharmacological profile of glycopyrrolate with other LAMAs and ipratropium
In an in vivo study on guinea pigs, glycopyrrolate, ipratropium, and aclidinium had a similar onset of action, which was more rapid than tiotropium.Citation16 However, in isolated human airways, glycopyrrolate had significantly more rapid onset of action than both aclidinium and tiotropium.Citation19 Glycopyrrolate had a similar duration of action at M3 receptors to that of aclidinium and tiotropium in vitro, but had a shorter duration of action than tiotropium and aclidinium in vivo in guinea pig studies.Citation16 Sykes et al proposed that drug molecules, once dissociated from their target receptors, may not be able to diffuse away from the receptor environment and thus are likely to rebind to localized receptors.Citation13 The authors suggested that this may explain why some LAMAs have a long duration of action, despite rapid dissociation rates.Citation13 In an in vitro study, aclidinium was shown to have lower stability than tiotropium, which in turn had lower stability than glycopyrrolate in rat, guinea pig, and human plasma.Citation16 This indicates that glycopyrrolate undergoes a slower rate of hydrolysis, which may result in a propensity to cause anticholinergic AEs in patients with COPD.Citation16 These findings are in agreement with a previous study that compared the plasma stability of aclidinium with tiotropium and ipratropium, with aclidinium found to be the least stable of the three bronchodilators evaluated.Citation20 Furthermore, a lower dose of glycopyrrolate and tiotropium than aclidinium is required to inhibit salivation effectively in rats, possibly because of the slower rate of hydrolysis in the blood.Citation16
Inhaled glycopyrrolate: effects on mucus secretion and mucociliary clearance in COPD
Mucus is secreted by submucosal mucus glands and goblet cells in the bronchi, and excessive mucus secretion is a feature of chronic bronchitis and COPD.Citation21 M1 and M3 receptors are expressed at a 1:2 ratio in submucosal glands.Citation22 The M3 receptor is involved primarily in mediating mucus secretion, whereas water and electrolyte secretion are likely to be regulated by M3 and M1 receptors in combination.Citation22 LABAs are known to enhance mucociliary clearance in patients with COPD,Citation23 probably via an effect on ciliary beat frequency,Citation24 and there are numerous theories regarding the alteration of mucus production by LAMAs.Citation25 However, the results from clinical trials testing these theories are conflicting.Citation25 In an open-label, non-placebo-controlled trial in 22 patients with COPD, tiotropium reduced cough symptoms and nasal clearance times.Citation26 The authors concluded that this effect may result from inhibition of mucus hypersecretion and an increase in mucociliary clearance in the airways.Citation26 However, this supposition is not supported by other studies: in a randomized, double-blind trial, Hasani et al failed to find an effect of tiotropium on mucociliary clearance in 34 patients with COPD,Citation27 whereas Meyer et al reported that tiotropium treatment slowed mucociliary clearance in their randomized, open-label, crossover study in 24 patients.Citation28 Furthermore, in a double-blind, crossover study, ipratropium was actually found to decrease cough clearance of secretions in patients with COPD.Citation29
Oral glycopyrrolate is known to reduce drooling in children, and glycopyrrolate injections can reduce preoperative salivation, as well as respiratory secretions during end-of-life care.Citation30,Citation31 However, on review of published literature cited in PubMed, no studies could be found that examined the role of inhaled glycopyrrolate in mucus secretion. There also appear to be limited published data examining the effect of glycopyrrolate on mucociliary clearance,Citation25 and hence, there is a need for further study in this area.
The effect of inhaled glycopyrrolate on cognition
Glycopyrrolate is a water-soluble, highly polar quaternary ammonium compound, which limits its passage across lipid membranes such as the blood–brain barrier; therefore, glycopyrrolate does not exhibit any central nervous system activity.Citation32 Inhaled glycopyrrolate is thus unlikely to have a significant effect on cognition in patients with COPD, although there is a lack of evidence to support this.
