Abstract
Background
Systemic corticosteroids (SC) are an integral part of managing acute exacerbations of COPD (AECOPD). However, the optimal dose and duration vary widely in clinical practice. We hypothesized that the use of a “PowerPlan” order set in the electronic health system (EHS) that includes a 5-day SC order would be associated with a reduced steroid dose and length of stay (LOS) for individuals hospitalized with AECOPD.
Patients and methods
We conducted a retrospective cohort study of Medicare recipients discharged with an AECOPD diagnosis from our University Hospital from 2014 to 2016. Our EHS-based “COPD PowerPlan” order set included admission, laboratory, pharmacy, and radiology orders for managing AECOPD. The default SC option included intravenous methyl-prednisolone for 24 hours followed by oral prednisone for 4 days. The primary endpoint was the difference in cumulative steroid dose between the PowerPlan and the usual care group. Secondary endpoints included hospital LOS and readmission rates.
Results
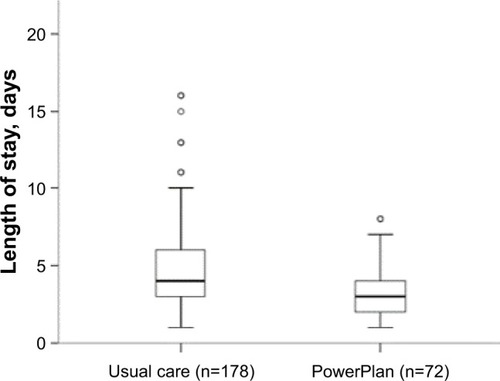
The 250 patients included for analysis were 62±11 years old, 58% male, with an FEV1 55.1%±23.6% predicted. The PowerPlan was used in 72 (29%) patients. Cumulative steroid use was decreased by 31% in the PowerPlan group (420±224 vs 611±462 mg, P<0.001) when compared with usual care. PowerPlan use was independently associated with decreased LOS (3 days; IQR 2–4 days vs 4 days; IQR 3–6 days, P=0.022) without affecting 30- and 90-day readmission rates.
Conclusion
Use of a standardized EHS-based order set to manage AECOPD was associated with a reduction in steroid dose and hospital LOS.
Introduction
COPD is a chronic and progressive disease, often punctuated by events of disease exacerbation. Acute exacerbations of COPD (AECOPD) are defined as an acute and sustained worsening beyond the normal day-to-day variation of the individual’s condition from a stable state and often warrants additional treatment.Citation1,Citation2 Admissions for AECOPD have an estimated in-patient mortality of 10%, and 4-year mortality can be as high as 45%.Citation3 AECOPD hospitalizations are also a major driver of COPD-related health care cost.Citation1,Citation4 Systemic corticosteroids (SC) use is an important component of treating severe exacerbations. SC provide symptomatic benefit, reduce exacerbation duration and hospital stay, and accelerate recovery in forced expiratory volume in one second (FEV1).Citation5–Citation7 However, the amount and duration of SC for treating an exacerbation vary widely across clinical practice. Prolonged steroid courses are associated with significant side effects such as muscle weakness, development of diabetes, loss of bone mineral density, and it is an independent risk factor for mortality.Citation8–Citation10 Given the adverse effect profile and high incidence of AECOPD, SC overuse should be minimized. The most updated GOLD (Global Initiative for Chronic Obstructive Lung Disease) guidelines now recommend a 5-day course of steroid for inpatient management of exacerbations based largely on results of the REDUCE trial.Citation11,Citation12
Despite the updated GOLD recommendation of 5-day course of SC, implementation into clinical practice has not been widely adopted. Electronic health system (EHS)-based preformatted order sets have been shown to be a tool to improve adherence to clinical guidelines and recommendations as well as to improve the daily workflow for providers. Prior studies have shown that order sets can be useful in patient assessment, guideline adherence, and standardization of treatment plans, which have resulted in improved clinical outcomes including benefits in hospital length of stay (LOS).Citation13–Citation15 However, many of these studies did not employ a short course of corticosteroids as recommended by GOLD, and there are limited data demonstrating the effectiveness of such an order set in a real-world setting.
