Abstract
Background
The chronic obstructive pulmonary disease (COPD) Assessment Test (CAT) questionnaire is a short patient-completed questionnaire, which is used to assess the health status of patients with stable COPD. However, whether it is a good tool to evaluate the response to treatment in acute exacerbation of COPD (AECOPD) has been less studied.
Methods
The patients were assessed at two visits, at admission and on the seventh day. Anthropometric variables were collected at admission. CAT and lung function were measured twice at the above time points. At the second visit, the health status of the patients were divided into five groups based on a 5-point Likert scale, ranging from 1 to 5, which represents “much better,” “slightly better,” “no change,” “slightly worse,” and “much worse.” Responders were those who reported “much better” or “slightly better,” and nonresponders were those who claimed “no change,” “worse,” or “much worse.”
Results
In total, 225 patients were recruited. The average CAT score at admission was 24.82±7.41, which declined to 17.41±7.35 on the seventh day. There were 81.33% responders, whose improvement in CAT score (9.37±5.24) was much higher than that of the nonresponders (−1.36±4.35). A moderate correlation was observed between the changes in CAT score and improvement in FEV1, FEV1%, and the length of hospital stay. There was a strong correlation between the changes in CAT score and health status. A 3.5-unit improvement in the CAT score, with highest area under the curve, was the cutoff to differentiate responders from nonresponders.
Conclusion
The evolution of CAT scores during exacerbation can provide useful information to assess the health status of patients with AECOPD. A 3.5-unit improvement in CAT score is the best cutoff to differentiate between patients who have a response or no response to treatment, which offers a convenient and easy way for clinicians to monitor the health status of patients with an AECOPD.
Keywords:
Introduction
COPD is defined by the GOLD as a disease characterized by airflow limitation, which is not fully reversible; it will represent the fourth leading cause of mortality worldwide by 2020.Citation1 The progress of COPD can always be deteriorated by the incidence of exacerbations. It was proved that exacerbation was an important life-threatening event for patients with COPD.Citation2,Citation3 Patients who suffer frequent and repeated exacerbations within 1 year have a poor prognosis,Citation4 low HRQOL,Citation5 rapid decline in lung function,Citation6–Citation8 and high mortality.Citation9 Effective treatment could improve the quality of life and decrease the economic burden of these patients. However, patients with AECOPD have various phenotypesCitation10 and often present different responses to treatment.Citation11,Citation12 Thus, to make timely and reasonable changes of the therapy for those who have no response to the treatment, it is essential to find an efficient tool to evaluate the curative effect of the therapy.
As we all know, the diagnosis, stage of severity, and treatment recommendations of COPD have been guided by the degree of airflow limitation (ie, the ratio of FEV1 and FVC, and FEV1%) for many years.Citation13 However, COPD is a heterogeneous disease, and spirometry only captures some of the disease variety.Citation14,Citation15 In addition, a studyCitation16 showed the airway function of some patients could not return to pre-exacerbation levels within 91 days, which indicates lung function was not able to sensitively reflect the health status of the patients. Thus, to better classify the patients for prognostic purposes and to guide treatment, the GOLD 2011Citation17 Executive Summary made great modifications in the disease classification. Instead of relying on FEV1 only, it classified the patients according to the level of dyspnea, exacerbation history, and FEV1, which pointed out the importance of clinical symptoms.
The CAT and mMRC dyspnea scale were the main questionnaires to evaluate the symptoms in the GOLD document. mMRC is a simple questionnaire that can only evaluate the dyspnea of the patient.Citation18 However, the impact of COPD on individuals is multifaceted and it causes impairment not only in the lungs but also in other organs, and even psychological conditions.Citation17 The CAT was designed by Jones et alCitation19 in 2009, and it consists of eight items, including cough, expectoration, dyspnea, chest tightness, confidence, limitation of daily activities, quality of sleep, and levels of energy. The score of each item ranges from 0 to 5 (0=no impairment, 5=greatest impairment). The total score is calculated by adding the points of the eight questions ranging from 0 to 40, where 0 means the best status and 40 means the worst status. This questionnaire is completed by the patients themselves, and it can assess the impact of COPD on the health status of patients within a few minutes. In recent years, the CAT has been proven to be very useful in evaluating the health status of patients with stable COPD.Citation20,Citation21 Also, it has been used to assess the severity of exacerbationsCitation22–Citation24 and the health status of patients with an AECOPD.Citation25–Citation27 The CAT score is a potential indicator to assess the response to treatment.