Clinical evidence for the use of inhaled glycopyrrolate in patients with COPD
In its inhaled form, glycopyrrolate and other approved LAMAs are used for the management of COPD.Citation1,Citation6,Citation33–Citation37 Glycopyrrolate is available as a monotherapy via a dry-powder inhaler (DPI)Citation6 and was approved by the FDA in 2017 as a nebulized monotherapy ().Citation38 Inhaled glycopyrrolate is also available in an FDC with formoterol, delivered using co-suspension delivery technology in a pressurized metered dose inhaler (pMDI),Citation34 and in a DPI FDC with indacaterol.Citation35,Citation36 Inhaled glycopyrrolate has been approved in Europe as a triple FDC with formoterol and beclomethasone, delivered via a pMDI.Citation39
Table 2 Available glycopyrrolate and other LAMA formulations
Efficacy of inhaled glycopyrrolate monotherapy
Onset of action
In a randomized, double-blind study to determine the most appropriate dose of inhaled glycopyrrolate monotherapy (delivered via a DPI) for patients with moderate–severe COPD, glycopyrrolate 50 µg once daily (QD) provided significant bronchodilation over 24 hours.Citation40 However, the efficacy of glycopyrrolate 50 µg QD was not significantly different from the same total daily dose administered twice daily (BID).Citation40 The randomized GLOW trials were conducted to evaluate the use of 50 µg QD of inhaled glycopyrrolate for treating patients with moderate–severe COPD.Citation41–Citation46 The GLOW1 study showed that glycopyrrolate 50 µg QD had a rapid onset of bronchodilation in patients with COPD, with a significant improvement from baseline in lung function vs placebo as early as 5 minutes after treatment (P<0.001).Citation41 Supporting the preclinical data, inhaled glycopyrrolate has been shown to have faster onset of action than tiotropium in several clinical trials. In the randomized GLOW2 study, glycopyrrolate 50 µg QD resulted in significantly more rapid bronchodilation than tiotropium 18 µg QD treatment at all time points from 5 minutes to 4 hours after the first dose on day 1 (P<0.01).Citation42 Similarly, in the randomized GLOW5 study, glycopyrrolate 50 µg QD resulted in significantly more rapid improvement in lung function than tiotropium 18 µg QD treatment at 5 and 15 minutes after first dose.Citation45 Additionally, in a post hoc analysis of the randomized SPRING study in patients with moderate–severe COPD, glycopyrrolate 50 µg QD resulted in a significantly greater improvement in lung function than tiotropium 18 µg QD at 5 minutes, 15 minutes, and 1 hour after the first dose on day 1 (P=0.015, P=0.026, and P=0.014, respectively).Citation47 In a further study in patients with moderate–severe COPD, single doses of both glycopyrrolate (50 µg) and aclidinium (400 µg) had more rapid onset of action than tiotropium (18 µg), resulting in greater levels of bronchodilation 90 minutes after treatment, although the authors concluded that faster onset of action seen in the clinic may not be relevant for patients who are undergoing long-term treatment for chronic disease.Citation19
Lung function and other efficacy end points
The efficacy of inhaled glycopyrrolate monotherapy (via a DPI) has been investigated in patients with COPD in numerous clinical studies, including the GLOW study series and the GEM studies ().Citation38,Citation41–Citation52 Two network meta-analyses have also been conducted to compare the efficacy of glycopyrrolate with other LAMAs.Citation53,Citation54 The first (including 21 trials) compared glycopyrrolate 50 µg QD with aclidinium 400 µg BID, tiotropium 18 µg QD (HandiHaler), and tiotropium 5 µg QD (Respimat) in patients with moderate–severe COPD.Citation53 After 24 weeks, glycopyrrolate 50 µg treatment resulted in a similar improvement in lung function compared with aclidinium 400 µg, tiotropium 18 µg, and tiotropium 5 µg.Citation53 At the same time point, glycopyrrolate 50 µg treatment resulted in similar improvements in St George’s Respiratory Questionnaire (SGRQ) scores from baseline compared with aclidinium 400 µg and tiotropium 18 µg, and a greater improvement compared with tiotropium 5 µg.Citation53 Improvements in breathlessness symptoms were similar for all treatments.Citation53
Table 3 Clinical evidence of the efficacy of glycopyrrolate monotherapy in patients with COPD
The second network meta-analysis (including 24 trials) compared the efficacy of glycopyrrolate 50 µg QD, tiotropium 18 µg QD, aclidinium 400 µg BID, and umeclidinium 62.5 µg QD in patients with COPD.Citation54 At weeks 12 and 24, all LAMA treatments evaluated resulted in clinically relevant (>100 mL) improvements in trough forced expiratory volume in 1 second (FEV1) compared with placebo.Citation54 Glycopyrrolate 50 µg treatment resulted in the greatest improvement over placebo in lung function at week 24 (FEV1 difference 135.8 mL).Citation54 For all LAMAs vs placebo, improvements were seen from baseline in 24-week SGRQ and Transition Dyspnea Index (TDI) scores.Citation54 Glycopyrrolate 50 µg QD treatment did not reach the minimal clinically important difference (MCID) in SGRQ score of 4 units compared with placebo, but did reach the MCID of ≥1 for TDI focal score.Citation54 However, aclidinium 400 µg and umeclidinium 62.5 µg treatments reached MCID for both SGRQ and TDI focal scores compared with placebo, whereas tiotropium did not reach MCID for either of the two measures.Citation54