We hypothesized that implementing a standardized EHS-based order set, termed the “COPD PowerPlan” would be associated with a lower total amount of SC used and LOS without impacting risk of future AECOPD.
Patients and methods
Study design and patients
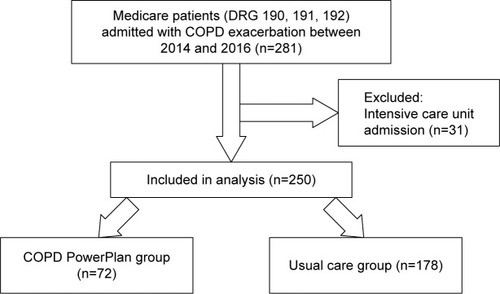
This was a retrospective cohort study of Medicare recipients who were discharged with a diagnosis of AECOPD from the University Hospital between January 1, 2014, and December 31, 2016. An AECOPD was defined by diagnosis related codes 190, 191, and 192 as the primary discharge diagnosis. Patients were excluded if they were admitted or transferred to the intensive care unit as the COPD PowerPlan was designed primarily to be used in nonintensive inpatient care units (). The study was approved by the University of Alabama at Birmingham (UAB) Institutional Review Board (IRB) (approval number: X121221005). Informed consent was waived because this study involved chart review only and the IRB determined that the research involved no more than minimal risk to the subjects, the research could not practicably be carried out without the waiver of informed consent, and the waiver will not adversely affect the rights and welfare of the subjects. To maintain confidentiality, data were collected, de-identified, and stored electronically on a secure server that was accessible only by study personnel. Study results were grouped and generalized and not directly linked to individual participants. Historic (prehospital) spirometry values were recorded if available.
Outcomes
The primary outcome of our study was to determine the difference in cumulative SC dose between individuals treated with COPD PowerPlan use vs those treated without using the PowerPlan, the “usual care” group. SC doses were converted to prednisone equivalents based on known equivalencies for ease of comparison. For example, 1 mg of methylprednisolone equates to 1.25 mg of prednisone.
Secondary outcomes included LOS, duration of intravenous steroid use in days, dose and duration of oral prednisone use, as well as all-cause hospital readmission rates at 30 and 90 days.
EHS-based order set: the COPD exacerbation PowerPlan
The UAB Hospital system uses IMPACT EHS software developed by Cerner (Millennium Version; Kansas City, MO, USA). The COPD PowerPlan is a comprehensive order set that was developed by a panel of experts including physicians, respiratory therapists, nurses, and pharmacists. The COPD PowerPlan was implemented as a part of the UAB EHS in November 2013 as part of the Bundled Payments for Care Improvement Initiative.Citation16
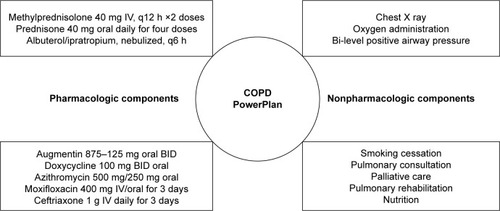
As shown in , the COPD PowerPlan order set contained orders for 1) SC therapy that included methylprednisolone 40 mg intravenous (IV) every 12 hours for two doses followed by 4 days of oral 40 mg prednisone, 2) options for nebulized therapies, 3) options for antibiotic regimens, 4) laboratory and imaging orders, 5) noninvasive ventilation, and 6) nonpharmacologic interventions.
Figure 2 Components included in the PowerPlan order set.
The PowerPlan was initiated and activated by providers at the time of hospital admission. Clinicians were educated and encouraged to use the PowerPlan at meetings, and using electronic reminders and through hospital administrative reminders in the 3 months prior to its implementation and quarterly in the first year of its launch. Use of the PowerPlan was encouraged but was optional. If providers chose to use the PowerPlan, they could use the default settings, including the short course of methylprednisolone or the provider could tailor the therapeutic regimen including corticosteroid type, dose, and duration based on personal preference and the clinical situation.
