Abstract
Introduction
Acute exacerbation of COPD (AECOPD) is associated with poor outcome. Noninvasive ventilation (NIV) is recommended to treat end-stage COPD. We hypothesized that changing breathing pattern of COPD patients on NIV could identify patients with severe AECOPD prior to admission.
Methods
This is a prospective monocentric study including all patients with COPD treated with long-term home NIV. Patients were divided in two groups: a stable group in which patients were admitted for the usual respiratory review and an exacerbation group in which patients were admitted for inpatient care of severe AECOPD. Data from the ventilator were downloaded and analyzed over the course of the 10 days that preceded the admission.
Results
A total of 62 patients were included: 41 (67%) in the stable group and 21 (33%) in the exacerbation group. Respiratory rate was higher in the exacerbation group than in the stable group over the 10 days preceding inclusion (18.2±0.5 vs 16.3±0.5 breaths/min, respectively) (P=0.034). For 2 consecutive days, a respiratory rate outside the interquartile limit of the respiratory rate calculated over the 4 preceding days was associated with an increased risk of severe AECOPD of 2.8 (95% CI: 1.4–5.5) (P<0.001). This assessment had the sensitivity, specificity, positive predictive, and negative predictive values of 57.1, 80.5, 60.0, and 78.6% respectively. Over the 10 days’ period, a standard deviation (SD) of the daily use of NIV >1.0845 was associated with an increased risk of severe AECOPD of 4.0 (95% CI: 1.5–10.5) (P=0.001). This assessment had the sensitivity, specificity, positive predictive, and negative predictive values of 81.0, 63.4, 53.1, and 86.7%, respectively.
Conclusion
Data from NIV can identify a change in breathing patterns that predicts severe AECOPD.
Introduction
Acute exacerbations of COPD (AECOPD) are associated with a higher mortality and have a negative impact on the quality of life.Citation1,Citation2 AECOPD-related hospitalizations have an important economic burden for the health care system. Their cost has been estimated to 3,090USD per patient per year and is the leading driver of overall costs (56.7%).Citation3 AECOPD-related hospitalizations are more frequent in patients with end-stage COPD.Citation4
Patients with end-stage COPD benefit from the use of home noninvasive ventilation (NIV).Citation5,Citation6 Murphy et alCitation5 have shown that NIV increases admission-free survival for AECOPD. In France and across Europe, COPD is one of the most common indications for home NIV.Citation7
Modern ventilators have a built-in software that records the usage of NIV and respiratory parameters such as respiratory rate, expired tidal volume, spontaneous inspirations, leaks, and residual apneic events.Citation8,Citation9 These data are reliable for the home monitoring of NIV.Citation9–Citation11 Their use is recommended by expert consensus.Citation11 Borel et alCitation12 have shown that data from built-in software could predict the onset of an AECOPD. They have shown that change in respiratory rate and in the percentage of triggered breaths was associated with the onset of AECOPD.Citation12 Such identification may trigger early therapy and alleviate the need of hospitalization.Citation13 Ventilators are now equipped with tele-transmission features that may authorize tele-monitoring of these patients with advanced chronic respiratory failure. The European Respiratory Society advocates for more research in that field for COPD patients.Citation13
Tele-monitoring is a promising tool for the management of COPD patients.Citation14 However, we still have a lack of positive results from randomized controlled trials.Citation15–Citation17 In Chatwin et al,Citation15 the negative results may be explained by day-to-day normal variability of respiratory pattern in COPD patients. Therefore, some normal changes may have triggered a non-required response from the tele-monitoring team.Citation15–Citation17
Our hypothesis was that changes in breathing pattern recorded by the NIV built-in software would occur in patients prior to admission for AECOPD.
Our primary endpoint was to investigate the association between the change in respiratory rate and severe AECOPD. Our secondary endpoints were to assess if changes in daily compliance to NIV, leaks, tidal volume, residual respiratory events, or overnight breaks from NIV use had a predictive value for detecting severe AECOPD.
Methods
We conducted a prospective observational monocentric case–control study approved by the local ethics committee for non-interventional research (Comité d’Ethique de la Recherche non-interventionnelle du CHU de Rouen) (E2016-79) and registered on ClinicalTrials.gov (NCT3018470). In accordance with French regulation, no written consent was required. Hence, only oral informed consent was obtained.
We included all patients admitted to Rouen University Hospital respiratory ward with a confirmed diagnosis of COPD and established on long-term NIV for >4 months regardless of the reason of their admission. Based on their reason of admission, we divided the patients into the following three groups: 1) stable group that included patients with stable COPD admitted for planned outpatient respiratory review and not presenting any feature for AECOPD, 2) severe AECOPD group that included patients hospitalized for AECOPD, and 3) moderate AECOPD group that included patients admitted for planned outpatient respiratory review but had clinical features of moderate AECOPD that did not require admission.
