Abstract
Airway mucus is part of the lung’s native immune function that traps particulates and microorganisms, enabling their clearance from the lung by ciliary transport and cough. Mucus hypersecretion and chronic productive cough are the features of the chronic bronchitis and chronic obstructive pulmonary disease (COPD). Overproduction and hypersecretion by goblet cells and the decreased elimination of mucus are the primary mechanisms responsible for excessive mucus in chronic bronchitis. Mucus accumulation in COPD patients affects several important outcomes such as lung function, health-related quality of life, COPD exacerbations, hospitalizations, and mortality. Nonpharmacologic options for the treatment of mucus accumulation in COPD are smoking cessation and physical measures used to promote mucus clearance. Pharmacologic therapies include expectorants, mucolytics, methylxanthines, beta-adrenergic receptor agonists, anticholinergics, glucocorticoids, phosphodiesterase-4 inhibitors, antioxidants, and antibiotics.
Introduction
Chronic obstructive pulmonary disease (COPD) is characterized by a persistent airflow limitation that is associated with an enhanced chronic inflammatory response to noxious particles or gases.Citation1 The World Health Organization estimates that over 200 million people have COPD worldwide, and it also predicts that COPD will be the third leading cause of death in the world by 2030, which is higher than its place in 2004 as the fourth leading cause of death.Citation2 The increased output from goblet cells and mucous glands in COPD patients is variably described as “chronic mucus hypersecretion”, “chronic sputum production”, or “chronic bronchitis” (CB). Sputum and mucus are commonly used interchangeably, but these are distinct substances. While mucus is generally cleared by cilia, the ciliated epithelium becomes damaged with chronic inflammation and the increased volume of secretions often requires clearance by cough. Sputum refers to the expectorated secretions.Citation3 CB is commonly defined as the presence of a chronic, productive cough and sputum production for at least 3 consecutive months in 2 consecutive years. This review presents the normal anatomy and physiology related to airway mucus and the pathophysiology of increased mucus production in COPD. Clinical consequences of mucus overproduction, as well as its therapeutic options, are also discussed.
Normal anatomy and physiology related to airway mucus
Airway mucus is secreted by goblet cells found in the superficial mucosa and the mucous glands in the submucosa. Goblet cells decrease in number further into the airways, and they eventually disappear at the level of the terminal bronchioles. The quantity of mucous glands, which produce the majority of the airway mucus, decrease distally as they approach the respiratory bronchioles. The mucosa is a surface layer of pseudostratified columnar epithelial cells that have cilia on their luminal surfaces. The rhythmic beating of the cilia enables the “mucociliary elevator” that transports mucus and liquid, as well as inhaled particles, pathogens, and dissolved chemicals, from distal to proximal airways. After the mucus ascends the trachea, it is driven through the vocal cords by the larynx ciliary epithelium. The mucus is then swallowed after the pharynx is entered. The vocal cords are covered by squamous epithelium, so they do not have cilia, but they promote cough clearance by closing, while expiratory pressure builds; they then open suddenly so airflow is forceful.Citation4,Citation5 The secreted mucins – in particular, the polymeric mucins MUC5AC and MUC5B – serve as the organizing framework of the mucus gel in the airways. The mucins also prevent barrier dehydration, present carbohydrate ligands to sequester the pathogens, and via binding to other components of the secretion, they have the potential to act as sinks for host-protective proteins and peptides.Citation6
Airway mucus is part of the lung’s innate immune function that traps particulates and microorganisms, facilitating their clearance from the lung by means of ciliary transport or cough.Citation3,Citation6 In normal conditions, mucin production efficiently defends the airways. However, in mucin secretory cell hyperplasia and metaplasia, there is overproduction, with pathological consequences.Citation6,Citation7
Mechanism of mucus accumulation in COPD
Mucus hypersecretion and chronic productive cough is a feature of CB.Citation1 The primary mechanisms responsible for excessive mucus production in CB in COPD are the overproduction and hypersecretion by goblet cells, and the decreased elimination of mucus.Citation7 There is also hypertrophy of the submucosal glands that ReidCitation8 described with a ratio of the thickness of the submucosal glands and the thickness between the epithelium and cartilage that covers the bronchi. The size of the submucosal glands correlates with the degree of airway inflammation ().Citation9
Figure 1 Causes of excessive mucus in COPD.
Abbreviations: PEF, peak expiratory flow; COPD, chronic obstructive pulmonary disease.
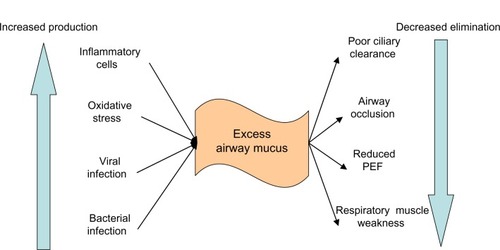
Mucus hypersecretion in COPD is a consequence of cigarette smoke exposure,Citation10,Citation11 acute and chronic viral infection,Citation12 bacterial infection,Citation13 or inflammatory cell activation of mucin gene transcription.Citation13 This leads to the overproduction of mucus and to hypersecretion from increased degranulation, primarily by neutrophil elastase. This is compounded by a difficulty in clearing secretions because of poor ciliary function, distal airway occlusion, and an ineffective cough that is secondary to respiratory muscle weakness and reduced peak expiratory flow.Citation13–Citation15
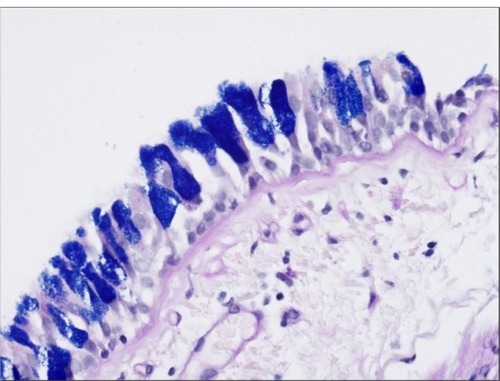
Under increased airway inflammation, the airway epithelium remodels and undergoes metaplasia, implying that there is a phenotypic change that occurs within an adult cell type, and that hyperplasia also occurs, denoting an increase in the total cell number within a given tissue type.Citation16 Saetta et al’sCitation17 study of surgical specimens shows that smokers with both CB and airflow limitation have an increased number of goblet cells and inflammatory cells in peripheral airway epithelium. Innes et alCitation18 revealed that goblet cell hypertrophy and hyperplasia occur in the large airways of habitual cigarette smokers, and this hypertrophy results in epithelial mucin stores that are significantly higher than normal; and show examples of airway epithelium remodeling.