Statistical analysis
Data were expressed as mean±SD. Continuous variables were evaluated for normality based on quantile–quantile (QQ) plots and Shapiro–Wilk testing. Independent t-tests were used to compare means of continuous variables and chi-square for categorical variables between groups.
Binary logistic regression was performed to determine if patient characteristics at the time of admission influenced provider’s decision to activate the PowerPlan. Factors associated with PowerPlan use (P-value of <0.1) on univariate logistic regression were entered into a multivariate model.
Univariate linear regression was performed to determine predictors of hospital LOS. Variables associated with LOS on univariate linear regression models with a P-value <0.1 were entered into the final multivariable models. Cox-proportional hazards models were used to define time to readmission. We used SPSS (version 24.0; IBM Corporation, Armonk, NY, USA) for all statistical analyses. Statistical significance was set at P-value <0.05.
Results
Of 281 Medicare patients discharged with an AECOPD diagnosis between January 2014 and December 2016, 250 were included in the analysis as shown in . The 250 patients were 62±11 years old, 58% males, 71% white with an FEV1 55.1%±23.6% predicted. Spirometry data were available in 70% of the patient population (66% [118/178] in the PowerPlan and 78% [56/72] in the usual care group; P=0.07). We had no other missing data for other variables. Baseline characteristics of the cohort are shown in and the PowerPlan was used in 72 patients (29%). Demographically, both the groups were similar. Prior AECOPD were more common in the PowerPlan group compared with the usual care group (44% vs 29%, P=0.021). The PowerPlan group had a higher pack-year history of smoking when compared with the usual care group. Patients in the PowerPlan group had a lower FEV1% and a higher proportion of current smokers, though these differences were not statistically significant. The incidence of comorbidities such as hypertension, diabetes, congestive heart failure, obstructive sleep apnea, and coronary artery disease did not differ between the two groups.
Table 1 Baseline characteristics
Factors associated with PowerPlan selection
As shown in , current smoking status and prior AECOPD were the only factors significantly associated with PowerPlan use on univariate analyses. In a multivariate model that included FEV1 percent predicted, smoking status, smoking history, or hospitalization for AECOPD within 12 months of the index visit, no variable was significantly associated with PowerPlan use.
Table 2 Associations between clinical factors and PowerPlan use
PowerPlan use and cumulative corticosteroid use
As shown in , cumulative steroid dose was 31% lower in the PowerPlan group when compared with the usual care group (420±224 vs 611±462 mg, P<0.001). This reduction was driven by a 47% decrease in intravenous SC administration and a 24% decrease in oral SC use in the PowerPlan group when compared with the usual care group.
Table 3 PowerPlan use and systemic corticosteroid therapy
The mean duration of intravenous SC use was lower by 1 day in the PowerPlan group (2.2±1.2 vs 3.2±1.9 days, P<0.001). Patients in the PowerPlan group had fewer days of total SC treatment compared with the usual care group, though this failed to reach statistical significance (9.6 vs 13 days, P=0.075).
PowerPlan use was associated with reduced length of stay
Patients in the PowerPlan group had shorter hospital LOS compared with those in the control group (). In unadjusted linear regression models, age, PowerPlan use, SC use, and cumulative steroid dose were inversely associated with hospital LOS. PowerPlan use was independently associated with LOS (beta =−0.92, P=0.006), when adjusted for age, sex, race, and smoking status as shown in .
Table 4 Predictors of hospital LOS
PowerPlan use and all-cause hospital readmission
The proportion of participants readmitted within 30 days was the same between the two groups (25% in each group, P=0.96). There were no differences in 90-day readmissions between groups (44% in the PowerPlan group vs 38% in the usual care group, P=0.37). There were no differences in time until readmission in the PowerPlan group vs the usual care group (90 vs 93 days, hazard ratio [HR] 1.31 95% CI 0.86–1.99, P=0.21).