For each patient, we retrieved their medical records and reported their comorbidities and the results of their sleep polygraphy before NIV initiation, last echocardiogram, and last lung function test. For the stable group, we used the results of the outpatient stay during which they were included in the trial. For patients with severe AECOPD, arterial blood gas and NIV settings were retrieved from their last outpatient assessment during which they had clinical stability. For patients with severe AECOPD, we collected results from admission blood samples, bacteriological sample, chest X-ray, length of stay, and outcome. Onset of exacerbation was determined using a standardized structured clinical questionnaire.
For each patient, we retrieved the memory card of their NIV devices and made a copy to a secured computer. Patients who had a mean compliance of <4 h/night were secondarily excluded.
After discharge, we performed an analysis of the data copied from the NIV memory card. For each patient, a dedicated Windows®-based software, ResScan v5.6.0 (Resmed, Moissy-Cramaye, France) or DirectView v2.4.1 (Philips Respironics, Carquefou, France), was used to analyze data from the NIV. We collected the data for each of the 10 days preceding the admission. Collected data were as follows: respiratory rate (breaths/min), daily compliance (h/day), expired tidal volume (mL), unintentional leaks (L), residual respiratory events (events/h), and overnight interruption of NIV use (interruption/night).
We assessed four different methods to evaluate a change in breathing pattern. Method A was derived from Borel’s method:Citation12 quartiles of each parameter were calculated over a 5-day moving window, if the value on the following day was below the first quartile or above the third quartile, it was identified as an abnormal value that had to be confirmed on 2 consecutive days. Method B was adapted from method A but included only a 4-day moving window. For method C, we performed the following analysis: standard deviation (SD) was calculated for each 2 consecutive days, if the SD varied for >5% the following day, the value was identified as abnormal and had to be confirmed for 2 consecutive days. For method D, we calculated the SD over the 10 days period.
Normal distribution was assessed using the Shapiro–Wilk test. Results are expressed as number and percentages, mean and SD when normally distributed or medians, and interquartile range (IQR) when not normally distributed. Comparisons were performed using the unpaired t-test for normally distributed continuous variables and a Mann–Whitney test for non-normally distributed continuous variables. Receiver-operator characteristic (ROC) analyses were used to identify predictors of admission for AECOPD. All tests were two-sided with type I error rate set at 0.05. The analyses were performed using GraphPad Prism 6 for Mac OS X (GraphPad Software, Inc., La Jolla, CA, USA) and IBM SPSS Statistics v20.0 (IBM Corporation, Armonk, NY, USA).
Results
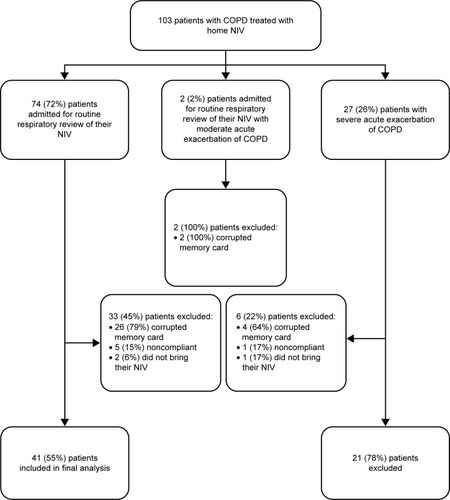
A total of 103 patients were included in the cohort, and 62 (60%) patients were included in the final analysis (). Reasons for exclusions are shown in . Patients’ baseline characteristics are summarized in . All patients were ventilated using a spontaneous timed mode except three patients in the stable group who were ventilated using a volume-targeted pressure-assured mode (P=0.519). Patients admitted for AECOPD were similar to those admitted for their routine assessment apart from: the number of admission in the year preceding inclusion: one (0–2) in the severe AECOPD vs one (0–1) in the stable group (P=0.021), control of hypoventilation in stable state assessed by daytime PaCO2 and bicarbonates following 1 h on NIV: 6.91±0.49 kPa and 30.9 (28.0–33.2) mmol/L in the severe AECOPD vs 6.06±0.17 kPa and 28.2 (25.3–30.5) mmol/L in the stable group (P=0.006 and 0.016, respectively) and for the level systolic pulmonary arterial pressure for those who had an echocardiography (n=39): 43±3 mmHg in the severe AECOPD vs 35±2 mmHg in the stable group (P=0.019).