Figure 2 MM and smooth muscle hypertrophy in a small airway from a COPD patient.
Note: Hematoxylin and eosin stain.
Abbreviations: MM, mucous metaplasia; SMH, smooth muscle hypertrophy; COPD, chronic pulmonary disease.
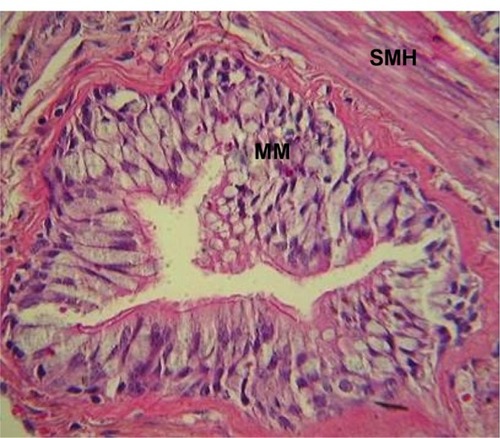
Figure 3 Goblet cell hyperplasia.
Cellular and molecular mechanisms in the pathogenesis of mucus hypersecretion in CB include acquired cystic fibrosis transmembrane conductance regulator dysfunctionCitation19 and activation of the epidermal growth factor receptor.Citation20 Smokers with and without COPD have reduced chloride conductance in the lower airway, and this ion transport abnormality is associated with the presence of CB and dyspnea.Citation19 Cigarette smoke also increases mucin MUC5AC synthesis via epidermal growth factor receptor activation in the airway epithelial cells.Citation20 Upregulation of the basic fibroblast growth factorCitation21 and transforming growth factor-β,Citation22 as well as a higher frequency of the tumor necrosis factor-α polymorphismCitation23 have also been implicated in the pathogenesis of CB.
Epidemiology
From a review of population-based studies, Kim and CrinerCitation7 estimated the prevalence of CB to be between 3.4%–22% among adults. The wide range of prevalence estimates may be due to varying definitions of CB (ie, chronic phlegm versus chronic cough and phlegm), as well as the possible inclusion of subjects with bronchiectasis.
The prevalence of CB is higher in COPD patients.Citation13,Citation24,Citation25 In the Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) cohort,Citation25 34.6% of the 2,161 subjects reported the presence of CB, as defined by the American Thoracic Society–Diffuse Lung Disease (ATS-DLD) questionnaire (“phlegm on most days for 3 or more consecutive months during the year and trouble with phlegm for 2 or more years”). Of the 1,061 Global initiative for chronic Obstructive Lung Disease (GOLD) 2–4 subjects in the COPDGene study,Citation24 CB, by the same definition, was found in 27.3% of patients. The sex predilection of CB is unclear, with some studies finding males more affected,Citation24,Citation26,Citation27 while others report a female predominance.Citation28–Citation30
Smoking is the primary risk factor for CB. In one study,Citation31 the 30-year cumulative incidence of CB was 42% in continuous smokers, 26% in ex-smokers, and 22% in never-smokers. Occupational exposure to biological dustCitation32 or to combustion byproducts, inorganic dusts, or fumes and organic dustsCitation33 may also be risk factors. Gastroesophageal reflux disease is another possible risk factor for CB.Citation34
Mucus accumulation and outcomes
CB affects important outcomes in COPD, including declines in lung function, health-related quality of life, as well as COPD exacerbations and hospitalizations, and mortality. summarizes the findings of select studies.
Table 1 Selected studies on chronic bronchitis and outcomes
Lung function
Several studies demonstrated that chronic mucus production is related to a progressive decline in lung function.Citation35,Citation36 In a longitudinal study that followed 1,757 men and 2,191 women for 12 years, the presence of chronic sputum production was associated with an accelerated loss in forced expiratory volume in one second (FEV1) in men of 4.5±2 mL/year after adjusting for height, age, and cigarette smoking, but a statistically insignificant decline in FEV1 in women of 1.7±1.5 mL/year.Citation35 Using data from the Copenhagen City Heart Study,Citation36 which was conducted with 5,354 females and 4,081 males, comparing two spirometry results 5 years apart, Vestbo et al found an excessive FEV1 decline of 22.8 mL/year (95% confidence interval [CI]: 8.2–37.4), after adjusting for age, height, weight change, and smoking, in males with chronic mucus hypersecretion compared with males without mucus hypersecretion; in women, the adjusted excess decline was not statistically significant at 12.6 mL/year (95% CI: 0.7–24.6).
The presence of CB may also predict the development of airflow obstruction.Citation37–Citation39 In an international population-based cohort study of young adults with normal lung function, chronic cough and phlegm predicts the development of COPD (defined as a ratio of FEV1 to forced vital capacity [FVC] <0.7) with an incidence rate ratio of 1.85 (95% CI: 1.17–2.93) after adjusting for smoking.Citation37 Subjects who reported chronic cough and phlegm have a nearly threefold increased risk of developing COPD (FEV1/FVC <0.7) with respect to asymptomatic subjects (incidence rate ratio: 2.88; 95% CI: 1.44–5.79). Among the 1,412 participants in the Tucson Epidemiological Study of Airway Obstructive Disease,Citation38 the presence of CB is an independent risk factor for incident airflow limitation among subjects <50 years old (adjusted hazard ratio [HR]: 2.2; 95% CI: 1.3–3.8), but not among subjects ≥50 years old (adjusted HR: 0.9; 95% CI: 0.6–1.4).