Discussion
We show that the use of a standardized EHS-based COPD PowerPlan was associated with lower amount and shorter duration of parenteral corticosteroids use for managing patients hospitalized with an AECOPD. The use of the PowerPlan and lower SC use were also associated with a reduction in hospital stay and were not associated with changes in 30- and 90-day readmission rates. To our knowledge, this is the first study to apply the lessons learned in the REDUCE trial in a quaternary care hospital in the United States using an EHS-based intervention.Citation12
SC are an integral component of managing COPD exacerbations. The VA SCCOPE trial published in 1999 was the first randomized controlled trial to evaluate the efficacy of glucocorticoids in managing AECOPD. In that study, a 2-week course of corticosteroid (methylprednisolone and prednisone) decreased the rate of treatment failure by 10% when compared with placebo and reduced the LOS in the hospital by 1.2 days.Citation7 In 2013, the REDUCE trial further demonstrated that a 5-day course of corticosteroid was non-inferior to a 14-day course with regards to re-exacerbation rates within the following 6 months.Citation12 These findings were later confirmed by a meta-analysis, which also showed no difference in readmission rates and outcomes with a shorter course of corticosteroids when compared with a conventional 10–14 days course.Citation17 However, real-world experience after implementation of short-course CS treatment for exacerbations is lacking and, despite the GOLD recommendations for a 5–7 days course of CS for management of AECOPD hospitalizations,Citation11 CS dosing and duration vary widely across clinical practice, and many patients are still treated with unnecessarily long steroid tapers.Citation18 Prolonged courses of systemic steroid use are associated with an increased risk of adverse effects including hypertension, hyperglycemia, edema, osteoporosis, and peripheral muscle weakness and can contribute to prolonged hospitalization.Citation7–Citation10
We showed that the use of an EHS-based intervention was associated with a 31% reduction in SC dose. This finding was driven by an earlier transition from intravenous to oral SC therapy. These findings are consistent with the results from a study by Sonstein et al in 2014 where the use of an EHS-based COPD order set significantly decreased the amount of parenteral steroid used in the first 48 hours of admission but did not affect hospital LOS.Citation19 This could be explained by the fact that their order set was developed in 2011 when a 10–14-day course of steroids was recommended for treating acute exacerbations.
Hospitalizations for COPD exacerbations account for up to 40%–60% of total COPD-related costs.Citation20,Citation21 These costs are also affected by disease severity, age, and presence of comorbidities.Citation22,Citation23 Average costs per hospitalization can range from $680 to $6,770 for stage 1 and stage 3 COPD.Citation23 A study from 2012 showed the annual inpatient expenditure for AECOPD admissions to be as high as $11.9 billion.Citation24 In addition to the financial burden, a prolonged hospital stay is also associated with cognitive disturbances, depression, social isolation, immobility, and increased risk of iatrogenic complications further contributing to deterioration in quality of life and increased readmissions.Citation25–Citation29 These factors are all associated with increased health care utilization which makes reducing LOS a priority for health care systems.
In our cohort, patients who were treated using the PowerPlan had a shorter LOS when compared with the usual care group by 1.1 days. This difference is significant in terms of reducing cost and morbidity associated with prolonged hospitalization. We believe this observed reduced LOS was driven by an earlier transition to oral corticosteroids and not due to other aspects of care included in the PowerPlan, such as smoking cessation, pulmonary rehabilitation, or antibiotic use. There is always a concern for increase in readmission rates with a shorter hospital stay. We did not find any difference in 30- or 90-day readmission rates between the two groups.
