Abstract
Purpose
Patients with severe COPD are at high risk of experiencing disease exacerbations, which require additional treatment and are associated with elevated mortality and increased risk of future exacerbations. Some patients continue to experience exacerbations despite receiving triple inhaled therapy (ICS plus LAMA plus LABA). Roflumilast is recommended by the Global Initiative for Chronic Obstructive Lung Disease as add-on treatment to triple inhaled therapy for these patients. This cost-effectiveness analysis compared costs and quality-adjusted life-years for roflumilast plus triple inhaled therapy vs triple inhaled therapy alone, using data from the REACT and RE2SPOND trials.
Patients and methods
Patients included in the analysis had severe to very severe COPD, FEV1 <50% predicted, symptoms of chronic bronchitis and ≥2 exacerbations per year. Our model was adapted from a previously published and validated model, and the analyses conducted from a UK National Health Service perspective. A scenario analysis considered a subset of patients who had experienced at least one COPD-related hospitalization within the previous year.
Results
Roflumilast as add-on to triple inhaled therapy was associated with non-significant reductions in rates of both moderate and severe exacerbations compared with triple inhaled therapy alone. The incremental cost-effectiveness ratio (ICER) for roflumilast as add-on to triple inhaled therapy was £24,976. In patients who had experienced previous hospitalization, roflumilast was associated with a non-significant reduction in the rate of moderate exacerbations, and a statistically significant reduction in the rate of severe exacerbations. The ICER for roflumilast in this population was £7,087.
Conclusions
Roflumilast is a cost-effective treatment option for patients with severe or very severe COPD, chronic bronchitis, and a history of exacerbations. The availability of roflumilast as add-on treatment addresses an important unmet need in this patient population.
Introduction
COPD is characterized by persistent, often progressive, airflow limitation, with symptoms including cough, dyspnea, and sputum production. COPD is a leading cause of death worldwide, and imposes a considerable humanistic and economic burden.Citation1 Patients with severe COPD, defined as post-bronchodilator forced expiratory volume in 1 second (FEV1) <50%,Citation2 are at particularly high risk of experiencing periods of disease exacerbation.Citation3 As well as requiring additional treatment, disease exacerbations contribute to a poor prognosis and increased mortality,Citation2 and are the most reliable predictor of future exacerbations, contributing to a worsened disease state.Citation3–Citation5 Thus, a major part of COPD management in the more severe disease states is the treatment of exacerbations, to limit their impact and frequency.Citation2
Treatment for COPD is based on bronchodilator therapy, often with a long-acting beta-2 agonist (LABA), which is also used in combination with a long-acting muscarinic antagonist (LAMA). Patients with severe or very severe COPD (defined as FEV1 <50%) who experience exacerbations may have inhaled corticosteroids (ICS) added to their existing treatment, either in combination with LABA, or with LABA and LAMA as triple inhaled therapy.Citation2 Despite this, some of these patients continue to experience exacerbations, and require additional treatment. Roflumilast (AstraZeneca) is a phosphodiesterase type four inhibitor that blocks inflammatory pathways in COPD. It is recommended by the Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines,Citation2 and is licensed by the European Medicines Agency,Citation6 as add-on maintenance treatment to bronchodilator therapy for patients with FEV1 <50% predicted and chronic bronchitis who have had at least one exacerbation in the past year. Roflumilast is also approved by the Food and Drug Administration in the United States of America (USA) for patients with severe COPD associated with chronic bronchitis and a history of exacerbations.Citation7 In the United Kingdom (UK), the National Institute for Health and Care Excellence (NICE) has recently recommended roflumilast as an add-on to bronchodilator maintenance treatment for severe COPD (post-bronchodilator FEV1 <50% predicted) in adult patients with chronic bronchitis and a history of exacerbations.Citation8
The safety and efficacy of roflumilast as an add-on to ICS/LABA ± LAMA were examined in the 52-week Roflumilast and Exacerbation in patients receiving Appropriate Combination Therapy (REACT) study (NCT1329029) and the 52-week Roflumilast Effect on Exacerbations in Patients on Dual therapy (RE2SPOND) study (NCT1443845).Citation9,Citation10 Patients enrolled in both studies had severe COPD and chronic bronchitis, and had experienced at least two moderate or severe exacerbations in the past year. The primary endpoint, reduction in the rate of moderate-to-severe COPD exacerbations, was not met in either trial. A reduction in the rate of moderate-to-severe exacerbations was observed, but did not reach statistical significance.Citation9,Citation10 Although the rates of adverse events were higher in the roflumilast group than in the placebo group in both trials, mortality was similar.