Health-related quality of life
Several analyses show that chronic sputum production correlates with a worse quality of life and more limitations due to physical health.Citation24–Citation26 Our analysis of the COPDGene study reports higher modified Medical Research Council (mMRC) dyspnea scores and St George’s Respiratory Questionnaire (SGRQ) scores in COPD subjects with CB symptoms.Citation24 In the ECLIPSE cohort, the presence of CB is related to worse total scores on the mMRC and SGRQ across all COPD disease severities (GOLD II to IV).Citation25 Among subjects with COPD in the Proyecto Latinoamericano de Investigación en Obstrucción Pulmonar (PLATINO) study, those with CB have a worse general health status and more physical activity limitations.Citation26
COPD exacerbations and hospitalization
Vestbo et alCitation36 showed that hospitalization due to COPD is associated with chronic mucus hypersecretion with a relative risk of 2.4 (95% CI: 1.3–4.5) for males and 2.6 (95% CI: 1.2–5.3) for females. A cross-sectional multicenter analysis of 433 COPD subjects found that chronic cough and sputum were associated with frequent COPD exacerbations during the previous year (adjusted odds ratio [OR]: 4.15 [95% CI: 2.43–7.08]; P<0.0001), including severe exacerbations requiring hospitalizations (adjusted OR: 4.08 [95% CI: 1.18–14.09]; P=0.03).Citation40 Examination of the COPDGene cohort concludes the fact that a history of exacerbations in the previous year is higher in the CB group (1.21±1.62 versus 0.63±1.12 per patient; P=0.027), and more subjects in that group reported a history of severe exacerbations (26.6% versus 20%; P=0.024).Citation24
However, not all studies link CB with exacerbations and hospitalizations. In ECLIPSE,Citation25 exacerbation frequency in the year before recruitment in those with and without CB was the same. In addition, an analysis of the PLATINO studyCitation26 showed a nonsignificant difference in exacerbation frequency between COPD subjects with and without CB.
The differing results could be explained by the numerous differences in the study population and in the design of the comparison of the selected studies that report COPD exacerbations or hospitalizations. Two studies are population-based and involve patients from DenmarkCitation36 or Latin America.Citation26 The other three are cross-sectional studies involving patients from France,Citation40 the United States,Citation24 or from multiple nations.Citation25 Vestbo et alCitation36 used a definition of chronic mucus hypersecretion, while the other studies use the more classic definition of CB.Citation24–Citation26,Citation40 The prevalence of CB in COPD patients using the classic definition is 14%,Citation26 27%,Citation24 35%,Citation25 and 74%.Citation40 Across the studies, there were also small differences in the percentage of current smokers and the severity of disease, as measured by airflow obstruction. In two studies with similar demographics and the number of current smokers, the study that showed a positive association between CB and exacerbations had more patients with frequent exacerbations,Citation24 while the other did not show an association and it had fewer patients with frequent exacerbations.Citation25 Therefore, prospective studies are needed to clarify the association between CB and COPD exacerbations and hospitalizations.
Mortality
CB is a risk factor for respiratory-relatedCitation27,Citation31,Citation41 and all-causeCitation31,Citation38,Citation42,Citation43 mortality. In a Finnish study of 1,711 middle-aged males with 40-year mortality data, persistent CB predicts risk of respiratory-related deaths (adjusted HR: 2.54; 95% CI: 1–6.46; P=0.049) and all-cause mortality (adjusted HR: 1.64; 95% CI: 1.23–2.19; P=0.001), after adjusting for pulmonary function.Citation31 Prescott et al,Citation41 who followed 14,223 participants for 10–12 years, reported that chronic mucus hypersecretion is a significant predictor of death from pulmonary infection, with a multivariate relative risk (RR) of 3.5 (95% CI: 1.8–7.1), but not of death without pulmonary infection. In a 9- to 12-year mortality follow-up of 8,427 Caucasian adults, Speizer et alCitation27 reported the association of cough or phlegm and an increase in COPD mortality with an adjusted OR of 3.75 (95% CI: 1.28–11) in men and 11.04 (95% CI: 2.52–48.5) in women. In the Tuscon Epidemiologic Survey of Airway Obstructive Disease,Citation38 the risk of all-cause mortality was higher in patients younger than 50 years of age with CB (HR: 2.2; 95% CI: 1.3–3.8), but not among subjects 50 years of age or older (HR: 1; 95% CI: 0.7–1.3). In an analysis of the 13,756 subjects in the Copenhagen City Heart Study,Citation42 the presence of chronic mucus hypersecretion increased mortality from all causes with an adjusted RR of 1.3 (95% CI: 1.1–1.4) in males and 1.1 (95% CI: 0.9–1.3) in females. An investigation of 1,061 French males showed a multivariate RR of 1.35 (P<0.01) for the relationship between chronic mucus hypersecretion and mortality during a 22-year follow-up.Citation43 The mechanism behind this association in unclear, but a possible cause could be the increased inflammatory state seen in those with CB, leading to increased cardiovascular events.Citation38
Other studies, however, have not shown a statistically significant relationship between chronic mucus production and mortality. There is a statistically insignificant trend towards death in Tockman and Comstock’s studyCitation44 of 10-year mortality in 884 males with chronic phlegm production (RR: 1.65; 95% CI: 0.95–2.89). Mannino et alCitation45 reported similar findings for risk of death in the presence of respiratory symptoms (cough, sputum, or wheeze) without obstructive lung disease.
Numerous differences are apparent after comparing the different studies that report mortality. These differences could account for the inconsistent relationship between CB and mortality. First, as mentioned previously, the definition used of mucus accumulation with or without spirometry-defined airflow obstruction varies. The negative studies use chronic phlegm productionCitation44 or a combination of respiratory symptoms (cough, sputum, or wheeze).Citation45 The positive studies use the classic CB definition,Citation31,Citation38 or symptoms of chronic mucus hypersecretion,Citation41,Citation42 or chronic phlegmCitation27,Citation43. All of the symptoms are self-reported, and so responses may be varied depending on how the question is phrased. Second, standard of care likely varies in the populations being studied, as the research on this subject matter includes patients from all areas of the globe at different points in time. Third, the association of mortality and chronic sputum, phlegm production, or CB may be influenced by factors such as the presence of COPD or smoking history. Most of the studies report results that have adjusted for these covariates, but unmeasured differences associated with the presence of these factors may influence the findings. Fourth, some authors suggest that the relationship between mucus accumulation and mortality is further affected by severity of airflow obstruction,Citation27 infection,Citation41 and age.Citation38
Treatment options
The main goals of therapy should target the different pathophysiologic mechanisms of CB by reducing mucus overproduction, decreasing mucus hypersecretion by controlling inflammation, facilitating mucus elimination by increasing ciliary transport, reducing mucus tenacity, increasing shear stress to augment mucus detachment, and modifying cough ().