For patients with moderate–severe COPD, clinical trial data for treatment with inhaled glycopyrrolate as a monotherapy indicate that glycopyrrolate 50 µg QD (the EMA-approved dose) improves lung function and health-related quality of life (HRQoL), decreases the severity of breathlessness and the risk of exacerbations, and improves morning symptoms.Citation41,Citation42,Citation45,Citation47,Citation51 Furthermore, glycopyrrolate 50 µg QD was found to be noninferior to tiotropium in its ability to increase airflow to the lungs.Citation45 In a short-term, crossover trial (3-week treatment periods), glycopyrrolate also significantly improved patients’ abilities to exercise vs placebo.Citation43 In Phase III trials of glycopyrrolate 15.6 µg BID (the FDA-approved dose) in patients with moderate–severe COPD, there were also significant improvements in FEV1 scores from baseline, TDI focal scores, and SGRQ scores vs placebo.Citation48,Citation49 In two Phase III trials of the nebulized form of glycopyrrolate, 50 µg BID treatment resulted in significant and clinically important increases from baseline in lung function and SGRQ scores vs placebo.Citation38 Finally, in two studies in patients with moderate–severe COPD, glycopyrrolate was delivered by pMDI using innovative co-suspension delivery technology, which allows aerosol delivery of micronized drug suspended with microsized, phospholipid-based porous particles.Citation52,Citation55–Citation57 In these studies, patients treated with glycopyrrolate (doses ≥2.4 µg BID) showed clinically relevant, significant improvements in lung function from baseline vs placebo.Citation52,Citation57 Furthermore, the highest glycopyrrolate dose tested in the first study (18 µg BID) was found to be noninferior to tiotropium 18 µg QD for improving lung function.Citation52 In the second study, all glycopyrrolate doses (delivered as glycopyrronium 28.8, 14.4, 7.2, and 3.6 µg BID) were found to be noninferior to ipratropium 34 µg 4 times daily.Citation57 Based on these studies, 18 µg (equivalent to 14.4 µg glycopyrronium) was selected as the optimal glycopyrrolate dose for the glycopyrrolate–formoterol combination studies.Citation52,Citation57
Efficacy of inhaled glycopyrrolate combinations
Combining a LAMA with a LABA can increase efficacy, as they have distinctly different mechanisms of action (ie, target different receptors). In addition, LABAs modify the release of ACh, leading to amplification of bronchial smooth muscle relaxation induced by the LAMA.Citation58 Moreover, LABAs act on presynaptic β2-receptors in the efferent cholinergic pathway, resulting in the inhibition of cholinergic transmission.Citation59
In 2016, glycopyrrolate–formoterol pMDI 18/9.6 µg BID was licensed by the FDA “for the long-term maintenance treatment of airflow obstruction in patients with COPD”.Citation34 Several randomized controlled trials have examined the efficacy of glycopyrrolate–formoterol 18/9.6 µg BID ().Citation60,Citation61 Five studies (PINNACLE-1, PINNACLE-2, PT03011, PT03012, and PINNACLE-3) showed that (as expected) glycopyrrolate–formoterol 18/9.6 µg BID significantly improved lung function compared with individual components and placebo.Citation60–Citation62 In addition, glycopyrrolate–formoterol 18/9.6 µg BID was at least as efficacious as open-label tiotropium 18 µg QD.Citation61,Citation62 Furthermore, the efficacy of glycopyrrolate–formoterol 18/9.6 µg BID was maintained over the 1-year treatment period.Citation60,Citation61 A post hoc analysis of PINNACLE-1 and -2 indicated that glycopyrrolate–formoterol improved FEV1 independently of baseline symptom severity, although high baseline symptom severity was significantly correlated with greater improvement in health outcomes after treatment.Citation63
Table 4 Recent clinical evidence (2015 to present) of efficacy of glycopyrrolate combinations in patients with COPD
Inhaled glycopyrrolate has also been combined with indacaterol for maintenance therapy in patients with COPD. Glycopyrrolate–indacaterol DPI was approved for use by the FDA in 2015 (at 15.6/27.5 µg BID)Citation36 and by the EMA in 2013 (at 50/110 µg QD).Citation35 Glycopyrrolate–indacaterol 50/110 µg QD significantly improved lung function compared with individual components (P<0.001)Citation64 and tiotropium 18 µg QD (P=0.0017).Citation65 The glycopyrrolate–indacaterol combination was also preferred to tiotropium by both patients (P=0.00004) and physicians (P<0.0001).Citation65 Furthermore, glycopyrrolate–indacaterol 50/110 µg QD significantly increased lung function, and a significantly greater proportion of patients achieved a clinically relevant improvement in dyspnea compared with tiotropium 18 µg QD plus formoterol 12 µg BID.Citation66 In two clinical trials, glycopyrrolate–indacaterol 50/110 µg QD was more effective at reducing COPD exacerbations than the ICS–LABA combination fluticasone propionate–salmeterol 500/50 µg BID.Citation67,Citation68 Additionally, glycopyrrolate–indacaterol 50/110 µg QD was found to be noninferior to tiotropium 18 µg QD plus formoterol 12 µg BID in improving HRQoL.Citation66
The 15.6/27.5 µg BID formulation of glycopyrrolate–indacaterol DPI has been shown to perform favorably in improving lung function when compared with its individual componentsCitation69,Citation70 and provide clinically meaningful improvements in breathlessness and HRQoL.Citation69 Two crossover studies of glycopyrrolate–indacaterol 15.6/27.5 µg BID vs umeclidinium–vilanterol 62.5/25 µg QD did not meet their primary efficacy end point of noninferiority of change from baseline in FEV1 area under the curve from 0 to 24 hours at week 12. The authors concluded that although glycopyrrolate–indacaterol was not non-inferior to umeclidinium–vilanterol, the differences in improvement in lung function between the combinations were not clinically relevant.Citation71 Several clinical trials have indicated that glycopyrrolate–indacaterol is effective at improving lung function in patients with moderate–severe COPD,Citation68,Citation69,Citation71–Citation73 and patients with COPD and a high risk of exacerbations.Citation67 One of these trials also demonstrated that glycopyrrolate–indacaterol decreases hyperinflation and improves patients’ ability to undertake daily physical activity.Citation73
Glycopyrrolate also showed potential as a COPD treatment when added to the ICS–LABA combination fluticasone propionate–salmeterol.Citation74 After 12 weeks’ treatment, glycopyrrolate 50 µg QD plus fluticasone propionate–salmeterol 500/50 µg BID significantly improved lung function when compared with placebo plus fluticasone propionate–salmeterol, and was noninferior to tiotropium (18 µg QD) plus fluticasone propionate–salmeterol.Citation74