Thus, in this study, we aim to assess the sensitivity of the CAT score to assess the response to the treatment of patients with an AECOPD and find the cutoff CAT score to define the responders among patients with an AECOPD.
Methods
The research protocol was approved by the local Ethics Committee of the Second Xiangya Hospital of Central South University (number: zay0410), and all subjects provided written informed consent to participate in the study. The study was approved by the ethics committee. The study was registered in the Chinese Clinical Trial Registry (ChiCTR-ROC-16009087; http://www.chictr.org.cn/).
Inclusions and exclusions of the patients
Patients with a clinician-diagnosed AECOPD from the Second Xiangya Hospital of Central South University in China from February 2016 to December 2017 were recruited. Patients with a history of COPD, confirmed by spirometry in stable phase showing a post-bronchodilator FEV1/FVC ratio <0.7, with a primary diagnosis of AECOPD without respiratory tract infection, and aged over 40 years were included in the study. Patients with a history of asthma or other respiratory diseases (ie, lung cancer, interstitial lung disease, bronchiectasis, or pulmonary thromboembolism), severe heart failure (New York Heart Association stage IV), and malignant comorbidities were excluded from the study. All diagnoses were established by the clinicians and were independently verified by physicians specializing in respiratory medicine. COPD was defined as progressive, irreversible airway obstruction associated with airway inflammation primarily caused by cigarette smoking, in agreement with GOLD 2013 guidelines. AECOPD was defined as increased dyspnea, cough, or sputum expectoration (quality or quantity) that led the subjects to seek medical care. Smoking subjects were defined as those who still smoked tobacco daily. Ex-smokers had stopped smoking at least 6 months prior to inclusion in the study.
Study design
Anthropometric and clinical variables were collected: smoking, drug treatment, and comorbidities, which included cardiovascular disease, OSAHS, diabetes, and hypertension. The comorbidities would be recorded, no matter whether a history of diagnosis at admission or diagnosed by clinicians during the hospital stay. CAT and lung function were measured at two time points: within 24 hours of hospital admission and on the seventh day. If the hospital stay was less than 7 days, the second visit would be performed at discharge. Treatment during hospitalization was determined by the clinicians based on GOLD guidelines. Treating clinicians were not directly involved in the study and were blinded to the results of the CAT. After treatment, the health status was divided into five groups based on a 5-point Likert scale, ranging from 1 to 5, which represents “much better,” “slightly better,” “no change,” “slightly worse,” and “much worse,” respectively. Responders were defined as reporting “much better” or “slightly better” at the second visit. Nonresponders were defined as claiming “no change,” “worse,” or “much worse.”
Statistical analyses
Sociodemographic and clinical characteristics of patients were summarized descriptively. SPSS software version 25.0 was used for statistical analysis. Data are reported as mean±SD. The level of statistical significance was set at 0.05 (two-sided). Changes in the CAT at hospital admission and discharge showing a normal distribution were tested using paired t-tests, while the variables showing non-normal distribution were analyzed by rank test. Spearman rank correlation coefficient method was used to analyze the relationship between the health status and CAT, and Pearson correlation analysis was used to analyze the relationship between the CAT and hospital stay. Group comparisons were tested using analysis of variance or t-tests. ROC curve analysis was performed to derive the optimum cutoff value for the CAT, and a value of the AUC above 0.8 on ROC analysis was considered to provide good discrimination.