Table 2 Summary of therapeutic interventions for chronic bronchitis
Nonpharmacological therapy
Smoking cessation
Smoking cessation can improve cough in many patients with CB by improving mucociliary function and by decreasing goblet cell hyperplasia.Citation46 Smoking cessation has also been shown to decrease airway injury and lower levels of mucus in exfoliated sputum tracheobronchial cells when compared to those that continued to smoke.Citation47 A large longitudinal follow-up study found that the incidence rates of CB were much higher in current smokers compared to ex-smokers (42% versus 26%, respectively).Citation31 Unfortunately, there is a paucity of data regarding the effects of smoking cessation on sputum symptomatology.
Physical measures
Mucus clearance is aided by maneuvers that promote coughing and increase minute ventilation. This augments shear stresses on mucosal surfaces generated by increased airflow. It also increases humidification of the airway and regulates mucus hydration. Thus, methods such as the application of positive expiratory pressure and use of flutter valves or high-frequency chest compression vests may be of value, but they have not been studied for use in COPD in large clinical trials. Although cystic fibrosis studies have demonstrated that chest percussion and postural drainage improve mucociliary clearance,Citation48 these methods have not been well studied in the COPD patient population. There are a few trials that have studied chest physiotherapy or directed coughing techniques in COPD.Citation49 These trials have shown some improvements in mucus clearance, but no changes in lung function.
Pharmacological therapy
Expectorants and mucolytics
Guaifenesin works by promoting vagally-mediated increases in airway secretions.Citation50 Long-term use of guaifenesin has not been shown to be of benefit in COPD or CB.Citation51 Inhaled hypertonic saline works by rehydrating mucus by drawing water from the epithelial cells and by promoting cough.Citation52,Citation53 While this method has shown improvement in lung function in cystic fibrosis, it has only been shown in one study in COPD to improve dyspnea and exercise capacity.Citation54 Inhaled dornase alfa hydrolyzes deoxyribonucleic acid (DNA), thereby improving lung function and decreasing exacerbation frequency in cystic fibrosis patients, in whom airway mucus concentrations of DNA are high. However, the concentration of DNA in the sputum of COPD patients is much lower,Citation55 and studies have shown that dornase alfa is not beneficial and, in fact, it may be harmful.Citation56
Methylxanthines and beta-adrenergic receptor agonists
Both methylxanthines and short-acting beta-adrenergic receptor agonists promote mucus clearance by increasing airway luminal diameter, increasing ciliary beat frequency via an increase in intracellular cyclic adenosine monophosphate levels, and increasing mucus hydration by stimulating airway Cl− secretion via activation of the cystic fibrosis transmembrane regulator. This decreases mucus viscosity, allowing for easier transport by airway cilia.Citation57–Citation59
In animal models, short-term administration of beta-agonists is associated with upregulation of mucociliary clearance.Citation60,Citation61 Similarly, methylxanthines improve mucociliary clearance not only via their bronchodilatory properties, but also by stimulating ciliary beat frequency, augmenting airway epithelial ion transport to increase mucus hydration, and promoting mucus secretion in the lower airways.Citation62 Clinical studies of theophylline in CB have shown improved lung function, but no consistent change in cough and sputum production.Citation63,Citation64
The effects of long-acting beta-adrenergic receptor agonists on mucociliary function have been attributed to their beneficial effects on lung function.Citation64–Citation67 Long-acting beta-adrenergic receptor agonists also reduce hyperinflation and increase peak expiratory flow, which are essential components of effective cough.Citation68 In vitro evidence has shown that salmeterol can stimulate ciliary beat frequency.Citation58 Similarly, formoterol significantly improves mucociliary clearance when compared with placebo in patients with bronchitis.Citation69
Anticholinergics
Anticholinergics, by their action on the muscarinic receptor, are believed to help mucus clearance by increasing luminal diameter and by decreasing surface and submucosal gland mucin secretion.Citation70–Citation72 They are also thought to facilitate cough-induced mucus clearance. However, anticholinergics may desiccate airway secretions by depleting airway surface liquid, thereby making secretions more difficult to expectorate. In vivo, the literature does not support the use of anticholinergics for the treatment of CB. Ipratropium bromide has been shown to reduce the quantity and severity of coughs in CB,Citation72 but it is not effective in improving the mucociliary clearance in COPD.Citation73 Tiotropium has been shown to improve lung functionCitation74 and reduce cough, but mucociliary clearance was not improved.Citation75
Glucocorticoids
There is in vitro evidence that glucocorticoids reduce inflammation and mucus production.Citation76,Citation77 In a murine model of asthma, inhaled corticosteroids decrease goblet cell hyperplasia.Citation78 Dexamethasone has also been shown to decrease epithelial mucin gene, MUC5AC, expression in human bronchial epithelial cells;Citation79 glucocorticoids may also hasten mucociliary clearance.Citation80 Inhaled corticosteroids reduce exacerbation frequency and improve quality of life scores in COPD.Citation81–Citation83 Whether inhaled corticosteroids are more beneficial in COPD patients with CB or in airway-predominant phenotypes remains to be determined.
Phosphodiesterase-4 inhibitors
Phosphodiesterase-4 (PDE-4) inhibition decreases inflammation and promotes smooth-muscle relaxation in the airways by preventing the hydrolysis of cyclic adenosine monophosphate to its inactive metabolite. Cilomilast and roflumilast are highly specific second-generation oral PDE-4 inhibitors. A meta-analysis of 23 randomized trials of roflumilast or cilomilast compared with placebo found that treatment with a PDE-4 inhibitor only modestly increased FEV1 (45.59 mL; 95% CI: 39.1–52.03), but it reduced the likelihood of an exacerbation (OR: 0.78; 95% CI: 0.72–0.85).Citation84 Roflumilast has been shown to significantly improve prebronchodilator FEV1, decrease the rate of moderate to severe exacerbations, and decrease the total number of exacerbations by 17% (95% CI: 8–25).Citation85 Two trials have evaluated the use of roflumilast in moderate to severe COPD patients.Citation86 The majority of patients (78%–100%) enrolled had chronic cough and sputum production at baseline. One study randomized patients to roflumilast plus salmeterol or salmeterol alone, and the second study randomized patients to roflumilast plus tiotropium or tiotropium alone.Citation87 In both trials, roflumilast significantly improved the primary endpoint, prebronchodilator FEV1, as well as the exacerbation rate. Thus, as CB increases the risk for exacerbation, PDE-4 inhibitors may play a preferential role in preventing the development of exacerbation in patients with CB and COPD.