An inhaled triple FDC, glycopyrrolate–beclomethasone–formoterol (12.5/100/6 µg BID pMDI) was approved for use by the EMA in 2017 for the maintenance treatment of patients with COPD.Citation39 Two long-term trials have demonstrated that this triple FDC is efficacious in this patient group.Citation75,Citation76 In the first trial, the triple FDC was significantly more effective at improving lung function vs beclomethasone–formoterol at week 26.Citation75 The triple FDC also decreased the risk of exacerbations from baseline to a greater extent than beclomethasone–formoterol by week 52.Citation75 The second trial provided evidence that this triple FDC was significantly superior to tiotropium in reducing the exacerbation rate and improving lung function from baseline.Citation76
Safety of inhaled glycopyrrolate monotherapy
Several trials have shown that glycopyrrolate 50 µg QD DPI is well tolerated,Citation41–Citation47 with a similar overall incidence of AEs to tiotropium 18 µg QD.Citation42,Citation44,Citation45,Citation47 In a study comparing glycopyrrolate 50 µg QD with open-label tiotropium 18 µg QD, the incidences of AEs and serious AEs were similar between groups; AEs with incidence >10% were worsening COPD (24% [glycopyrrolate] vs 33% [tiotropium]) and nasopharyngitis (31% vs 33%, respectively).Citation44 Glycopyrrolate also had an acceptable safety profile at 15.6 µg BID,Citation48–Citation50 with AE incidence after 52 weeks’ treatment similar to that seen after treatment with indacaterol 75 µg QD.Citation50 Furthermore, nebulized glycopyrrolate (50 µg BID) was also shown to have an acceptable safety profile over 48 weeks’ treatment compared with tiotropium 18 µg QD.Citation51 Treatment discontinuation due to treatment-emergent AEs was higher for nebulized glycopyrrolate than for tiotropium (10% vs 3%, respectively); the authors suggested several reasons for this, including the open-label nature of the trial.Citation51 In another study, nebulized glycopyrrolate was well tolerated at 25 and 50 µg BID, as measured by the incidences of AEs and cardiovascular (CV) events.Citation38 Finally, in two chronic-dosing trials of glycopyrrolate pMDI delivered using co-suspension delivery technology, glycopyrrolate had an acceptable safety profile at all doses evaluated, with no unexpected safety findings reported.Citation52,Citation57
Newly prescribed LAMAs and LABAs have been associated with a greater risk of CV events compared with nonuse in patients with COPD (adjusted OR 1.14 [95% CI 1.01–1.28, P=0.03] and 1.31 [95% CI 1.12–1.52, P<0.001] for LAMAs and LABAs, respectively).Citation77 However, the studies discussed in this review provide no evidence for an increased risk of CV events with glycopyrrolate monotherapy vs tiotropium or indacaterol monotherapy.Citation45,Citation50 Furthermore, in one of the trials investigating nebulized glycopyrrolate, fewer patients treated with glycopyrrolate experienced major CV AEs than those treated with tiotropium (0.5% vs 1.7%).Citation51
Safety of inhaled glycopyrrolate combinations
The FDC glycopyrrolate–formoterol is designed to minimize the risk of AEs associated with high doses of LAMA or LABA monotherapies. In its Phase III clinical development program, glycopyrrolate–formoterol pMDI 18/9.6 µg BID showed a safety profile consistent with that of the individual components (PINNACLE-1 and -2), placebo (PINNACLE-1 and -2, PT03011, and PT03012), and open-label tiotropium 18 µg QD (PINNACLE-1 and PT03011).Citation60–Citation62
Furthermore, several clinical trials have indicated that the glycopyrrolate–indacaterol DPI combination at both EMA- and FDA-approved doses (50/110 µg QD and 15.6/27.5 µg QD, respectively) has good tolerability and an acceptable safety profile in patients with moderate–severe COPD.Citation65,Citation67–Citation69,Citation71–Citation73 A trial by Ferguson et al showed that the risk of CV events was similar with the glycopyrrolate–indacaterol FDC to with indacaterol alone.Citation70
Safety end points have also been assessed in triple FDC therapy regimens that incorporate glycopyrrolate. For example, in a study comparing glycopyrrolate plus fluticasone propionate–salmeterol with the ICS–LABA combination administered with either tiotropium 18 µg QD or placebo, the authors reported no significant differences between the number of AEs or severe AEs in any of the treatment groups evaluated.Citation74 In a study comparing the glycopyrrolate–beclomethasone–formoterol FDC with both tiotropium and beclomethasone–formoterol plus tiotropium, AE incidence was similar among treatment groups.Citation76 A further study comparing glycopyrrolate–beclomethasone–formoterol with beclomethasone–formoterol also reported similar rates of AEs between treatment groups, although one serious treatment-emergent AE (atrial fibrillation) occurred in a patient from the triple-FDC group.Citation75
Conclusion
Inhaled glycopyrrolate is a fast-acting, efficacious treatment option for patients with moderate–severe COPD and is available in a variety of doses. It improves lung function, reduces the risk of exacerbations, and alleviates the symptoms of breathlessness, which in turn may explain the improvement seen in patients’ QoL. Glycopyrrolate has comparable effects on lung function to tiotropium, and glycopyrrolate and aclidinium showed more rapid onset of action than tiotropium. Formulations containing inhaled glycopyrrolate are well tolerated and, despite being an anticholinergic, few CV-related events have been reported, with glycopyrrolate showing a similar safety profile to tiotropium. Inhaled glycopyrrolate is thus of value both as monotherapy and for optimizing bronchodilation when used in FDCs with LABAs and ICS–LABAs for maintenance treatment of COPD.