The objective of this analysis was to assess the lifetime costs, outcomes, and cost-effectiveness of roflumilast as an add-on to triple inhaled therapy, compared with triple inhaled therapy alone, in patients with FEV1 <50% predicted and chronic bronchitis who continued to experience exacerbations while receiving triple inhaled therapy. Eligible patients were pooled from the intention-to-treat (ITT) populations of the REACT and RE2SPOND studies, and the model used was adapted from a previously published and validated economic model by Samyshkin et al, 2014.Citation11
Methods
Setting and patient population
This analysis compared costs and outcomes between patients with severe to very severe COPD with FEV1 <50% predicted, symptoms of chronic bronchitis, and frequent exacerbations (≥2 per year), receiving either roflumilast maintenance treatment added on to triple inhaled therapy (ICS/LABA + LAMA), or triple inhaled therapy alone. The analysis was conducted from a UK National Health Service (NHS) and Personal Social Services perspective.
Patient-level data pooled from the ITT populations in the REACT and RE2SPOND trials were used in this analysis. Patient baseline characteristics are shown in . Only patients who received LAMA in addition to ICS/LABA (70% of the REACT population; 47% of the RE2SPOND population) were eligible for inclusion in this analysis. The 2018 GOLD guidelines recognize that roflumilast should be considered as an add-on to triple inhaled therapy in patients with an FEV1 <50% predicted and chronic bronchitis, particularly if they have experienced at least one hospitalization for an exacerbation in the previous year, which represents the patient population used in this analysis.Citation2 This is supported by clinical data indicating that ~90% of patients who receive roflumilast in clinical practice do so as add-on to triple inhaled therapy.Citation12 Therefore, the data used in this analysis were from those patients who are most likely to benefit from roflumilast treatment. In total, 1,225 patients receiving roflumilast plus triple inhaled therapy and 1,215 patients receiving triple inhaled therapy alone were included in the base case analysis.
Table 1 Baseline characteristics in the pooled REACT/RE2SPOND population (ITT population receiving ICS/LABA + LAMA)
Model structure and clinical parameters
A cohort state transition (Markov) model adapted from a previous study by Samyshkin et al, 2014,Citation11 which examined the cost-effectiveness of roflumilast as add-on to LABA alone, was used in this analysis.Citation11 The model has three states: severe COPD, very severe COPD, and death. COPD severity was defined by the GOLD airflow limitation criteria,Citation2 expressed as FEV1 relative to the general population. COPD is characterized by chronic lung function decline; therefore, once patients had transitioned to the very severe state in the model, there was no possibility of this being reversed. In the base case analysis, all patients entered the model in the severe state; thus, patients could transition from severe COPD to very severe COPD, and from either COPD state to death. Key model inputs for the base case are given in .
Table 2 Key model inputs for the base case
Transition probabilities for progression from severe to very severe COPD were calculated using the estimated time taken to reach an FEV1 of 30% predicted. The general population FEV1 level was predicted separately for men and women,Citation13 using reference equations based on a previous study conducted in the USA, and actual FEV1 was assumed to decline at a rate of 52 mL per year, based on the findings of Tantucci and Modina, 2012.Citation14 Transition probabilities were also estimated separately for men and women, weighted by the proportion of men and women in the model. The predicted average transition time to very severe COPD was 6.97 years, with a monthly transition probability from severe to very severe COPD of 1.2% ().
Figure 1 Calculation of average time to very severe COPD state, used to calculate transition probabilities.