Antioxidants
Given that oxidative stress is crucial to the pathogenesis of COPD,Citation87 antioxidant therapy may be of benefit in COPD treatment. Thiol compounds are powerful antioxidants and include N-acetylcysteine (NAC), N-acestyln, carbocysteine, erdosteine, and fudosteine. The two most extensively studied antioxidant medications for COPD are NAC and carbocysteine. NAC is a precursor of L-cysteine and reduced glutathione, which reduces the cellular levels of oxidative stress and the production of reactive oxygen species. NAC also reduces disulfide bonds and sulfhydryl bonds that link together mucin polymers, thereby reducing sputum viscosity. Carbocysteine is a blocked thiol derivative of L-cysteine with in vitro free radical scavenging and anti-inflammatory properties, and it may work on the fucose and sialic acid content in mucus.Citation88
The Bronchitis Randomized On NAC Cost-Utility StudyCitation89 (BRONCHUS) is the largest trial of N-acetylcysteine use in COPD to date. In this multicenter study, 523 patients with a mean predicted FEV1 of 57% were randomized to NAC 600 mg daily or placebo, and they were followed for 3 years. The mean exacerbation rates of the subjects were 2.4–2.5 exacerbations/year. There were no differences in FEV1 decline in terms of time or health-related quality of life between the two groups. There was also no overall difference in the number of exacerbations. However, in a post hoc analysis, those without inhaled corticosteroids (about 30% of the entire group) had a significant reduction in exacerbations with NAC when compared to placebo.
A more recent study, the High-Dose N-Acetylcysteine in Stable COPD (HIACE) study,Citation90 enrolled 120 subjects with stable COPD who were randomized to receive NAC 600 mg twice daily or placebo daily for 1 year. The primary outcomes were change in small airway function, as assessed by forced expiratory flow at 25% to 75% (FEF 25%–75%) and forced oscillation technique parameters, which were measured by applying external oscillatory pressure during tidal breathing. The secondary outcomes measurements were change in symptoms, as assessed by the mMRC dyspnea scale and SGRQ scores, exacerbation frequency, and hospitalizations. The patients were predominantly male (93.2%) with a mean age of 70.8±0.74 years and a predicted FEV1% of 53.9%±2.0%. There were no differences in baseline FEF25–75, mMRC dyspnea score, and exacerbation frequency within the previous year prior to enrollment between the two groups. Patients in the NAC group had exhibited decreased small airway resistance over the duration of the study when compared to the placebo group. The NAC group had a statistically significant increase (P=0.037) in FEF25–75 (0.72±0.07 L/second versus 0.80±0.07 L/second) compared to placebo (0.679±0.07 L/second to 0.677±0.07 L/second), and the reactance at 6 Hz improved in the NAC group by 23%, which was compared to a decrease in the placebo group by 10.7% (P=0.04). Additionally, the mean exacerbation frequency in the NAC group was lower (0.96/year) compared to the placebo group (1.71/year) (P=0.19).
The Effect of Carbocysteine on Acute Exacerbation of Chronic Obstructive Pulmonary Disease (PEACE) study randomized 709 patients with at least two exacerbations within the 2 years prior to enrollment to carbocysteine 500 mg three times daily or placebo, with the primary endpoint of exacerbation rate over 1 year.Citation91 Numbers of exacerbations per patient per year declined significantly in the carbocysteine group when compared with the placebo group (1.01 [standard error: 0.06] versus 1.35 [standard error: 0.06]); RR: 0.75 (95% CI: 0.62–0.92; P=0.004). There were no significant interactions between COPD severity, smoking, and use of inhaled corticosteroids, and the primary endpoint. These three studies provided conflicting results on the efficacy of antioxidants on exacerbation frequency. A 600 mg daily dose of NAC may be too low to see a clinical effect when compared to a 600 mg twice daily dose, which was used in the HIACE study,Citation90 and a 600 mg three times daily dose used in patients with idiopathic pulmonary fibrosis. Additionally, patients in the PEACE trial and HIACE trial had a lower mean predicted FEV1 (44% and 53.9%, respectively) compared to 57% in the BRONCHUS trial.Citation89,Citation90,Citation91 Therefore, it is possible that antioxidant therapy is more efficacious in those with lower lung function.
Poole et alCitation92 performed a meta-analysis of mucolytic agents for CB or COPD. They included randomized, placebo-controlled studies of at least 2 months’ duration. They found 30 trials involving 7,436 participants to be methodologically acceptable for further analysis. The majority of the studies involved the use of NAC (n=15) or carbocysteine (n=4). Compared to placebo, there was a 17% reduction in the number of exacerbations per patient with oral mucolytics (a reduction of 0.04 exacerbations per participant per month; 95% CI: –0.04 to –0.03). This may not be clinically relevant since there was very high heterogeneity (I2=87%) in assessing the exacerbation frequency outcome. There was no overall effect on lung function or increase in adverse effects from the medications.
Antibiotics
Chronic antibiotic therapy is generally not indicated for patients with emphysema or CB. Macrolide therapy, however, has been shown to have anti-inflammatory properties and may play a role in the treatment of those with CB. These therapies have been shown to inhibit proinflammatory cytokines, decrease neutrophil burst, inhibit migration and increase apoptosis, decrease eosinophilic inflammation, increase mucociliary transport, reduce goblet cell secretion, and decrease bronchoconstriction.Citation93 The effect of chronic macrolide therapy on COPD exacerbations was assessed in 109 patients with COPD who were randomly assigned to receive erythromycin 250 mg or placebo twice daily for 1 year.Citation94 The erythromycin group had significantly fewer exacerbations than the placebo group. A recent, large, prospective, placebo-controlled, randomized trial on the use of azithromycin (250 mg daily for 1 year) to prevent acute exacerbations of COPD showed that azithromycin was associated with a significant decrease in exacerbation frequency and an improvement in health-related quality of life.Citation95 There was, however, no significant additional benefit of azithromycin in those with CB at baseline.