Author contributions
DPT and NJG made substantial contributions to the conception of this article, drafted, and critically revised it for important intellectual content; provided final approval of the version to be published; and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of the work are appropriately investigated and resolved.
Acknowledgments
We thank Johan Karlberg, MD, PhD, of the Clinical Trial Magnifier Newsletter for some of the information in this manuscript. Medical writing support was provided by Carly Hayes, PhD, of Core (London, UK) and editorial support was provided by Maryam Vahdat, PGDip of Core (London, UK), which was in accordance with Good Publication Practice (GPP3) guidelines and funded by AstraZeneca LP (Wilmington, DE, USA). AstraZeneca LP reviewed the manuscript for medical accuracy prior to submission.
Disclosure
DPT serves on advisory boards for AstraZeneca, Sunovion, Mylan, and Theravance/Innoviva, and as a speaker for AstraZeneca, Boehringer-Ingelheim, and Sunovion. NJG has no conflicts of interest.
References
- Global Initiative for Chronic Obstructive Lung Disease (GOLD)Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary DiseaseBethesda, MDGOLD2017
- QaseemAWiltTJWeinbergerSEDiagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory SocietyAnn Intern Med2011155317919121810710
- SchroeckensteinDCBushRKChervinskyPBusseWWTwelve-hour bronchodilation in asthma with a single aerosol dose of the anticholinergic compound glycopyrrolateJ Allergy Clin Immunol19888211151193392363
- WalkerFBKaiserDLKowalMBSurattPMProlonged effect of inhaled glycopyrrolate in asthmaChest198791149513792086
- JohnsonBESurattPMGalTJWilhoitSCEffect of inhaled glycopyrrolate and atropine in asthma: precipitated by exercise and cold air inhalationChest19848533253286697786
- FoodUSAdministrationDrugSeebri Neohaler [prescribing Information]2015 Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/207923lbl.pdfAccessed March 20, 2018
- European Medicines AgencyEuropean public assessment report: Seebri Breezhaler2012 Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002430/WC500133771.pdfAccessed March 20, 2018
- CazzolaMPageCPCalzettaLMateraMGPharmacology and therapeutics of bronchodilatorsPharmacol Rev201264345050422611179
- RackéKMatthiesenSThe airway cholinergic system: physiology and pharmacologyPulm Pharmacol Ther200417418119815219263
- FrankoBVLunsfordCDDerivatives of 3-pyrrolidinols – III: the chemistry, pharmacology, and toxicology of some N-substituted-3-pyrrolidyl α-substituted phenylacetatesJ Med Pharm Chem1960252354013701449
- BarnesPJAnticholinergicsCelliBRPharmacotherapy in Chronic Obstructive Pulmonary DiseaseNew YorkMarcel Dekker2004201216
- BartelsCLoobyMSechaudRKaiserGDetermination of the pharmacokinetics of glycopyrronium in the lung using a population pharmacokinetic modelling approachBr J Clin Pharmacol201376686887923506208
- SykesDADowlingMRLeighton-DaviesJThe influence of receptor kinetics on the onset and duration of action and the therapeutic index of NVA237 and tiotropiumJ Pharmacol Exp Ther2012343252052822854200
- KaltialaEPenttiläAVapaataloHLarmiTThe fate of intravenous (3H) glycopyrrolate in manJ Pharm Pharmacol19742653523544153115
- SechaudRRenardDZhang-AubersonLde la MotteSDrollmannAKaiserGPharmacokinetics of multiple inhaled NVA237 doses in patients with chronic obstructive pulmonary disease (COPD)Int J Clin Pharmacol Ther201250211812822257577
- GavaldàARamosICarcasonaCThe in vitro and in vivo profile of aclidinium bromide in comparison with glycopyrronium bromidePulm Pharmacol Ther201428211412124928173
- HanselTTBarnesPJTiotropium bromide: a novel once-daily anti-cholinergic bronchodilator for the treatment of COPDDrugs Today (Barc)200238958560012582447
- OgodaMNiiyaRKoshikaTYamadaSComparative characterization of lung muscarinic receptor binding after intratracheal administration of tiotropium, ipratropium, and glycopyrrolateJ Pharmacol Sci2011115337438221358117
- RoglianiPCalzettaLOraJPharmacological assessment of the onset of action of aclidinium and glycopyrronium versus tiotropium in COPD patients and human isolated bronchiEur J Pharmacol201576138339025952728
- SentellasSRamosIAlbertiJAclidinium bromide, a new, long-acting, inhaled muscarinic antagonist: in vitro plasma inactivation and pharmacological activity of its main metabolitesEur J Pharm Sci201039528329020093184
- RamosFLKrahnkeJSKimVClinical issues of mucus accumulation in COPDInt J Chron Obstruct Pulmon Dis2014913915024493923
- GosensRZaagsmaJMeursHHalaykoAJMuscarinic receptor signaling in the pathophysiology of asthma and COPDRespir Res200677316684353