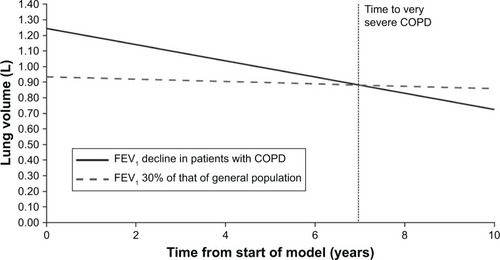
Although roflumilast is associated with an improvement in lung function,Citation13 this is not included in the model base case, which adopted a common FEV1 trajectory for both the roflumilast and placebo arms. Reductions in exacerbations in the model are therefore driven entirely by observed exacerbation rate ratios, and not by any possible effect through improved lung function.
Analysis of exacerbations
Patients could experience a moderate, a severe, or no exacerbation in each model cycle. Exacerbation rates were predicted using data from the pooled ITT populations from REACT and RE2SPOND. In these trials, a moderate exacerbation was defined as requiring treatment with oral or parenteral corticosteroids, and a severe exacerbation was defined as necessitating hospital admission or causing death.Citation9,Citation10 In the cost-effectiveness model the rate of moderate or severe exacerbations was predicted using a predefined negative binomial regression model. Explanatory variables included randomized treatment, background treatment stratum (LAMA or no LAMA), trial (REACT, RE2SPOND) and COPD severity (defined by GOLD status).
Note that the primary efficacy analysis in the REACT trial employed a Poisson regression model. This was consistent with the pivotal M2-124 and M2-125 trials and the approach to determine the sample size (following regulatory guidance). This model did not find significant differences between the groups; however, a predefined sensitivity analysis using a negative binomial regression to assess the robustness of the primary analysis showed that the effect was statistically significant.Citation9 A negative binomial regression model was preferred in the RE2SPOND trial, and there were no significant differences between the two treatments for the primary efficacy variable.Citation10
The lack of significant treatment effect for roflumilast in RE2SPOND might be due to the fact that patients in this study had less severe COPD than the study population in REACT. Two key differences between the study populations suggest that there may have been a disparity in disease severity. Firstly, a lower proportion of patients in RE2SPOND received triple inhaled therapy (47%) than in REACT (70%), and secondly patients were only required to have received LAMA for 3 months prior to randomization in RE2SPOND, whereas patients in REACT had received LAMA for at least 12 months.
Cost-effectiveness, time horizon and discounting
Cost-effectiveness was expressed as incremental cost per quality-adjusted life year (QALY) gained. The base case time horizon was 40 years, representing a lifetime time horizon, and costs and outcomes were discounted at 3.5% per annum in line with NICE guidance.Citation15
Resource use and costs
Drug costs and dosing for roflumilast, LAMA, ICS/LABA, and prednisolone (used in the treatment of moderate exacerbations), were taken from the British National Formulary (July 2016).Citation16 Resource use and monthly maintenance costs for severe and very severe COPD were taken from British Medical Journal (BMJ) Best Practice Guidance (2016) and previous publications,Citation17,Citation18 and included General Practitioner (GP) consultations, spirometry, influenza vaccinations, and oxygen therapy. Resource use and costs associated with exacerbations were taken from BMJ Best Practice Guidance (2016), NHS reference costs, and previous publications,Citation17,Citation19–Citation21 and included GP consultations, taken as one visit for a moderate exacerbation and none for a severe exacerbation; prednisolone treatment; and, for severe exacerbations, hospital admission and ambulance costs. Full details of costs and resource use are included in Tables S1 and S2.