Table 1 Population characteristics (data reported as mean or median with SD or interquartile range where appropriate)
Patients admitted for AECOPD had a median onset of their symptoms 2 (IQR; 1–3) days prior to admission. Vital signs, results from admission arterial blood gas, and venous blood samples are reported in . Patients admitted for AECOPD remained acidotic for a median period of 1 (IQR; 1–4) days. Their length of stay was 9 (IQR; 5–12) days. None of them required invasive ventilation or died.
Table 2 Vital observations, arterial blood gas, and venous sample at admission for patients with severe AECOPD (n=21)
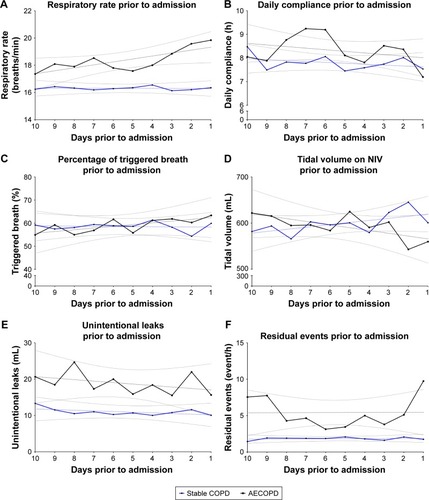
Data from built-in software are reported in and are represented over time in . Patients with severe AECOPD had a significantly higher respiratory rate than stable patients over the 10 days preceding inclusion (18.2±0.5 vs 16.3±0.5 breaths/min, respectively) (P=0.034). Using linear regression, the only significant change over time was the respiratory rate in patients with severe AECOPD (y=−0.2168×X+19.55, P=0.049). SD over 10 days was significantly higher for patients with severe AECOPD for respiratory rate and daily compliance when compared with the stable group (2.1 [0.8–2.6] vs 0.7 [0.4–1.2] breaths/min and 1.5 [1.0–2.4] vs 0.9 [0.7–1.3] h/day) (P=0.003 vs 0.021, respectively).
Table 3 Comparison of mean and SD values over the 10 days prior to admission in the stable group and in the group with severe AECOPD
Figure 2 Change in the 10 days preceding admission (A) change in respiratory rate, (B) change in daily compliance, (C) change in triggered breath, (D) change in tidal volume on NIV, (E) change in unintentionnal leaks, (F) change in residual events (mean value per day for each parameter).
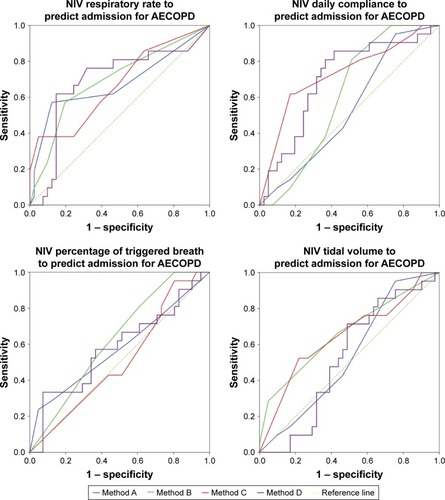
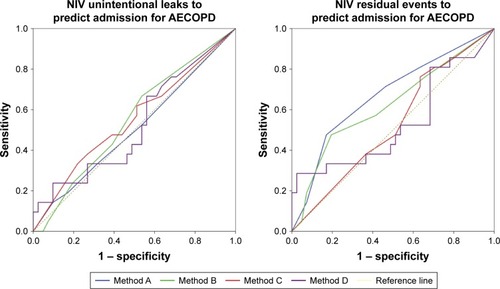
Usefulness of data from built-in software to predict admission for severe AECOPD according to prespecified analysis methods are reported in and . Respiratory rate was the parameter that gave the more consistent results regardless of the analysis method. Variability in the daily use of NIV assessed by methods C and D was the best predictor for severe AECOPD.
Table 4 Performance of parameters recorded by ventilator to predict severe AECOPD
Discussion
We have shown that change in the breathing pattern analyzed with data provided by NIV built-in software could predict admission for AECOPD. An increased variability in respiratory rate prior to admission was consistently able to predict admission for AECOPD regardless of the method used whereas an increased variability in daily use was the strongest predictor.