Future therapy
There is a novel inhaled therapy, BIO-11006, that is currently undergoing Phase II testing (BREATH 1 trialCitation96) in patients with CB. This drug inhibits the function of the myristoylated alanine-rich C kinase substrate protein (MARCKS), which has been shown to be a vital component for the secretion of mucus and inflammatory mediators. Preliminary results indicated that patients have improvements in lung function and reductions in both cough and sputum production.Citation96
Microbiology
There is no available evidence of the benefit of performing routine microbiologic cultures of mucus from COPD patients. Sputum production is one of the characteristic symptoms of COPD, and a change in amount or quality of sputum beyond a day-to-day variation may indicate an exacerbation.Citation1 The presence of purulent sputum is 94.4% sensitive and 77% specific for the yield of a high bacterial load, and it indicates a clear subset of patient episodes identified at presentation that is likely to benefit most from antibiotic therapy.Citation97 Thus, the 2013 GOLD guidelinesCitation1 recommend that the presence of purulent sputum during an exacerbation can be a sufficient indication for starting empirical antibiotics, and that a sputum culture with antibiotic sensitivity testing should be performed when there is lack of response to the initial antibiotic treatment.
Future directions
We believe more studies are needed on why some smokers develop CB and others do not, and on how smoking cessation affects the natural history of CB. In addition, more research on the pathophysiology of this disease process will help in the development of better therapies that directly target CB in order to improve symptoms, while decreasing exacerbations and mortality. The effects of the presence of radiology-confirmed bronchiectasis, a clinically similar phenotype, on the symptoms and outcomes of CB deserve further study.
We believe that the additional study of higher doses of antioxidants such as NAC, and more in-depth studies of selective PDE-4 inhibitors like roflumilast are needed. The identification of more therapeutic targets is necessary for drug development in order to improve outcomes specifically related to CB.
Disclosure
The authors report no conflicts of interest in this work.
References
- VestboJHurdSSAgustíAGGlobal strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summaryAm J Respir Crit Care Med2013187434736522878278
- World Health OrganizationWorld Health Statistics 2008Geneva, SwitzerlandWorld Health Organization2008 Available from: http://www.who.int/whosis/whostat/EN_WHS08_Full.pdfAccessed July 23, 2013
- VoynowJARubinBKMucins, mucus, and sputumChest2009135250551219201713
- ShadeDJrCordovaFCNormal lung structureCrinerGJD’AlonzoGEPulmonary Pathophysiology1st ed199920
- FahyJVDickeyBFAirway mucus function and dysfunctionN Engl J Med2010363232233224721121836
- ThorntonDJRousseauKMcGuckinMAStructure and function of the polymeric mucins in airways mucusAnnu Rev Physiol20087045948617850213
- KimVCrinerGJChronic bronchitis and chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2013187322823723204254
- ReidLMeasurement of the bronchial mucous gland layer: A diagnostic yardstick in chronic bronchitisThorax19601513214114437095
- MullenJBWrightJLWiggsBRParePDHoggJCReassessment of inflammation of airways in chronic bronchitisBr Med J (Clin Res Ed)1985291650412351239
- EbertRVTerracioMJThe bronchiolar epithelium in cigarette smokers. Observations with the scanning electron microscopeAm Rev Respir Dis197511114111111397
- DeshmukhHSCaseLMWesselkamperSCMetalloproteinases mediate mucin 5AC expression by epidermal growth factor receptor activationAm J Respir Crit Care Med2005171430531415531749
- HoltzmanMJTynerJWKimEYAcute and chronic airway responses to viral infection: implications for asthma and chronic obstructive pulmonary diseaseProc Am Thorac Soc20052213214016113481
- BurgelPRNadelJARoles of epidermal growth factor receptor activation in epithelial cell repair and mucin production in airway epitheliumThorax2004591199299615516478
- HoggJCChuFUtokaparchSThe nature of small-airway obstruction in chronic obstructive pulmonary diseaseN Engl J Med2004350262645265315215480
- VerraFEscudierELebargyFBernaudinJFDe CrémouxHBignonJCiliary abnormalities in bronchial epithelium of smokers, ex-smokers, and nonsmokersAm J Respir Crit Care Med19951513 Pt 16306347881648
- WilliamsOWSharafkhanehAKimVDickeyBFEvansCMAirway mucus: From production to secretionAm J Respir Cell Mol Biol200634552753616415249
- SaettaMTuratoGBaraldoSGoblet cell hyperplasia and epithelial inflammation in peripheral airways of smokers with both symptoms of chronic bronchitis and chronic airflow limitationAm J Respir Crit Care Med20001613 Pt 11016102110712357
- InnesALWoodruffPGFerrandoREEpithelial mucin stores are increased in the large airways of smokers with airflow obstructionChest200613041102110817035444
- DransfieldMTWilhelmAMFlanaganBAcquired cystic fibrosis transmembrane conductance regulator dysfunction in the lower airways in COPDChest2013144249850623538783
- TakeyamaKJungBShimJJActivation of epidermal growth factor receptors is responsible for mucin synthesis induced by cigarette smokeAm J Physiol Lung Cell Mol Physiol20012801L165L17211133506
- GuddoFVignolaAMSaettaMUpregulation of basic fibroblast growth factor in smokers with chronic bronchitisEur Respir J200627595796316510460
- VignolaAMChanezPChiapparaGTransforming growth factor-beta expression in mucosal biopsies in asthma and chronic bronchitisAm J Respir Crit Care Med19971562 Pt 15915999279245
- HuangSLSuCHChangSCTumor necrosis factor-alpha gene polymorphism in chronic bronchitisAm J Respir Crit Care Med19971565143614399372657
- KimVHanMKVanceGBCOPDGene InvestigatorsThe chronic bronchitic phenotype of COPD: an analysis of the COPDGene StudyChest2011140362663321474571
- AgustiACalverleyPMCelliBEvaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) investigatorsRespir Res20101112220831787
- de OcaMMHalbertRJLopezMVThe chronic bronchitis phenotype in subjects with and without COPD: the PLATINO studyEur Respir J2012401283622282547