Results
Patient demographics
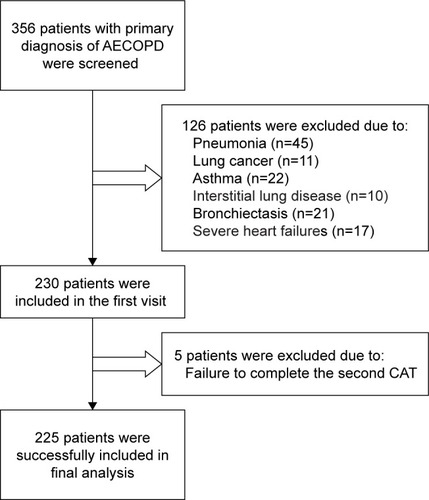
A total of 356 subjects were screened, and 126 patients were excluded because of pneumonia, lung cancer, asthma, interstitial lung disease, bronchiectasis, and severe heart failure. In total, 230 subjects were recruited in the first visit. Among them, five patients failed to complete the second visit because of serious deterioration (). Thus, just 225 patients succeeded to complete two visits and 55 of them completed lung function testing. Demographic and clinical data are presented in . Mean±SD age was 67.08±10.03 years, with 89.3% males and 10.7% females in the study. Mean±SD FEV1% was 42.13%±16.14% and mean±SD FEV1/FVC ratio was 44.87±11.07, which included 103 smokers and 122 ex-smokers. Most of the patients were also diagnosed with hypertension (44.89%) and cardiovascular disease (40.89%). Patients receiving regular ICS therapy and bronchodilators totaled 195, while 190 patients received antibiotics. The type of antibiotic was decided by the clinicians. Because of dyspnea, 116 patients were given doxofylline, and 43 of them accepted intravenous prednisone due to wheezing ().
Table 1 Clinical characteristics of the patients
The changes of CAT
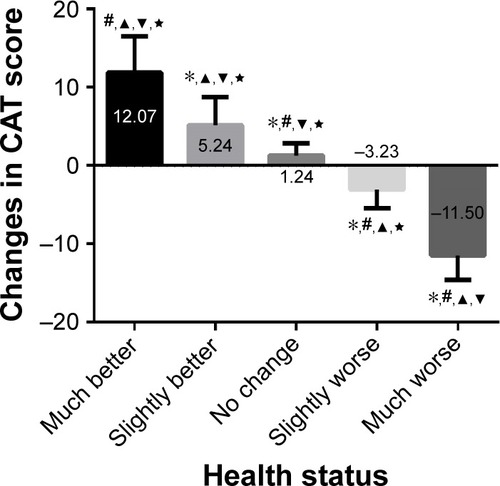
Most of the patients with an AECOPD (81.33%) reported improved health status at the second visit. Among these patients, 49.78% reported their health status as much improved and 31.56% reported slightly improved. The mean±SD of changes in the CAT score of all patients was 7.37±6.60. The mean±SD of changes in the much improved group was 12.07±4.48 and slightly better was 5.24±3.51, while the mean±SD of changes in the no change, slightly worse, and much worse groups were 1.24±1.53, −3.23±2.31, and −11.50±3.11, respectively. The changes between two visits in each group presented great differences, except in the much worse group (). In addition, the comparison of changes in the CAT between the two visits among the five groups is shown in . There are significant differences between the five groups.
Table 2 Changes in CAT scores between the two visits in different health statuses
Figure 2 The comparison of the changes in CAT score in AECOPD patients with different health statuses.
Abbreviations: AECOPD, acute exacerbation of COPD; CAT, COPD assessment test.
The comparison between responders and nonresponders
Of the 225 patients, 183 (81.33%) were responders and 42 (18.67%) were nonresponders. The mean initial CAT values before treatment in the responders and nonresponders were 25.87±6.94 and 20.26±7.75, respectively. There was a statistically significant difference between the groups. After treatment, the CAT score improvement in the responders group was 9.37±5.24, which was significantly higher than the nonresponders group (−1.36±4.35). There was a statistically significant difference between the two groups ().
Table 3 Changes in CAT scores between responders and nonresponders
The correlation between CAT score both with health status and lung function
Spearman rank correlation coefficient method was used to analyze the relationship between the health status and CAT score. The initial CAT score in the acute exacerbation period was negatively correlated with the patient’s self-assessment of health status, and the correlation coefficient was −0.331. In addition, there was a significant negative correlation between the CAT score changes and the health status of the patients. The correlation coefficient was −0.824. A positive relationship was observed between the CAT score at discharge and the health status of the patients. The correlation coefficient was 0.333. As for the lung function, a total of 55 patients completed lung function testing during the exacerbations. Pearson correlation analysis was used to analyze the relationship between the CAT score and FEV1 and FEV1%. The changes in the CAT score had moderate correlation with increases of FEV1 and FEV1%. The correlation index was 0.363 and 0.387, respectively, while the CAT score either at admission or at the second visit had no relationship with changes in FEV1 and FEV1% ().