Our results confirmed that respiratory rate was a good predictor to detect AECOPD. Borel et al also identified a change in the percentage of trigger breaths as a predictor for AECOPD. Such difference can be explained by the design of our respective study.Citation12 In Borel’s study, data from NIV were collected during a long period of stable state which was used for the calculation of the quartiles. In our study, we performed all of our estimations using the data from the 10 days preceding admission. We chose this 10-day window based on the hypothesis that given the frailty of the study population, they were unlikely to exacerbate for more than 10 days prior to admission and based on technical limitations on the built-in software of ventilators used in our population. This difference could also be explained by the fact that we aimed to identify severe AECOPD whereas Borel et alCitation12 aimed to identify all exacerbations regardless of their severity.
Our results also differ from Borel’s regarding the criteria that we identified as predictors of severe AECOPD. In his study, a change in the percentage of triggered breaths was associated with an increased risk of AECOPD. In our study, despite having a similar set back-up rate, patients with AECOPD trended to have a lower percentage of triggered breaths. This result has to be interpreted cautiously given the number of patients and the large interquartile of percentage of triggered breaths in this group (39%–90%). We hypothesize this trend to be explained by patient ventilator asynchrony that may be more frequent in exacerbating patients. In our cohort, variation in the daily use of NIV was able to predict outcome unlike in Borel’s study where daily use was only trending toward statistical significance. Daily use of NIV was only able to predict AECOPD by measuring daily variability assessed by the SD over 10 days or every 2 days but for at least 6 consecutive days. Such variability can be explained by the fact that some patients would use their NIV more during an AECOPD and that some others would not be able to tolerate it longer due to phlegm or cough. In our cohort, we have also shown that a change in residual apneic events detected by the built-in software could predict severe AECOPD that could be explained by the fact that 38% of our cohort of patients who had a severe AECOPD had concomitant obstructive sleep apnea. Rostral fluid distribution is known to increase apneic events. In patient with AECOPD, we hypothesize that acute hypoxemia induced an increase in the right ventricular pressure that leads to fluid retention. Indeed, our patients with severe AECOPD had stable higher pulmonary arterial pressure. As a result of their fluid retention and rostral redistribution, the number of apneic events may increase.
We had a significant number of patients for whom data from built-in software could not be retrieved. This can be explained by the fact that some of the memory cards were defective or missing. For others, data were not usable because of an incomplete copy of the data contained in the memory card in the computer used for the trial. As the first analyses of the memory card were carried out 1 month after initiation of the study, we were not able to retrieve the complete data set secondarily for those with missing data. Finally, some patients did not attend to the hospital with their NIV or had insufficient use of their NIV prior to the admission.
We focused on identifying severe AECOPD in a population with severe chronic respiratory insufficiency. Identification of such an event is crucial as it could help trigger earlier treatment and avoid admission. In our cohort, patients stayed 9 (IQR; 5–12) days. Therefore, despite not using a questionnaire-based assessment of AECOPDCitation18 as Borel et al,Citation12 we are confident that our patients had severe AECOPD.
New ventilators now have built-in transmission unit that allows tele-monitoring, and this has been shown to be feasible in NIV patients.Citation19 Therefore, our results could be used for remote early identification of severe AECOPD that may avoid hospitalization. Given the costs of hospitalization, such strategy would be likely to be cost-effective. One of the advantages of such an approach would be the fact that patients would not have to participate actively to their monitoring. This may prevent withdrawal from the tele-monitoring.Citation20,Citation21 However, to be generalized, the methods used to detect AECOPD in our study would need to be based on automatic algorithm in order to alert health care provider. This would require interoperable platforms between manufacturers’ software or homogeneity of the ventilators used in each center’s cohort of patients. Given the low sensitivity of the detection methods that we used, we suggest that changes in breathing pattern should trigger an alarm to the health care provider who would then make telephone contact with the patient to further evaluate symptoms suggesting the onset of an AECOPD. Such strategy would need clinical validation and economical validation.
Our study provides new data on the ability to predict admission for AECOPD. However, the sensitivity of all the methods used remains low. Two main reasons are likely to explain this limit. First, the onset of an AECOPD is variable. This is highlighted by the IQR (1–3) of symptoms’ onset in our patients admitted for AECOPD. Hence, if the detection method requires 2 days outside the normal values, it may not detect the change before the patient’s admission. In contrast, using a detection method that assesses day-to-day variability may produce too many false positives. Second, very little is known about the normal variability of breathing pattern of patients on NIV. In this study, we only assessed individual variability over a 10-day period, but Borel et alCitation12 assessed normality over a more prolonged period as data were collected from inclusion to the onset of an AECOPD. Moreover, as NIV was mainly used by our patients while asleep, we think that variability in respiratory rate was minimal. However, we advocate for larger dataset to be collected in line with the recommendations of the European Respiratory Society that highlight the needs of research in the field of tele-monitoring.Citation13
Conclusion
We have shown that data from NIV can identify a change in breathing patterns that predicts severe AECOPD. The clinical impact of the identification of such change remains to be evaluated.