Utilities
Health-related quality of life weights (utilities) associated with COPD health states were taken from the study of Ruttenvan Mölken et al, 2006,Citation22 which sampled 1,235 patients in 13 countries using the 5-dimension EuroQoL questionnaire (EQ-5D) and applied the UK general population tariff. All patients in this study had FEV1 less than 70%, and approximately 49% of the population had severe or very severe COPD. Decrements (disutilities) associated with exacerbations were taken from Hoogendoorn et al, 2011, based on patient-reported EQ-5D scores using the UK tariff.Citation23
Mortality
Within the model, death could occur as a consequence of a severe exacerbation or from a cause not related to COPD (background mortality). Mortality due to a severe exacerbation was obtained from the UK National COPD Audit Report,Citation24 with an adjustment for patient age.Citation11 Background mortality for severe and very severe COPD was calculated using age- and sex-specific UK life tables and standardized mortality ratios as employed in the previous study by Samyshkin et al, 2014.Citation11 We also included a 90-day post-COPD hospitalization mortality risk of 15.3% (Connolly et al, 2006).Citation25 This modification arose in the context of the recent appraisal of roflumilast by NICE. Rather than modify the model to accommodate tunnel states (during which additional mortality could apply) in the post-discharge period, this was applied as an immediate mortality penalty at the point of exacerbation.
Treatment-emergent adverse events
Rates of treatment-emergent severe adverse events were obtained from the ITT populations of the REACT and RE2SPOND trials. Adverse events occurred in approximately 16% of patients, and were comparable between treatment groups. The most frequently reported grade three adverse events were pneumonia, diarrhea, nausea, and weight decrease. The incidence of pneumonia was similar between the roflumilast and placebo groups, but comparatively more patients in the roflumilast group reported nausea, weight decrease, and abdominal pain.
Uncertainty
Probabilistic sensitivity analyses (10,000 iterations) were performed to assess uncertainty in the model inputs for the base case scenario. One-way deterministic sensitivity analyses were also used to assess the impact of varying individual parameters. Probability distributions for relevant model inputs are given in Table S3.
Two scenario analyses were also performed:
Post-hospitalization mortality
Scenario analyses were conducted to assess the impact of mortality assumptions on the incremental cost-effectiveness ratio (ICER) by varying mortality risk. Mortality risks were taken from Roberts et al, 2002 (90-day post-hospitalization mortality risk of 13.7%),Citation26 Soler-Cataluna et al, 2005 (permanent post-hospitalization hazard ratio of 2.235),Citation27 Hartl et al, 2016 (90-day post-hospitalization mortality risk of 10.8%),Citation28 and Wildman et al, 2009 (180-day post-hospitalization mortality risk of 37.9%),Citation29 termed scenarios 1–4 respectively.
Patients with severe COPD and prior hospitalization
A second scenario analysis assessed the effect of roflumilast in a subgroup of patients with severe COPD, who had experienced two or more exacerbations and at least one COPD-related hospitalization within the previous year, despite treatment with triple inhaled therapy. Data were available for 444 patients randomized to roflumilast and 405 patients randomized to placebo. Baseline characteristics for this population are shown in . Treatment-emergent adverse events were reported by 23% of this population: 20.5% of patients receiving roflumilast and 24.9% of patients receiving placebo. The most frequently reported adverse effect was pneumonia, followed by diarrhea and nausea.
Software
The model in our analysis was developed in Microsoft Excel. Regression analyses were performed in SAS 9.2 (SAS Institute, Cary, NC, USA).
Results
Exacerbation rates in the REACT/RE2SPOND pooled population
Exacerbation rates for the base case analysis are shown in . It was estimated that roflumilast as an add-on to triple inhaled therapy was associated with a non-significant reduction in the combined rate of moderate or severe exacerbations, compared with triple inhaled therapy alone (rate ratio [RR]: 0.89, 95% CI: 0.78–1.00, P=0.056, 11% reduction). When considered separately, estimates for both moderate (RR: 0.92, 95% CI: 0.81–1.05, P=0.220; 8% reduction) and severe exacerbation rates (RR: 0.86, 95% CI: 0.70–1.05, P=0.137; 14% reduction) were also lower with roflumilast than with triple therapy alone, although without statistical significance.