Table 4 The correlation between CAT score and health status, and improvement in FEV1% and FEV1
The correlation between CAT and hospital stay
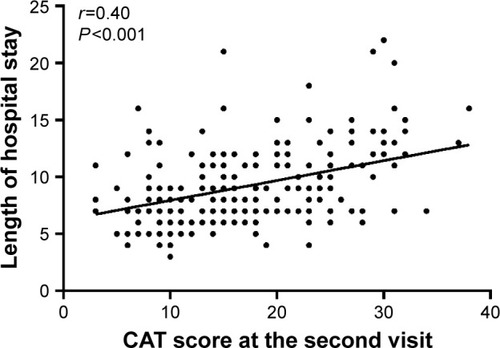
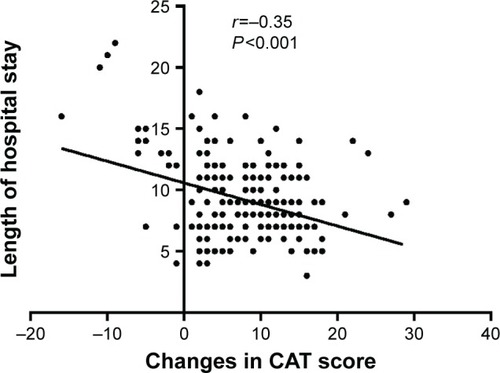
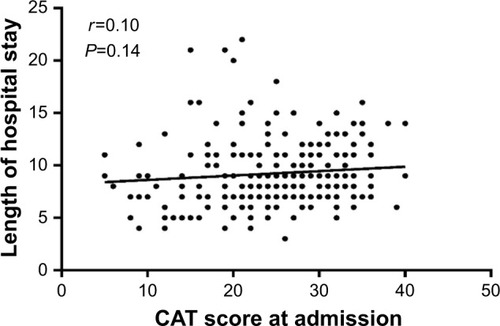
Pearson correlation analysis was used to analyze the relationship between the CAT and hospital stay. The average length of stay was 9.24±5.36 days. There was no significant relationship between the CAT score obtained at admission and length of hospital stay (r=0.10; P=0.143; ), and there was a moderate correlation between the CAT score obtained on the second visit and the length of hospital stay (r=0.403; P<0.001; ). In addition, a slightly negative relationship was observed between the changes in CAT score and length of hospital stay (r=−0.35; P<0.001; ).
Figure 3 Relationship between the CAT score at admission and length of hospital stay.
Abbreviation: CAT, COPD assessment test.
Cutoff of changes in CAT to predict response to treatment
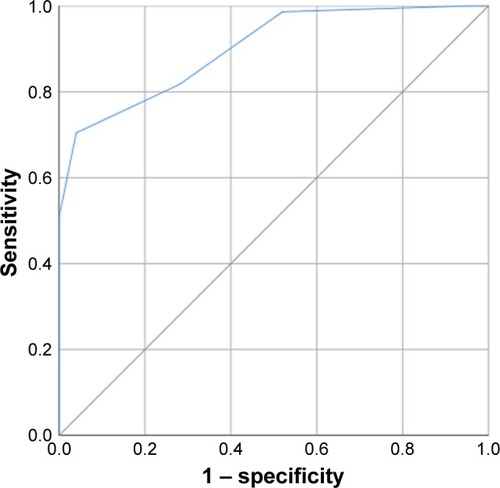
The ROC curve analysis identified a decrease of 3.5 units in the CAT score between admission and discharge as the cutoff point with the greatest predictive value for treatment failure (AUC=0.973, sensitivity=86.9%, and specificity=97.4%; ). Moreover, a decrease of 3.5 units in the CAT score was also the optimum cutoff to differentiate patients reporting slightly better and no change (AUC=0.900, sensitivity=70%, and specificity=96%; ).
Figure 6 The ROC curve for the cutoff point of CAT score improvements to differentiate responders and nonresponders.
Notes: The AUC was 0.973; the sensitivity and specificity were 86.9% and 97.4%, respectively.