- MelloniBGermoutyJThe influence of a new β-agonist: formoterol on mucociliary functionRev Mal Respir199295503507 French1439090
- LindbergSKhanRRunerTThe effects of formoterol, a long-acting β2-adrenoceptor agonist, on mucociliary activityEur J Pharmacol199528532752808575514
- RestrepoRDInhaled adrenergics and anticholinergics in obstructive lung disease: do they enhance mucociliary clearance?Respir Care20075291159117317716384
- TagayaEYagiOSatoAEffect of tiotropium on mucus hypersecretion and airway clearance in patients with COPDPulm Pharmacol Ther201639818427350218
- HasaniATomsNAgnewJESarnoMHarrisonAJDilworthPThe effect of inhaled tiotropium bromide on lung mucociliary clearance in patients with COPDChest200412551726173415136383
- MeyerTReitmeirPBrandPEffects of formoterol and tiotropium bromide on mucus clearance in patients with COPDRespir Med2011105690090621397483
- BennettWDChapmanWFMascarellaJMThe acute effect of ipratropium bromide bronchodilator therapy on cough clearance in COPDChest199310324884958432142
- HugelHEllershawJGamblesMRespiratory tract secretions in the dying patient: a comparison between glycopyrronium and hyoscine hydrobromideJ Palliat Med20069227928416629557
- US Food and Drug AdministrationMedical review: glycopyrrolate2009 Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2010/022571Orig1s000MedR.pdfAccessed March 20, 2018
- MirakhurRKDundeeJWJonesCJEvaluation of the anticholinergic actions of glycopyrronium bromideBr J Clin Pharmacol1978517784619938
- European Medicines AgencySeebri Breezhaler [summary of product characteristics]2012 Available from: https://www.medicines.org.uk/emc/medicine/27138Accessed March 20, 2018
- AstraZeneca PharmaceuticalsLPBevespi Aerosphere [prescribing information]2016 Available from: https://www.azpicentral.com/bevespi/bevespi_pi.pdfAccessed March 20, 2018
- European Medicines AgencyUltibro Breezhaler [summary of product characteristics]2016 Available from: https://www.medicines.org.uk/emc/medicine/29533Accessed March 20, 2018
- US Food and Drug AdministrationUtibron Neohaler [prescribing information]2017 Available from: https://www.accessdata.fda.gov/drug-satfda_docs/label/2015/207930s000lbl.pdfAccessed March 20, 2018
- European Medicines AgencyTrimbow [summary of product characteristics]2017 Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/004257/WC500233163.pdfAccessed March 20, 2018
- KerwinEDonohueJFGoodinTTosielloRWheelerAFergusonGTEfficacy and safety of glycopyrrolate/eFlow CS (nebulized glycopyrrolate) in moderate-to-very-severe COPD: Results from the glycopyrrolate for obstructive lung disease via electronic nebulizer (GOLDEN) 3 and 4 randomized controlled trialsRespir Med201713223825028838685
- European Medicines Agency Trimbow [initial authorization]2017 Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Summary_of_opinion_-_Initial_authorisation/human/004257/WC500228080.pdfAccessed March 20, 2018
- ArievichHOverendTRenardDA novel model-based approach for dose determination of glycopyrronium bromide in COPDBMC Pulm Med2012127423217058
- D’UrzoAFergusonGTvan NoordJAEfficacy and safety of once-daily NVA237 in patients with moderate-to-severe COPD: the GLOW1 trialRespir Res20111215622151296
- KerwinEHébertJGallagherNEfficacy and safety of NVA237 versus placebo and tiotropium in patients with COPD: the GLOW2 studyEur Respir J20124051106111423060624
- BeehKMSinghDDi ScalaLDrollmannAOnce-daily NVA237 improves exercise tolerance from the first dose in patients with COPD: the GLOW3 trialInt J Chron Obstruct Pulmon Dis2012750351322973092
- SekiyaMKawayamaTFukuchiYSafety and efficacy of NVA237 once daily in Japanese patients: the GLOW4 trialEur Respir J201240Suppl 56P2013
- ChapmanKRBeehKMBeierJA blinded evaluation of the efficacy and safety of glycopyrronium, a once-daily long-acting muscarinic antagonist, versus tiotropium, in patients with COPD: the GLOW5 studyBMC Pulm Med201414424438744
- WangCSunTHuangYEfficacy and safety of once-daily glycopyrronium in predominantly Chinese patients with moderate-to-severe chronic obstructive pulmonary disease: the GLOW7 studyInt J Chron Obstruct Pulmon Dis201510576825609940
- MarinJMBeehKMClemensAEarly bronchodilator action of glycopyrronium versus tiotropium in moderate-to-severe COPD patients: a cross-over blinded randomized study (Symptoms and Pulmonary Function in the Morning)Int J Chron Obstruct Pulmon Dis2016111425143427418815
- LaForceCFeldmanGSpangenthalSEfficacy and safety of twice-daily glycopyrrolate in patients with stable, symptomatic COPD with moderate-to-severe airflow limitation: the GEM1 studyInt J Chron Obstruct Pulmon Dis2016111233124327354782