Table 3 Exacerbation rates in the pooled REACT and RE2SPOND population (base case) and in the scenario analyses
Cost-effectiveness in the REACT/RE2SPOND pooled population (base case)
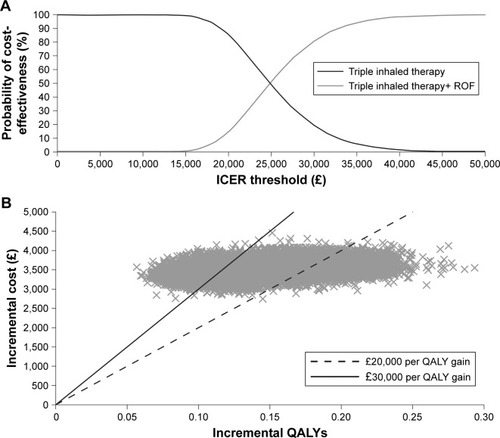
In the full patient population, triple inhaled therapy+ roflumilast accumulated total costs of £19,524 and 5.23 QALYs, whereas triple inhaled therapy alone accumulated costs of £16,016 and 5.09 QALYs (). This equated to an additional 0.14 QALYs at an incremental cost of £3,508 for triple inhaled therapy+ roflumilast, generating a deterministic ICER of £24,976. In probabilistic analyses, triple inhaled therapy+ roflumilast accumulated an additional 0.14 QALYs at an incremental cost of £3,528, generating an ICER of £24,682 ( and ). Triple inhaled therapy had a 15% probability of being cost-effective at a threshold of £20,000 per QALY gained, increasing to 81% at £30,000 per QALY gained ().
Table 4 Costs and QALYs in the pooled REACT and RE2SPOND population (base case) and in the scenario analyses
Figure 2 Probabilistic sensitivity analyses for the base case scenario.
Abbreviations: ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life year; ROF, roflumilast.
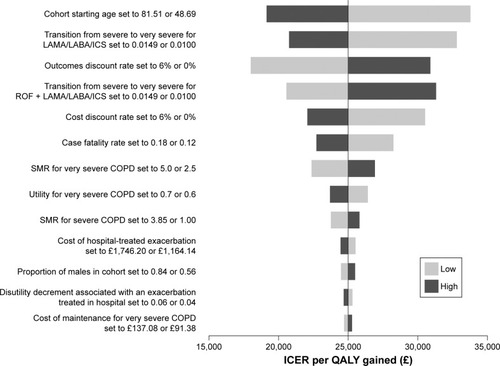
Deterministic sensitivity analyses showed that the most influential parameter in the model was the starting age of the patient. Other influential parameters were the transition from the severe to very severe state in those receiving tripled inhaled therapy alone and the discount rate. The ICER remained under £35,000 per QALY gained for all sensitivity analyses ().
Scenario analyses varying the assumptions made for post-hospitalization mortality
The ICERs generated for four populations using different post-hospitalization mortality assumptions are shown in . The lowest ICER generated was £16,293 in scenario 4, using the 180-day post-hospitalization mortality rate in Wildman et al, 2009,Citation29 and the highest ICER generated was £31,202 in scenario 2, using the permanent post-hospitalization hazard ratio in Soler-Cataluna et al, 2005.Citation27
Scenario analysis including a subpopulation of patients with prior hospitalization Exacerbation rates
In the patient population with prior hospitalization it was estimated that, in comparison to placebo, the addition of roflumilast to triple inhaled therapy was associated with a significant reduction in the combined rate of moderate or severe exacerbations (RR: 0.74, CI: 0.60–0.92, P=0.005; 26% reduction; ). In this analysis, the addition of roflumilast to triple inhaled therapy was also associated with a statistically significant reduction in the rate of severe exacerbations (RR: 0.66, CI: 0.49–0.88, P=0.004; 34% reduction), and a non-statistically significant reduction in the rate of moderate exacerbations (RR: 0.86, CI: 0.68–1.09, P=0.214, 14% reduction).
Cost-effectiveness analysis
When all patients who entered the model had experienced prior hospitalization due to COPD, triple inhaled therapy+ roflumilast was associated with an additional 0.48 QALYs at an incremental cost of £3,401, generating an ICER of £7,087 ().