Abbreviations: AUC, area under the curve; CAT, COPD assessment test; ROC, receiver operating characteristic.
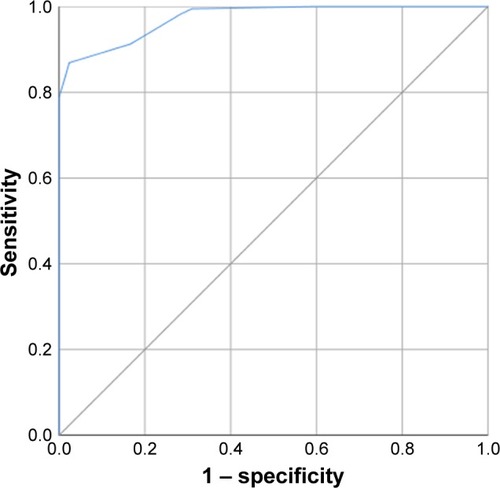
Figure 7 The ROC curve for the cutoff point of CAT score improvements to differentiate between patients in the slightly better group and no-change group.
Abbreviations: AUC, area under the curve; CAT, COPD assessment test; ROC, receiver operating characteristic.
Discussion
This study showed that monitoring the changes of the CAT score during an AECOPD could assess the curative effect of treatment. Previous studies have observed a difference in the CAT score of between 4 and 10 points for patients with COPD on different time points during exacerbation.Citation22,Citation25,Citation28,Citation29 In our study, the difference in CAT scores observed was 7.37±6.60, which was similar to previous studies. In addition, we also found the changes in CAT scores in the much better to much worse groups declined in turn, with statistically significant differences, while the changes of CAT scores in the responder group were much higher than those in the nonresponder group, indicating CAT score was very sensitive to the changes of the health status of patients. However, we found the CAT score in the much better group was significantly improved by 12.07±4.48, which was much higher than that in other studies.Citation25,Citation31 We suppose it may have relation to the fact that most patients in China do not have sufficient knowledge of the disease and always have difficulty in adhering to regular treatment. One of our previous prospective studies showed only one-third of patients adhered to the treatments suggested by doctors (Figure S1). In addition, another study showed that the patients who received regular treatment achieved a significant decrease in CAT score.Citation30 Therefore, we speculate that the CAT score would be a little higher than expected when the patients presented for medical care because of the absence of receiving regular treatment. Moreover, the distribution of medical resources in China is unbalanced. The primary care units and community hospitals are not always equipped with very professional physicians and advanced instruments. Our hospital is a comprehensive third-grade hospital, which represents the highest medical level in Hunan Province. Thus, most of the patients admitted to our hospital had significant improvement. Actually, in another Chinese study, the CAT score changed by as much as 15 units.Citation29 Interestingly, in the patients reporting no change, there was also a statistically significant difference between the CAT values during the two visits. We suppose it may have relation to the complex condition of the patients. Nearly half of the patients had some comorbidities, which would affect their self-assessment. In this study, six patients reported a three-unit improvement of CAT values but reported no change in their health status. We found three of them also had coronary heart disease, two of them also had diabetes, and one also had OSAHS (Table S1). Although respiratory symptoms, including cough and sputum, were relieved, some other manifestations, such as angina and dizziness, were not improved, which may have resulted in the final reply of no change in patients.