- SpeizerFEFayMEDockeryDWFerrisBGJrChronic obstructive pulmonary disease mortality in six US citiesAm Rev Respir Dis19891403 Pt 2S49S552782760
- American Lung AssociationTrends in COPD (Chronic Bronchitis and Emphysema): Morbidity and MortalityWashington, DCAmerican Lung Association2013
- HarmsenLThomsenSFIngebrigtsenTChronic mucus hypersecretion: prevalence and risk factors in younger individualsInt J Tuberc Lung Dis20101481052105820626952
- EhrlichRIWhiteNNormanRPredictors of chronic bronchitis in South African adultsInt J Tuberc Lung Dis20048336937615139477
- PelkonenMNotkolaILNissinenATukiainenHKoskelaHThirty-year cumulative incidence of chronic bronchitis and COPD in relation to 30-year pulmonary function and 40-year mortality: a follow-up in middle-aged rural menChest200613041129113717035447
- MathesonMCBenkeGRavenJBiological dust exposure in the workplace is a risk factor for chronic obstructive pulmonary diseaseThorax200560864565116061705
- TrupinLEarnestGSan PedroMThe occupational burden of chronic obstructive pulmonary diseaseEur Respir J200322346246914516136
- SmyrniosNAIrwinRSCurleyFJChronic cough with a history of excessive sputum production. The spectrum and frequency of causes, key components of the diagnostic evaluation, and outcome of specific therapyChest199510849919977555175
- ShermanCBXuXSpeizerFEFerrisBGJrWeissSTDockeryDWLongitudinal lung function decline in subjects with respiratory symptomsAm Rev Respir Dis199214648558591416410
- VestboJPrescottELangePAssociation of chronic mucus hypersecretion with FEV1 decline and chronic obstructive pulmonary disease morbidity. Copenhagen City Heart Study GroupAm J Respir Crit Care Med19961535153015358630597
- de MarcoRAccordiniSCerveriIIncidence of chronic obstructive pulmonary disease in a cohort of young adults according to the presence of chronic cough and phlegmAm J Respir Crit Care Med20071751323917008642
- GuerraSSherrillDLVenkerCCeccatoCMHalonenMMartinezFDChronic bronchitis before age 50 years predicts incident airflow limitation and mortality riskThorax2009641089490019581277
- LindbergAErikssonBLarssonLGRönmarkESandströmTLundbäckBSeven-year cumulative incidence of COPD in an age-stratified general population sampleChest2006129487988516608933
- BurgelPRNesme-MeyerPChanezPInitiatives Bronchopneumopathie Chronique Obstructive Scientific CommitteeCough and sputum production are associated with frequent exacerbations and hospitalizations in COPD subjectsChest2009135497598219017866
- PrescottELangePVestboJChronic mucus hypersecretion in COPD and death from pulmonary infectionEur Respir J199588133313387489800
- LangePNyboeJAppleyardMJensenGSchnohrPRelation of ventilatory impairment and of chronic mucus hypersecretion to mortality from obstructive lung disease and from all causesThorax19904585795852402719
- AnnesiIKauffmannFIs respiratory mucus hypersecretion really an innocent disorder? A 22-year mortality survey of 1,061 working menAm Rev Respir Dis198613446886933767125
- TockmanMSComstockGWRespiratory risk factors and mortality: longitudinal studies in Washington County, MarylandAm Rev Respir Dis19891403 Pt 2S56S632782761
- ManninoDMBuistASPettyTLEnrightPLReddSCLung function and mortality in the United States: data from the First National Health and Nutrition Examination Survey follow up studyThorax200358538839312728157
- MullenJBWrightJLWiggsBRParéPDHoggJCStructure of central airways in current smokers and ex-smokers with and without mucus hypersecretion: relationship to lung functionThorax198742118438483424265
- SwanGEHodgkinJERobyTMittmanCJacoboNPetersJReversibility of airways injury over a 12-month period following smoking cessationChest199210136076121541120
- WarnockLGatesAvan der SchansCPChest physiotherapy compared to no chest physiotherapy for cystic fibrosisCochrane Database Syst Rev20139CD00140124006212
- van der SchansCPConventional chest physical therapy for obstructive lung diseaseRespir Care200752911981206 discussion 1206–120917716386
- RubinBKMucolytics, expectorants, and mucokinetic medicationsRespir Care200752785986517594730
- ThomsonMLPaviaDMcNicolMWA preliminary study of the effect of guaiphenesin on mucociliary clearance from the human lungThorax19732867427474595814
- LevinMHSullivanSNielsonDYangBFinkbeinerWEVerkmanASHypertonic saline therapy in cystic fibrosis: Evidence against the proposed mechanism involving aquaporinsJ Biol Chem200628135258032581216829520
- BoucherRCCystic fibrosis: a disease of vulnerability to airway surface dehydrationTrends Mol Med200713623124017524805
- ValderramasSRAtallahANEffectiveness and safety of hypertonic saline inhalation combined with exercise training in patients with chronic obstructive pulmonary disease: a randomized trialRespir Care200954332733319245725
- FahyJVSteigerDJLiuJBasbaumCBFinkbeinerWEBousheyHAMarkers of mucus secretion and DNA levels in induced sputum from asthmatic and from healthy subjectsAm Rev Respir Dis19931475113211378484621
- O’DonnellAEBarkerAFIlowiteJSFickRBTreatment of idiopathic bronchiectasis with aerosolized recombinant human DNase I. rhDNase Study GroupChest19981135132913349596315
- TamaokiJKondoMTakizawaTEffect of cAMP on ciliary function in rabbit tracheal epithelial cellsJ Appl Physiol (1985)1989663103510392468639
- DevaliaJLSapsfordRJRusznakCToumbisMJDaviesRJThe effects of salmeterol and salbutamol on ciliary beat frequency of cultured human bronchial epithelial cells, in vitroPulm Pharmacol1992542572631362105
- SalatheMEffects of beta-agonists on airway epithelial cellsJ Allergy Clin Immunol2002110Suppl 6S275S28112464936
- NguyenLPOmoluabiOParraSChronic exposure to beta-blockers attenuates inflammation and mucin content in a murine asthma modelAm J Respir Cell Mol Biol200838325626218096872
- NguyenLPLinRParraSBeta2-adrenoceptor signaling is required for the development of an asthma phenotype in a murine modelProc Natl Acad Sci U S A200910672435244019171883
- WannerAEffects of methylxanthines on airway mucociliary functionAm J Med1985796A16213002175
- AndersonGPeelETPardoeTJonesRSustained-release theophylline in chronic bronchitisBr J Dis Chest19827632612656751366