Discussion
Roflumilast as an add-on to triple inhaled therapy was found to be cost-effective for the treatment of patients in the UK with severe to very severe COPD, chronic bronchitis, and a history of exacerbations, compared with triple inhaled therapy alone. This analysis formed part of the submission to NICE to support the reimbursement of roflumilast, following which NICE updated its guidance (Technology Appraisal guidance 461 [TA461]) to recommend the use of roflumilast in England and Wales as an add-on to triple inhaled therapy for patients with severe COPD and a history of two or more exacerbations in the previous 12 months despite receiving triple inhaled therapy.
This analysis is the first to assess exacerbation rates and cost-effectiveness specifically in a patient population receiving roflumilast in addition to triple inhaled therapy. Although no statistically significant interaction was found between roflumilast and the use of LAMA in addition to ICS/LABA on exacerbation rate, patients receiving concomitant LAMA were pre-specified as a subgroup prior to the unblinding of both the REACT and RE2SPOND trials, justifying the use of this population in our analysis. The analysis was conducted using a de novo economic model based on a previously published and validated model by Samyshkin et al, 2014.Citation11 Although previous analyses also used data from the REACTCitation9 and RE2SPONDCitation10 trials, they included a mixed population of patients receiving roflumilast as an add-on to ICS/LABA with or without concomitant LAMA. In our analysis, roflumilast as add-on to triple inhaled therapy was associated with a reduction in the rate of moderate/severe COPD disease exacerbations compared with triple inhaled therapy alone; the P-value for this comparison was 0.056, indicating that the difference between groups is just outside the boundaries of conventional statistical significance. The deterministic ICER for roflumilast was £24,976 per QALY gained, and probabilistic analyses generated an ICER of £24,682.
The earlier analysis by Samyshkin et al, 2014 examined the cost-effectiveness of roflumilast as add-on treatment to ICS/LABA in a patient population with chronic bronchitis and a history of exacerbations, using data from the pivotal M2-124 and M2-125 trials, and reported an ICER of £19,505 per QALY gained for roflumilast as add-on therapy.Citation11 The model structure used by Samyshkin et al, 2014, formed the basis of the model in our study; however, there were important differences between the models, not limited to the alternative clinical evidence bases. Although exacerbation case-fatality was an important feature in Samyshkin et al, 2014, and the general mortality risk in patients with COPD relative to the general population was incorporated in the model, mortality in the period following hospital discharge was not addressed. During the recent NICE appraisal of roflumilast, an interest was expressed in considering post-discharge mortality in the model. We therefore modified the model to incorporate a 90-day post-hospitalization mortality risk. Our model also used different utility values from those in the previous study, and adopted a slightly longer time horizon. A reconciliation with the original model was successfully performed, however, and indicated that the models were comparable.
The effect of roflumilast in improving lung function was not incorporated in our model, which may have meant that the treatment benefit of roflumilast was underestimated. Furthermore, the model included the assumption that the occurrence of an exacerbation did not affect FEV1 or increase the probability of future exacerbations, meaning that the true impact of exacerbations may also have been underestimated. Therefore, the treatment effect and cost-effectiveness of roflumilast generated by our analyses may be conservative estimates.
The results of scenario analyses indicated that varying the post-hospitalization mortality risk incorporated into the model impacts the ICER generated. As the post-hospitalization mortality assumption used in the base case analysis may be an underestimate of mortality, the ICER generated in this study may be a conservative estimate of cost-effectiveness.
In the scenario analysis in which all patients had previously experienced hospitalization following an exacerbation, roflumilast as an add-on to triple therapy was associated with a significant reduction in exacerbation rates compared with triple therapy alone. Roflumilast as add-on to triple therapy was also considered a cost-effective treatment option. Hospitalizations following an exacerbation are associated with a poor long-term prognosis, higher mortality, and increased resource use.Citation2 Furthermore, the incidence of an exacerbation is itself considered to perpetuate future exacerbations, leading to a further decline in lung function and a worsened health state.Citation4 This indicates that roflumilast is cost-effective in a patient population that may be at a particularly high risk of adverse outcomes.