Acknowledgments
The authors thank Miss Gill Arbane for her careful reading and correction of the article.
Disclosure
Dr MP reports grants from B&D Electromedical, personal fees from ResMed, grants and nonfinancial support from Fisher & Paykel, nonfinancial support from MSD, nonfinancial support from Asten, and grants from ADIR Association, outside the submitted work. The other authors report no conflicts of interest in this work.
References
- GroenewegenKHScholsAMWoutersEFMortality and mortality-related factors after hospitalization for acute exacerbation of COPDChest2003124245946712907529
- MiravitllesMFerrerMPontAEffect of exacerbations on quality of life in patients with chronic obstructive pulmonary disease: a 2 year follow up studyThorax200459538739515115864
- KhakbanASinDDFitzgeraldJMTen-year trends in direct costs of COPD: a population-based studyChest2015148364064626043120
- JouneauSAcute exacerbation of COPD: prediction, biology and becomingRev Mal Respir2006235, pt 23236
- MurphyPBRehalSArbaneGEffect of home noninvasive ventilation with oxygen therapy vs oxygen therapy alone on hospital readmission or death after an acute COPD exacerbation: a randomized clinical trialJAMA201731721217728528348
- KöhnleinTWindischWKöhlerDNon-invasive positive pressure ventilation for the treatment of severe stable chronic obstructive pulmonary disease: a prospective, multicentre, randomised, controlled clinical trialLancet Respir Med20142969870525066329
- Lloyd-OwenSJDonaldsonGCAmbrosinoNPatterns of home mechanical ventilation use in Europe: results from the Eurovent surveyEur Respir J20052561025103115929957
- PasquinaPAdlerDFarrPBourquiPBridevauxPOJanssensJPWhat does built-in software of home ventilators tell us? An observational study of 150 patients on home ventilationRespiration201283429329921952176
- GeorgesMAdlerDContalOReliability of apnea-hypopnea index measured by a home bi-level pressure support ventilator versus a polysomnographic assessmentRespir Care20156071051105625737571
- ContalOVignauxLCombescureCPepinJLJollietPJanssensJPMonitoring of noninvasive ventilation by built-in software of home bilevel ventilators: a bench studyChest2012141246947621778253
- JanssensJPBorelJCPépinJLSomnoNIV GroupNocturnal monitoring of home non-invasive ventilation: the contribution of simple tools such as pulse oximetry, capnography, built-in ventilator software and autonomic markers of sleep fragmentationThorax201166543844520971980
- BorelJPelletierJTaleuxNRespiratory parameters recorded by built-in ventilator software: a new tool for the early detection of exacerbation in COPD patients treated with long-term non-invasive ventilation?Rev Mal Respir201532A31
- AmbrosinoNVitaccaMDreherMERS Tele-Monitoring of Ventilator-Dependent Patients Task ForceTele-monitoring of ventilator-dependent patients: a European respiratory society statementEur Respir J201648364866327390283
- EstebanCMorazaJIriberriMOutcomes of a telemonitoring-based program (telEPOC) in frequently hospitalized COPD patientsInt J Chron Obstruct Pulmon Dis2016112919293027920519
- ChatwinMHawkinsGPanicchiaLRandomised crossover trial of telemonitoring in chronic respiratory patients (TeleCRAFT trial)Thorax201671430531126962013
- TuggeyJMPlantPKElliottMWDomiciliary non-invasive ventilation for recurrent acidotic exacerbations of COPD: an economic analysisThorax2003581086787114514940
- WalkerPPPompilioPPZanaboniPTelemonitoring in COPD: the CHROMED study, a randomized clinical trialAm J Respir Crit Care Med Epub2018320
- LeidyNKWilcoxTKJonesPWEXACT-PRO Study GroupDevelopment of the EXAcerbations of chronic obstructive pulmonary disease tool (EXACT): a patient-reported outcome (PRO) measureValue Health201013896597520659270
- KesslerRCasan-ClaraPKoehlerDCOMET: a multicomponent home-based disease-management programme versus routine care in severe COPDEur Respir J2018511170161229326333
- CruzJBrooksDMarquesAHome telemonitoring in COPD: a systematic review of methodologies and patients’ adherenceInt J Med Inform201483424926324529402
- ParéGJaanaMSicotteCSystematic review of home telemonitoring for chronic diseases: the evidence baseJ Am Med Inform Assoc200714326927717329725