In this study, we analyzed the correlation between CAT score and FEV1, FEV1%, health status, and the length of hospital stay. We found that when compared with CAT score, no matter whether at admission or at discharge, the changes in CAT score had better correlation with health status, which indicated dynamically monitoring CAT score would be more useful than a one-time measure of CAT score in predicting the health status of the patients. In addition, the changes in CAT score also had a positive correlation with FEV1 or FEV1%, which was similar to other studies.Citation24,Citation32 As we all know, in other previous studies, FEV1 and FEV1% were often used to assess the response to treatment not only in stable COPD but also in acute exacerbations.Citation33–Citation35 In this study, a positive moderate correlation was observed between the improvement in CAT score and ΔFEV1 and ΔFEV1%, suggesting that for those patients who could not afford lung function testing or who were too ill, the CAT was a good alternative. In terms of the length of hospital stay, there was a relationship with the CAT score at the second visit and the improvement of the CAT score, indicating the changes of CAT and the CAT value at the second visit can predict the length of hospital stay for patients with an AECOPD. This result was similar to Dai’s study.Citation36 However, there was no relationship between the CAT score at admission and length of hospital stay, which was different from another study.Citation37 We speculate the reason was related to the fact that nearly one-third of the patients with COPD did not obey physician recommendations to get regular treatment, resulting in a higher CAT score at admission (Figure S1). After receiving professional treatment, they were likely to have obvious improvement as well as a shorter hospital stay. In addition, the hospital stay can be influenced by many factors, including the financial condition, education background, insurance policy, and even family relationship of the patient.Citation38
An improvement of more than 3.5 units in the CAT score between hospital admission and discharge was the cutoff with the highest predictive value to differentiate responders from nonresponders. In one previous study, García-Sidro et alCitation39 collected CAT scores on 106 patients with an AECOPD on the first day, third day of admission, and at discharge. They found that patients with a CAT improvement value of <4 were more likely to suffer another exacerbation and readmission. Kon et alCitation40 used a different method to calculate the MCID value for the CAT. The distribution method recommended 3.75 units, whereas the ROC curve identified 2 units. Two recent studies suggested 2–3 and 3 units, respectively, as the MCID for the CAT.Citation41,Citation42 In this study, the ROC curve was used to differentiate patients reporting slightly better and no change, with the cutoff being 3.5 units. The above differences may be related to many factors, such as the difference in the sample size, design of the study, and calculation method for MCID.
Our study has some limitations. The sample size in the nonresponder group is much smaller than that in the responder group owing to the medical condition in our hospital, which may result in the initial CAT score in nonresponders being lower than that in responders. In the future, a larger study, which includes patients with stable COPD, is required to address this issue. Moreover, there is no gold standard to assess the curative effect of the treatment. We used the self-reported health status of patients as the standard to assess the role of the CAT score, which may be affected by the subjective consciousness of the patients. But we believe that it would have a small effect on the relationship between the CAT score and health status, since both of them were completed by the patients themselves. Finally, this is not a multicenter study, which will limit the application of the conclusion. For this issue, a multicenter study should be conducted, which may be beneficial to the management of COPD.
In conclusion, this study found that the evaluation of CAT scores between admission and discharge can provide useful additional information to assess the health status of patients with an AECOPD. Also, CAT score would be a good alternative for lung function testing, especially for those who cannot afford the expense of lung function testing. CAT scores that improved 3.5 units were regarded as the cutoff to differentiate responders from nonresponders, which would be useful to guide clinicians toward a timely change in therapy.
Author contributions
PC and AZ contributed to the study design; AZ contributed to the drafting of the manuscript; PC contributed to critically revising the manuscript for important intellectual content. AZ, ZZ, YP, YZ, and JD contributed to data collection. All authors contributed toward data analysis, drafting, and revising the paper, and agree to be accountable for all aspects of the work.
Abbreviations
| AECOPD | = | acute exacerbation of chronic obstructive pulmonary disease |
| AUC | = | area under the curve |
| CAT | = | COPD assessment test |
| COPD | = | chronic obstructive pulmonary disease |
| GOLD | = | Global Initiative for Chronic Obstructive Lung Disease |
| HRQOL | = | health-related quality of life |
| ICS | = | inhaled corticosteroid |
| MCID | = | minimum clinically important difference |
| mMRC | = | modified Medical Research Council |
| OSAHS | = | obstructive sleep apnea–hypopnea syndrome |
| ROC | = | receiver operating characteristic |
Acknowledgments
This study was funded by grants from the National Natural Science Foundation of China (NSFC, Grants 81770046 to Prof Ping Chen) and Fundamental Research Funds for the Central Universities of Central South University (2017zzts228 to Dr Aiyuan Zhou).
Supplementary materials
Figure S1 The number of the patients who received standard treatment.
Notes: This was a one-year follow-up study. In total, 189 patients were recruited into our study. All of them accepted three visits after being recruited (at 3 months, at 6 months, and at 12 months). If the patients reported they took their drugs everyday based on the prescription at every visit, they would be classified as those who received regular treatment. The rest were those who didn’t receive regular treatment. We found that only one-third of the patients received regular treatment.