Conclusion
In this analysis, roflumilast as an add-on to triple inhaled therapy was associated with a reduction in both moderate and severe exacerbations, and was a cost-effective option when compared with triple inhaled therapy alone. This analysis formed the basis of the submission to NICE, which has since made a positive recommendation for the use of roflumilast as an add-on to triple inhaled therapy in patients with severe COPD, chronic bronchitis, and a history of exacerbations.Citation8 Disease exacerbations can impose a considerable burden on individuals with COPD, resulting in reduced quality of life, elevated risk of future exacerbations and increased mortality. Therefore, the availability of a new add-on treatment shown to reduce exacerbation rates addresses an important unmet need for patients in this subgroup.
Acknowledgments
Medical writing support was provided by Caroline Freeman and Lucia Giles from PharmaGenesis Oxford Central, Oxford, UK. Funding was provided by AstraZeneca UK Limited.
Supplementary materials
Table S1 Drug costs used in the model
Table S2 Costs associated with COPD maintenance and exacerbations
Table S3 Probability distributions used in probabilistic sensitivity analyses
Disclosures
Chris Kiff was employed by ICON plc at the time that these analyses were conducted. ICON plc was funded by AstraZeneca UK Limited to provide analytical support for this study. Chris Kiff is currently employed by Bristol Myers-Squibb, Uxbridge, UK. Sandrine Ruiz is an employee of AstraZeneca, Barcelona, Spain, and does not hold any stocks. Nebibe Varol was an employee of AstraZeneca UK Limited at the time that these analyses were conducted, and does not hold any stocks. Nebibe Varol is currently employed by Bristol Myers-Squibb, Uxbridge, UK. Danny Gibson is an employee of AstraZeneca UK Limited. Andrew Davies was employed by ICON plc at the time that these analyses were conducted. ICON plc was funded by AstraZeneca UK Limited to provide analytical support for this study. Andrew Davies is currently employed by Stockbridge Economic Appraisal Ltd., Edinburgh, UK. Debasree Purkayastha is an employee of Phastar, and was funded by AstraZeneca UK Limited to provide statistical support for this study.
References
- World Health OrganizationChronic obstructive pulmonary disease (COPD). Fact sheet2016 Available from: http://www.who.int/media-centre/factsheets/fs315/en/Accessed June 2017
- Global Initiative for Chronic Obstructive Lung DiseaseGlobal strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease – 2018 Available from: http://goldcopd.org/wp-content/uploads/2017/11/GOLD-2018-v6.0-FINAL-revised-20-Nov_WMS.pdfAccessed December 2017
- HurstJRVestboJAnzuetoASusceptibility to exacerbation in chronic obstructive pulmonary diseaseN Engl J Med2010363121128113820843247
- HurstJRDonaldsonGCQuintJKGoldringJJBaghai-RavaryRWedzichaJATemporal clustering of exacerbations in chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2009179536937419074596
- SuissaSDell’anielloSErnstPLong-term natural history of chronic obstructive pulmonary disease: severe exacerbations and mortalityThorax2012671195796322684094
- European Medicines AgencyRoflumilast. Summary of Product Characteristics18112016 Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/001179/WC500095209.pdfAccessed June 2017
- Food and Drug AdministrationDaliresp (roflumilast) highlights of prescribing information2011 Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/022522s003lbl.pdfAccessed June 2017
- National Institute for Health and Care ExcellenceRoflumilast for treating chronic obstructive pulmonary disease2017 Available from: https://www.nice.org.uk/guidance/ta461Accessed August 27, 2018
- MartinezFJCalverleyPMGoehringUMBroseMFabbriLMRabeKFEffect of roflumilast on exacerbations in patients with severe chronic obstructive pulmonary disease uncontrolled by combination therapy (REACT): a multicentre randomised controlled trialLancet2015385997185786625684586