EHS-based algorithms and order sets have shown to improve provider adherence to practice guidelines.Citation13–Citation15 Two recent studies also showed a reduction in LOS with implementation of EHS-based COPD order sets.Citation13,Citation30 These interventions mainly focused on improving adherence to the multidisciplinary components of the order set to reduce LOS and avoid prescription errors rather than promoting a tailored corticosteroid regimen. However, our focus was to educate providers and reduce the corticosteroid dosing by encouraging the use of PowerPlan. This is particularly important as COPD GOLD guidelines have evolved over time and their implementation in clinical practice still lags behind.Citation31
Our study has the limitations of being a cohort study, and thus we are not able to assign causation to our intervention. Additionally, patients were allocated into groups based on individual provider decision making, introducing selection bias, and the potential for confounding by indication. While this may be the case, we did not observe an association between clinical factors (demographics, lung function, smoking status, and prior AECOPD) and the use of the PowerPlan in logistic regression models adjusted for these covariates, though participants in the PowerPlan group were more often current smokers and were more likely to have had a hospitalization for an AECOPD within the 12 months prior to the index admission, factors that could potentially bias the results against the intervention. Furthermore, we did not observe that comorbidities or baseline COPD interventions (home oxygen use or chronic steroid use) influenced selection of PowerPlan by the provider. Another limitation was that the PowerPlan did not control the dose and duration of outpatient corticosteroids prescribed by the provider at the time of hospital discharge, which may have accounted for the longer than expected duration of steroid use in the PowerPlan group. Despite the longer overall duration of corticosteroid use, the PowerPlan group did receive fewer days of outpatient corticosteroids when compared with the usual care group (5.1±4.6 vs 6.9±6.3 days, P=0.018). Lastly, only 29% of patients were treated with PowerPlan. Although this reflects a relatively low usage rate by the providers, it is higher than prior reports showing a 19% utilization rate of order sets in their first few years of launch.Citation32 We also noted that the use of PowerPlan increased from 24% in 2014 to 31% in 2016. This reflects a positive trend in practice change over the years.
Conclusion
We have demonstrated the real-world applicability of using a standardized EHS-based intervention on reducing corticosteroid exposure and hospital LOS in managing patients hospitalized with AECOPD without adversely affecting hospital readmissions. These findings suggest health systems can safely adopt EHS-based COPD treatment plans using currently accepted standard treatment regimens.
Author contributions
All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Acknowledgments
SPB was supported by the NIH/NHLBI (5K23HL133438), CSW was supported by the NIH (5TL1TR1418), and JMW was supported by the NIH/NHLBI (K08HL123940).
Disclosure
The authors report no conflicts of interest in this work.
References
- Rodriguez-RoisinRToward a consensus definition for COPD exac-erbationsChest20001175 Suppl 2398S401S10843984
- BurgeSWedzichaJACOPD exacerbations: definitions and classificationsEur Respir J Suppl20034146S53S12795331
- PiquetJChavaillonJMDavidPHigh-risk patients following hospitalisation for an acute exacerbation of COPDEur Respir J201342494695523349446
- YuAPYangHWuEQSetyawanJMocarskiMBlumSIncremental third-party costs associated with COPD exacerbations: a retrospective claims analysisJ Med Econ201114331532321500975
- DaviesLAngusRMCalverleyPMOral corticosteroids in patients admitted to hospital with exacerbations of chronic obstructive pulmonary disease: a prospective randomised controlled trialLancet1999354917745646010465169
- ThompsonWHNielsonCPCarvalhoPCharanNBCrowleyJJControlled trial of oral prednisone in outpatients with acute COPD exacerbationAm J Respir Crit Care Med19961542 Pt 14074128756814