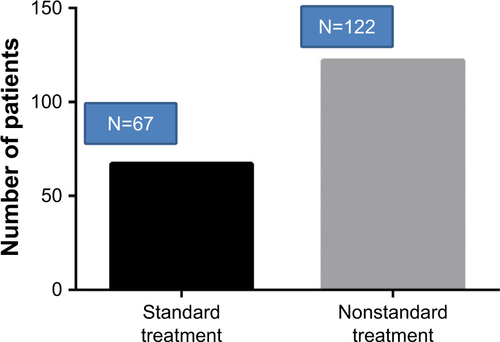
Table S1 The detailed information of the comorbidities of patients who reported no change
Disclosure
The authors report no conflicts of interest in this work.
References
- AgustiACalverleyPMCelliBCharacterisation of COPD heterogeneity in the ECLIPSE cohortRespir Res20101112220831787
- VogelmeierCFCrinerGJMartinezFJGlobal Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report. GOLD Executive SummaryAm J Respir Crit Care Med2017195555758228128970
- GroenewegenKHScholsAMWoutersEFMortality and mortality-related factors after hospitalization for acute exacerbation of COPDChest2003124245946712907529
- SeemungalTAHurstJRWedzichaJAExacerbation rate, health status and mortality in COPD – a review of potential interventionsInt J Chron Obstruct Pulmon Dis2009420322319554195
- SpencerSCalverleyPMBurgePSJonesPWImpact of preventing exacerbations on deterioration of health status in COPDEur Respir J200423569870215176682
- CelliBRThomasNEAndersonJAEffect of pharmacotherapy on rate of decline of lung function in chronic obstructive pulmonary disease: results from the TORCH studyAm J Respir Crit Care Med2008178433233818511702
- MakrisDMoschandreasJDamianakiAExacerbations and lung function decline in COPD: new insights in current and ex-smokersRespir Med200710161305131217112715
- DonaldsonGCSeemungalTABhowmikAWedzichaJARelationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary diseaseThorax2002571084785212324669
- Soler-CataluñaJJMartínez-GarcíaMARomán SánchezPSevere acute exacerbations and mortality in patients with chronic obstructive pulmonary diseaseThorax2005601192593116055622
- ZhouAZhouZZhaoYChenPThe recent advances of phenotypes in acute exacerbations of COPDInt J Chron Obstruct Pulmon Dis2017121009101828392685
- Lopez-CamposJLAgustíAHeterogeneity of chronic obstructive pulmonary disease exacerbations: a two-axes classification proposalLancet Respir Med20153972973426165134
- CalverleyPMAnzuetoARDusserDTreatment of exacerbations as a predictor of subsequent outcomes in patients with COPDInt J Chron Obstruct Pulmon Dis2018131297130829719385
- RabeKFHurdSAnzuetoAGlobal strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summaryAm J Respir Crit Care Med2007176653255517507545
- GrouseLNew studies address urgent need for early COPD diagnosisJ Thorac Dis201241192122295163
- LutchmedialSMCreedWGMooreAJHow common is airflow limitation in patients with emphysema on CT scan of the chest?Chest2015148117618425539080
- SeemungalTADonaldsonGCBhowmikAJeffriesDJWedzichaJATime course and recovery of exacerbations in patients with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med200016151608161310806163
- Global Initiative for Chronic Obstructive Lung Disease (GOLD)Globalstrategy for the diagnosis, management, and prevention of chronicobstructive pulmonary disease (Update 2011) Available from: http://goldcopd.org/
- BestallJCPaulEAGarrodRGarnhamRJonesPWWedzichaJAUsefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary diseaseThorax199954758158610377201
- JonesPWHardingGBerryPDevelopment and first validation of the COPD Assessment TestEur Respir J200934364865419720809
- ZhouZZhouAZhaoYDuanJChenPA comparison of the assessment of health status between CCQ and CAT in a Chinese COPD clinical population: a cross-sectional analysisInt J Chron Obstruct Pulmon Dis2018131675168229872285
- TsiligianniIGvan der MolenTMoraitakiDAssessing health status in COPD. A head-to-head comparison between the COPD assessment test (CAT) and the clinical COPD questionnaire (CCQ)BMC Pulm Med2012122022607459