- MartinezFJRabeKFSethiSEffect of Roflumilast and Inhaled Corticosteroid/Long-Acting β2-Agonist on Chronic Obstructive Pulmonary Disease Exacerbations (RE(2)SPOND). A Randomized Clinical TrialAm J Respir Crit Care Med2016194555956727585384
- SamyshkinYKotchieRWMörkACBriggsAHBatemanEDCost-effectiveness of roflumilast as an add-on treatment to long-acting bronchodilators in the treatment of COPD associated with chronic bronchitis in the United KingdomEur J Health Econ2014151698223392624
- National Institute for Health and Care ExcellenceAppraisal consultation document. Roflumilast for treating chronic obstructive pulmonary disease12017 Available from: https://www.nice.org.uk/guidance/GID-TA10062/documents/appraisal-consultation-documentAccessed June 2017
- CrapoROMorrisAHGardnerRMReference spirometric values using techniques and equipment that meet ATS recommendationsAm Rev Respir Dis198112366596647271065
- TantucciCModinaDLung function decline in COPDInt J Chron Obstruct Pulmon Dis20127959922371650
- National Institute for Health and Care ExcellenceGuide to the methods of technology appraisal2013 Available from: https://www.nice.org.uk/process/pmg9/chapter/forewordAccessed June 2017
- Joint Formulary CommitteeBritish National Formulary2016
- British Medical Journal. Best Practice. COPD2016 Available from: http://bestpractice.bmj.com/best-practice/monograph/7.htmlAccessed June 2017
- OostenbrinkJBRutten-van MölkenMPMonzBUFitzgeraldJMProbabilistic Markov model to assess the cost-effectiveness of broncho-dilator therapy in COPD patients in different countriesValue Health200581324615841892
- CurtisLBurnsAUnit costs of health and social care. Canterbury: personal social services research unit2015 Available from: http://www.pssru.ac.uk/project-pages/unit-costs/2015/Accessed June 2017
- Department of HealthNHS reference costs 2014–2015 Available from: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/477919/2014-15_Reference_costs_publication.pdfAccessed December 2017
- ThomasMRadwanAStonhamCMarshallSCOPD exacerbation frequency, pharmacotherapy and resource use: an observational study in UK primary careCOPD201411330030924152210
- Rutten-van MölkenMPOostenbrinkJBTashkinDPBurkhartDMonzBUDoes quality of life of COPD patients as measured by the generic EuroQol five-dimension questionnaire differentiate between COPD severity stages?Chest200613041117112817035446
- HoogendoornMHoogenveenRTRutten-van MölkenMPVestboJFeenstraTLCase fatality of COPD exacerbations: a meta-analysis and statistical modelling approachEur Respir J201137350851520595157
- National COPD Audit ProgrammeNational COPD Audit Programme. COPD: Who cares matters National Chronic Obstructive Pulmonary Disease (COPD) Audit Programme: Clinical audit of COPD exacerbations admitted to acute units in England and Wales 2014. National clinical audit report2015 Available from: http://www.hqip.org.uk/public/cms/253/625/24/77/COPD%20National%20audit%20report%20-%20exacerbations%20-%202015.PDF?realName=zTtPO9.pdfAccessed August 27, 2018
- ConnollyMJLoweDAnsteyKAdmissions to hospital with exacerbations of chronic obstructive pulmonary disease: Effect of age related factors and service organisationThorax2006611084384816928716
- RobertsCMLoweDBucknallCERylandIKellyYPearsonMGClinical audit indicators of outcome following admission to hospital with acute exacerbation of chronic obstructive pulmonary diseaseThorax200257213714111828043
- Soler-CataluñaJJMartínez-GarcíaMARomán SánchezPSalcedoENavarroMOchandoRSevere acute exacerbations and mortality in patients with chronic obstructive pulmonary diseaseThorax2005601192593116055622
- HartlSLopez-CamposJLPozo-RodriguezFRisk of death and readmission of hospital-admitted COPD exacerbations: European COPD AuditEur Respir J201647111312126493806
- WildmanMJSandersonCFGrovesJSurvival and quality of life for patients with COPD or asthma admitted to intensive care in a UK multicentre cohort: the COPD and Asthma Outcome Study (CAOS)Thorax200964212813218852157