- NiewoehnerDEErblandMLDeupreeRHEffect of systemic glucocorticoids on exacerbations of chronic obstructive pulmonary disease. Department of Veterans Affairs Cooperative Study GroupN Engl J Med1999340251941194710379017
- McevoyCEEnsrudKEBenderEAssociation between corticosteroid use and vertebral fractures in older men with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med19981573 Pt 17047099517579
- DecramerMLacquetLMFagardRRogiersPCorticosteroids contribute to muscle weakness in chronic airflow obstructionAm J Respir Crit Care Med1994150111168025735
- SmyllieHCConnollyCKIncidence of serious complications of corticosteroid therapy in respiratory disease. A retrospective survey of patients in the Brompton hospitalThorax19682365715815304705
- VogelmeierCFCrinerGJMartinezFJGlobal Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report. GOLD Executive SummaryAm J Respir Crit Care Med2017195555758228128970
- LeuppiJDSchuetzPBingisserRShort-term vs conventional glucocorticoid therapy in acute exacerbations of chronic obstructive pulmonary disease: the REDUCE randomized clinical trialJAMA2013309212223223123695200
- BrownKEJohnsonKJDeronneBMParentiCMRiceKLOrder set to improve the care of patients hospitalized for an exacerbation of chronic obstructive pulmonary diseaseAnn Am Thorac Soc201613681181527058777
- TerasakiJSinghGZhangWWagnerPSharmaGUsing EMR to improve compliance with clinical practice guidelines for management of stable COPDRespir Med2015109111423142926475055
- KitchluAAbdelshaheedTTullisEGuptaSGaps in the inpatient management of chronic obstructive pulmonary disease exacerbation and impact of an evidence-based order setCan Respir J201522315716225886627
- BhattSPWellsJMIyerASResults of a medicare bundled payments for care improvement initiative for chronic obstructive pulmonary disease readmissionsAnn Am Thorac Soc201714564364828005410
- WaltersJAETanDJWhiteCJSystemic corticosteroids for acute exacerbations of chronic obstructive pulmonary diseaseCochrane Database Syst Rev20149CD001288
- VondracekSFHemstreetBARetrospective evaluation of systemic corticosteroids for the management of acute exacerbations of chronic obstructive pulmonary diseaseAm J Health Syst Pharm200663764565216554288
- SonsteinLClarkCSeidenstickerSZengLSharmaGImproving adherence for management of acute exacerbation of chronic obstructive pulmonary diseaseAm J Med2014127111097110424927911
- HalpinDMMiravitllesMChronic obstructive pulmonary disease: the disease and its burden to societyProc Am Thorac Soc20063761962316963544
- MiravitllesMMurioCGuerreroTGisbertRCosts of chronic bronchitis and COPD: a 1-year follow-up studyChest2003123378479112628879
- TorabipourAHakimAAhmadi AngaliKDolatshahMYusofzadehMCost Analysis of Hospitalized Patients with Chronic Obstructive Pulmonary Disease: A State-Level Cross-Sectional StudyTanaffos2016152758227904538
- HillemanDEDewanNMaleskerMFriedmanMPharmacoeconomic evaluation of COPDChest200011851278128511083675
- PereraPNArmstrongEPSherrillDLSkrepnekGHAcute exacerbations of COPD in the United States: inpatient burden and predictors of costs and mortalityCOPD20129213114122409371
- CreditorMCHazards of hospitalization of the elderlyAnn Intern Med199311832192238417639
- GudmundssonGGislasonTJansonCDepression, anxiety and health status after hospitalisation for COPD: a multicentre study in the Nordic countriesRespir Med20061001879315893921
- GillTMAlloreHGHolfordTRGuoZHospitalization, restricted activity, and the development of disability among older personsJAMA2004292172115212415523072
- IbañezMAguilarJJMaderalMASexuality in chronic respiratory failure: coincidences and divergences between patient and primary caregiverRespir Med2001951297597911778795
- IyerASBhattSPGarnerJJDepression is associated with readmission for acute exacerbation of chronic obstructive pulmonary diseaseAnn Am Thorac Soc201613219720326599286
- ParikhRShahTGTandonRCOPD exacerbation care bundle improves standard of care, length of stay, and readmission ratesInt J Chron Obstruct Pulmon Dis20161157758327042046
- SharifRCuevasCRWangYAroraMSharmaGGuideline adherence in management of stable chronic obstructive pulmonary diseaseRespir Med201310771046105223639271
- SandhuSKChuJYurkovichMHarrimanDTaraboantaCFitzgeraldJMVariations in the management of acute exacerbations of chronic obstructive pulmonary diseaseCan Respir J201320317517923762887