- KerwinESilerTMKorenblatPEfficacy and safety of twice-daily glycopyrrolate versus placebo in patients with COPD: the GEM2 studyChronic Obstr Pulm Dis20163254955928848879
- MahlerDAGiffordAHSattiALong-term safety of glycopyrrolate: a randomized study in patients with moderate-to-severe COPD (GEM3)Respir Med2016115394527215502
- FergusonGTGoodinTTosielloRWheelerAKerwinELong-term safety of glycopyrrolate/eFlow CS in moderate-to-very-severe COPD: results from the Glycopyrrolate for Obstructive Lung Disease via Electronic Nebulizer (GOLDEN) 5 randomized studyRespir Med201713225126028919143
- FabbriLMKerwinEMSpangenthalSDose-response to inhaled glycopyrrolate delivered with a novel Co-Suspension Delivery Technology metered dose inhaler (MDI) in patients with moderate-to-severe COPDRespir Res201617110927586537
- KarabisALindnerLMocarskiMHuismanEGreeningAComparative efficacy of aclidinium versus glycopyrronium and tiotropium, as maintenance treatment of moderate to severe COPD patients: a systematic review and network meta-analysisInt J Chron Obstruct Pulmon Dis2013840542324043936
- IsmailaASHuismanELPunekarYSKarabisAComparative efficacy of long-acting muscarinic antagonist monotherapies in COPD: a systematic review and network meta-analysisInt J Chron Obstruct Pulmon Dis2015102495251726604738
- Lechuga-BallesterosDNogaBVehringRCummingsRHDwivediSKNovel cosuspension metered-dose inhalers for the combination therapy of chronic obstructive pulmonary disease and asthmaFuture Med Chem20113131703171821942257
- VehringRLechuga-BallesterosDJoshiVNogaBDwivediSKCosuspensions of microcrystals and engineered microparticles for uniform and efficient delivery of respiratory therapeutics from pressurized metered dose inhalersLangmuir20122842150151502322985189
- KerwinEMSpangenthalSKollarCRoseEStReisnerCA phase IIB randomized, chronic-dosing, incomplete block, cross-over study of glycopyrronium, delivered via metered dose inhaler, compared with a placebo and an active control in patients with moderate-to-severe COPDRespir Res20181913829506504
- CazzolaMMateraMGThe effective treatment of COPD: anticholinergics and what else?Drug Discov Today Ther Strateg200633277286
- BarisioneGBaroffioMCrimiEBrusascoVBeta-adrenergic agonistsPharmaceuticals (Basel)2010341016104427713285
- MartinezFJRabeKFFergusonGTEfficacy and safety of glycopyrrolate/formoterol metered dose inhaler formulated using Co-Suspension Delivery Technology in patients with COPDChest2017151234035727916620
- HananiaNATashkinDPKerwinEMLong-term safety and efficacy of glycopyrrolate/formoterol metered dose inhaler using novel Co-Suspension Delivery Technology in patients with chronic obstructive pulmonary diseaseRespir Med201712610511528427541
- ReisnerCGottschlichGFakihF24-h bronchodilation and inspiratory capacity improvements with glycopyrrolate/formoterol fumarate via co-suspension delivery technology in COPDRespir Res20171815728821260
- MartinezFJFabbriLMFergusonGTBaseline symptom score impact on benefits of glycopyrrolate/formoterol metered dose inhaler in COPDChest201715261169117828720336
- BatemanEDFergusonGTBarnesNDual bronchodilation with QVA149 versus single bronchodilator therapy: the SHINE studyEur Respir J20134261484149423722616
- KardosPHagedorn-PeinzIThe impact of indacaterol/glycopyrronium fixed-dose combination versus tiotropium monotherapy on lung function and treatment preference: a randomized crossover study: the FAVOR studyInt J Chron Obstruct Pulmon Dis201813697729317812
- BuhlRGessnerCSchuermannWEfficacy and safety of once-daily QVA149 compared with the free combination of once-daily tiotropium plus twice-daily formoterol in patients with moderate-to-severe COPD (QUANTIFY): a randomised, non-inferiority studyThorax201570431131925677679
- WedzichaJABanerjiDChapmanKRIndacaterol-glycopyrronium versus salmeterol-fluticasone for COPDN Engl J Med2016374232222223427181606
- ZhongNWangCZhouXLANTERN: a randomized study of QVA149 versus salmeterol/fluticasone combination in patients with COPDInt J Chron Obstruct Pulmon Dis2015101015102626082625
- MahlerDAKerwinEAyersTFLIGHT1 and FLIGHT2: efficacy and safety of QVA149 (indacaterol/glycopyrrolate) versus its monocomponents and placebo in patients with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med201519291068107926177074
- FergusonGTTaylorAFThachCLong-term maintenance bronchodilation with indacaterol/glycopyrrolate versus indacaterol in moderate-to-severe COPD patients: the FLIGHT 3 studyChronic Obstr Pulm Dis20163471672828848898
- KerwinEFergusonGTSanjarSDual bronchodilation with indacaterol maleate/glycopyrronium bromide compared with umeclidinium bromide/vilanterol in patients with moderate-to-severe COPD: results from two randomized, controlled, cross-over studiesLung2017195673974728993871