- MiravitllesMGarcía-SidroPFernández-NistalACourse of COPD assessment test (CAT) and clinical COPD questionnaire (CCQ) scores during recovery from exacerbations of chronic obstructive pulmonary diseaseHealth Qual Life Outcomes20131114723987232
- ChettaAOlivieriDThe COPD Assessment Test in the evaluation of chronic obstructive pulmonary disease exacerbationsExpert Rev Respir Med20126437337522971062
- MackayAJDonaldsonGCPatelARUsefulness of the Chronic Obstructive Pulmonary Disease Assessment Test to evaluate severity of COPD exacerbationsAm J Respir Crit Care Med2012185111218122422281834
- AgustíASolerJJMolinaJIs the CAT questionnaire sensitive to changes in health status in patients with severe COPD exacerbations?COPD20129549249822958111
- Folch AyoraAMacia-SolerLOrts-CortésMIHernándezCSeijas-BabotNComparative analysis of the psychometric parameters of two quality-of-life questionnaires, the SGRQ and CAT, in the assessment of patients with COPD exacerbations during hospitalization: a multicenter studyChron Respir Dis2018 479972318761645:147997231876164
- KardosPMokrosISauerRVogelmeierCFHealth status in patients with COPD treated with roflumilast: two large noninterventional real-life studies: DINO and DACOTAInt J Chron Obstruct Pulmon Dis2018131455146829765213
- Feliz-RodriguezDZudaireSCarpioCEvolution of the COPD Assessment Test score during chronic obstructive pulmonary disease exacerbations: determinants and prognostic valueCan Respir J2013205e92e9724093119
- TuYHZhangYFeiGHUtility of the CAT in the therapy assessment of COPD exacerbations in ChinaBMC Pulm Med2014144224618290
- PapaioannouMPitsiouGManikaKCOPD assessment test: a simple tool to evaluate disease severity and response to treatmentCOPD201411548949524766370
- JonesPWHardingGWiklundITests of the responsiveness of the COPD assessment test following acute exacerbation and pulmonary rehabilitationChest2012142113414022281796
- HuangWCWuMFChenHCHsuJYTOLD Group, MfW, Group TFeatures of COPD patients by comparing CAT with mMRC: a retrospective, cross-sectional studyNPJ Prim Care Respir Med2015251506326538368
- AntusBBartaIHorvathICsiszerERelationship between exhaled nitric oxide and treatment response in COPD patients with exacerbationsRespirology201015347247720210889
- MargaySMFarhatSKaurSTeliHATo study the efficacy and safety of doxophylline and theophylline in bronchial asthmaJ Clin Diagn Res201594FC05FC08
- DecramerMDekhuijzenPNTroostersTThe Bronchitis Randomized On NAC Cost-Utility Study (BRONCUS): hypothesis and design. BRONCUS-trial CommitteeEur Respir J200117332933611405507
- DaiMYQiaoJPXuYHFeiGHRespiratory infectious phenotypes in acute exacerbation of COPD: an aid to length of stay and COPD Assessment TestInt J Chron Obstruct Pulmon Dis2015102257226326527871
- GiffordSZambonJPOrlandoGRecycling organs – growing tailor-made replacement kidneysRegen Med201510891391526542737
- GhodsAAKhabiriRRaeisdanaNPredictors of inappropriate hospital stay: experience from IranGlob J Health Sci201473828925948427
- García-SidroPNavalEMartinez RiveraCThe CAT (COPD Assessment Test) questionnaire as a predictor of the evolution of severe COPD exacerbationsRespir Med2015109121546155226542727
- KonSSCanavanJLJonesSEMinimum clinically important difference for the COPD Assessment Test: a prospective analysisLancet Respir Med20142319520324621681
- SmidDEFranssenFMHouben-WilkeSResponsiveness and MCID estimates for CAT, CCQ, and HADS in patients with COPD undergoing pulmonary rehabilitation: a prospective analysisJ Am Med Dir Assoc2017181535827624705
- AlmaHde JongCJelusicDHealth status instruments for patients with COPD in pulmonary rehabilitation: defining a minimal clinically important differenceNPJ Prim Care Respir Med2016261604127597571