- TaylorDRBuickBKinneyCLowryRCMcDevittDGThe efficacy of orally administered theophylline, inhaled salbutamol, and a combination of the two as chronic therapy in the management of chronic bronchitis with reversible air-flow obstructionAm Rev Respir Dis198513157477514003919
- FosterWMLangenbackEGBergofskyEHLung mucociliary function in man: interdependence of bronchial and tracheal mucus transport velocities with lung clearance in bronchial asthma and healthy subjectsAnn Occup Hyg1982261–42272447181267
- JonesPWBoshTKQuality of life changes in COPD patients treated with salmeterolAm J Respir Crit Care Med19971554128312899105068
- MahlerDADonohueJFBarbeeRAEfficacy of salmeterol xinafoate in the treatment of COPDChest1999115495796510208192
- van NoordJAAumannJLJanssensEEffects of tiotropium with and without formoterol on airflow obstruction and resting hyperinflation in patients with COPDChest2006129350951716537846
- MelloniBGermoutyJThe influence of a new beta agonist: formoterol on mucociliary functionRev Mal Respir199295503507 French1439090
- BatemanEDRennardSBarnesPJAlternative mechanisms for tiotropiumPulm Pharmacol Ther200922653354219635581
- WineJJJooNSSubmucosal glands and airway defenseProc Am Thorac Soc200411475316113412
- GhafouriMAPatilKDKassISputum changes associated with the use of ipratropium bromideChest19848633873936236043
- BennettWDChapmanWFMascarellaJMThe acute effect of ipratropium bromide bronchodilator therapy on cough clearance in COPDChest199310324884958432142
- CasaburiRBriggsDDJrDonohueJFSerbyCWMenjogeSSWitekTJJrThe spirometric efficacy of once-daily dosing with tiotropium in stable COPD: a 13-week multicenter trial. The US Tiotropium Study GroupChest200011851294130211083677
- HasaniATomsNAgnewJESarnoMHarrisonAJDilworthPThe effect of inhaled tiotropium bromide on lung mucociliary clearance in patients with COPDChest200412551726173415136383
- HattotuwaKLGizyckiMJAnsariTWJefferyPKBarnesNCThe effects of inhaled fluticasone on airway inflammation in chronic obstructive pulmonary disease: a double-blind, placebo-controlled biopsy studyAm J Respir Crit Care Med2002165121592159612070058
- InnesALCarringtonSDThorntonDJEx vivo sputum analysis reveals impairment of protease-dependent mucus degradation by plasma proteins in acute asthmaAm J Respir Crit Care Med2009180320321019423716
- LeungSYEynottPNathPChungKFEffects of ciclesonide and fluticasone propionate on allergen-induced airway inflammation and remodeling featuresJ Allergy Clin Immunol2005115598999615867856
- ChenYNickolaTJDiFronzoNLColberg-PoleyAMRoseMCDexamethasone-mediated repression of MUC5AC gene expression in human lung epithelial cellsAm J Respir Cell Mol Biol200634333834716239644
- O’RiordanTGMaoYOteroRLopezJSabaterJRAbrahamWMBudesonide affects allergic mucociliary dysfunctionJ Appl Physiol (1985)1998853108610919729587
- BurgePSCalverleyPMJonesPWSpencerSAndersonJAMaslenTKRandomised, double blind, placebo controlled study of fluticasone propionate in patients with moderate to severe chronic obstructive pulmonary disease: the ISOLDE trialBMJ200032072451297130310807619
- CalverleyPPauwelsRVestboJTRial of Inhaled STeroids ANd long-acting beta2 agonists study groupCombined salmeterol and fluticasone in the treatment of chronic obstructive pulmonary disease: a randomised controlled trialLancet2003361935644945612583942
- SzafranskiWCukierARamirezAEfficacy and safety of budesonide/formoterol in the management of chronic obstructive pulmonary diseaseEur Respir J2003211748112570112
- ChongJPoolePLeungBBlackPNPhosphodiesterase 4 inhibitors for chronic obstructive pulmonary diseaseCochrane Database Syst Rev2011CD00230921563134
- CalverleyPMRabeKFGoehringUMKristiansenSFabbriLMMartinezFJM2-124 and M2-125 study groupsRoflumilast in symptomatic chronic obstructive pulmonary disease: two randomised clinical trialsLancet2009374969168569419716960
- FabbriLMCalverleyPMIzquierdo-AlonsoJLM2-127 and M2-128 study groupsRoflumilast in moderate-to-severe chronic obstructive pulmonary disease treated with longacting bronchodilators: two randomised clinical trialsLancet2009374969169570319716961
- MacNeeWOxidants/antioxidants and COPDChest20001175 Suppl 1303S317S10843965
- IshibashiYTakayamaGInouyeYTaniguchiACarbocisteine normalizes the viscous property of mucus through regulation of fucosylated and sialylated sugar chain on airway mucinsEur J Pharmacol20106412–322622820553908
- DecramerMRutten-van MölkenMDekhuijzenPNEffects of N-acetylcysteine on outcomes in chronic obstructive pulmonary disease (Bronchitis Randomized on NAC Cost-Utility Study, BRONCUS): a randomised placebo-controlled trialLancet200536594701552156015866309
- TseHNRaiteriLWongKYHigh-dose N-acetylcysteine in stable COPD: the 1-year, double-blind, randomized, placebo-controlled HIACE studyChest2013144110611823348146
- ZhengJPKangJHuangSGEffect of carbocisteine on acute exacerbation of chronic obstructive pulmonary disease (PEACE Study): a randomised placebo-controlled studyLancet200837196292013201818555912
- PoolePBlackPNCatesCJMucolytic agents for chronic bronchitis or chronic obstructive pulmonary diseaseCochrane Database Syst Rev20128CD00128722895919
- GotfriedMHMacrolides for the treatment of chronic sinusitis, asthma, and COPDChest2004125Suppl 252S60S quiz 60S–61S14872001
- SeemungalTAWilkinsonTMHurstJRPereraWRSapsfordRJWedzichaJALong-term erythromycin therapy is associated with decreased chronic obstructive pulmonary disease exacerbationsAm J Respir Crit Care Med2008178111139114718723437
- AlbertRKConnettJBaileyWCCOPD Clinical Research NetworkAzithromycin for prevention of exacerbations of COPDN Engl J Med2011365868969821864166
- BioMarck Pharmaceuticals [webpage on the Internet]BioMarck Pharmaceutical Reports Phase II Study Results for COPD Inhalation Solution: BREATH 1 study assessed effectiveness and safety of BIO-11006 inhalation solutionRaleigh, NCBioMarck Pharmaceuticals2011 Available from: http://www.biomarck.com/downloads/biomarck-pharmaceuticals-reports-phase-ii.swfAccessed October 21, 2013
- StockleyRAO’BrienCPyeAHillSLRelationship of sputum color to nature and outpatient management of acute exacerbations of COPDChest200011761638164510858396