- VogelmeierCZhongNHumphriesMJIndacaterol/glycopyrronium in symptomatic patients with COPD (GOLD B and GOLD D) versus salmeterol/fluticasone: ILLUMINATE/LANTERN pooled analysisInt J Chron Obstruct Pulmon Dis2016113189319728008244
- WatzHMailänderCBaierMKirstenAEffects of indacaterol/glycopyrronium (QVA149) on lung hyperinflation and physical activity in patients with moderate to severe COPD: a randomised, placebo-controlled, crossover study (the MOVE study)BMC Pulm Med20161619527301417
- FrithPAThompsonPJRatnavadivelRGlycopyrronium once-daily significantly improves lung function and health status when combined with salmeterol/fluticasone in patients with COPD: the GLISTEN study, a randomised controlled trialThorax201570651952725841237
- SinghDPapiACorradiMSingle inhaler triple therapy versus inhaled corticosteroid plus long-acting β2-agonist therapy for chronic obstructive pulmonary disease (TRILOGY): a double-blind, parallel group, randomised controlled trialLancet20163881004896397327598678
- VestboJPapiACorradiMSingle inhaler extrafine triple therapy versus long-acting muscarinic antagonist therapy for chronic obstructive pulmonary disease (TRINITY): a double-blind, parallel group, randomised controlled trialLancet2017389100821919192928385353
- GershonACroxfordRCalzavaraACardiovascular safety of inhaled long-acting bronchodilators in individuals with chronic obstructive pulmonary diseaseJAMA Intern Med2013173131175118523689820
- FoodUSAdministrationDrug Lonhala Magnair [prescribing information]2017 Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/208437lbl.pdfAccessed March 20, 2018
- Boehringer Ingelheim Spiriva HandiHaler [prescribing information]2016 Available from: http://docs.boehringer-ingelheim.com/Prescribing%20Information/PIs/Spiriva/Spiriva.pdfAccessed March 20, 2018
- Boehringer IngelheimSpiriva Respimat [prescribing information]2017 Available from: http://docs.boehringer-ingelheim.com/Prescribing%20Information/PIs/Spiriva%20Respimat/spirivarespimat.pdfAccessed March 20, 2018
- Boehringer IngelheimStiolto Respimat [prescribing information]2016 Available from: http://docs.boehringer-ingelheim.com/Prescribing%20Information/PIs/Stiolto%20Respimat/stiolto.pdfAccessed March 20, 2018
- European Medicines Agency Spiolto Respimat [summary of product characteristics]2015 Available from: https://www.medicines.org.uk/emc/medicine/30495Accessed March 20, 2018
- European Medicines AgencyIncruse [summary of product characteristics]2017 Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002809/WC500167430.pdfAccessed March 20, 2018
- GlaxoSmithKlineIncruse Ellipta [prescribing information]2017 Available from: https://www.gsksource.com/pharma/content/dam/GlaxoSmithKline/US/en/Prescribing_Information/Incruse_Ellipta/pdf/INCRUSE-ELLIPTA-PI-PIL.PDFAccessed March 20, 2018
- European Medicines AgencyAnoro [summary of product characteristics]2017 Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002751/WC500168424.pdfAccessed March 20, 2018
- GlaxoSmithKlineAnoro Ellipta [prescribing information]2013 Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/203975s000lbl.pdfAccessed March 20, 2018
- GlaxoSmithKlineTrelegy Ellipta [prescribing information]2017 Available from: https://www.gsksource.com/pharma/content/dam/GlaxoSmithKline/US/en/Prescribing_Information/Trelegy/pdf/TRELEGY-PI-MG-IFU.PDFAccessed March 20, 2018
- European Medicines AgencyEklira Genuair [summary of product characteristics]2017 Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002211/WC500132661.pdfAccessed March 20, 2018
- AstraZeneca PharmaceuticalsLPTudorza Pressair [prescribing information]2012 Available from: http://www.azpicentral.com/tudorza/pi_tudorza.pdfAccessed March 20, 2018
- European Medicines AgencyBretaris Genuair [summary of product characteristics]2017 Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002706/WC500132732.pdfAccessed March 20, 2018
- European Medicines AgencyDuaklir Genuair [summary of product characteristics]2017 Available from: https://ec.europa.eu/health/documents/community-register/2014/20141119130022/anx_130022_en.pdfAccessed March 20, 2018
- Theravance BiopharmaRevefenacin peak inspiratory flow rate (PIFR) study in COPD Available from: https://clinicaltrials.gov/ct2/show/NCT3095456. NLM identifier: NCT3095456Accessed March 20, 2018
- Theravance BioPharmaTheravance Biopharma and Mylan submit new drug application to FDA for revefenacin (TD-4208) in adults with chronic obstructive pulmonary disease2017 Available from: http://investor.theravance.com/news-releases/news-release-details/theravance-biopharma-and-mylan-submit-new-drug-application-fdaAccessed March 20, 2018
