Abstract
Background
COPD patients with increased airway eosinophilic inflammation show a favorable response to inhaled corticosteroids (ICS) in combination with a long-acting bronchodilator. Recent studies have demonstrated a significant correlation of sputum eosinophilia with blood eosinophils and periostin. We investigated whether high blood eosinophils and plasma periostin were associated with an improvement in forced expiratory volume in 1 second (FEV1) after 3-month treatment with ICS/long-acting beta2-agonist (LABA) in stable COPD patients.
Patients and methods
Blood eosinophils and plasma periostin levels were measured in 130 stable COPD subjects selected from the Korean Obstructive Lung Disease cohort. Subjects began a 3-month ICS/LABA treatment after washout period.
Results
High blood eosinophils (>260/µL, adjusted odds ratio =3.52, P=0.009) and high plasma periostin (>23 ng/mL, adjusted odds ratio =3.52, P=0.013) were significantly associated with FEV1 responders (>12% and 200 mL increase in FEV1 from baseline after treatment). Moreover, the addition of high blood eosinophils to age, baseline positive bronchodilator response, and FEV1 <50% of the predicted value significantly increased the area under the curve for prediction of FEV1 responders (from 0.700 to 0.771; P=0.045).
Conclusion
High blood eosinophils and high plasma periostin were associated with improved lung function after 3-month ICS/LABA treatment. In particular, high blood eosinophils, in combination with age and baseline lung function parameters, might be a possible biomarker for identification of COPD patients with favorable FEV1 improvement in response to ICS/LABA treatment.
Keywords:
Introduction
COPD is a progressive inflammatory disorder that is characterized by persistent airflow limitation.Citation1 It affects approximately 10% of adults over 40 years of age and is the third leading cause of death worldwide.Citation2 Although most COPD guidelines advocate the use of inhaled corticosteroids (ICS) for only those with frequent exacerbations, in real clinical practice, they are widely used especially in combination with a long-acting beta2-agonist (LABA).Citation3,Citation4 Because only a small fraction of COPD patients are responsive to ICS-based therapy,Citation5,Citation6 identifying characteristics associated with ICS-responsiveness is crucial for clinicians to make therapeutic decisions. Nonetheless, little information is available on which clinical features might predict a favorable ICS response. Although neutrophils are thought to play a prominent role in the pathogenesis of COPD,Citation7 Christenson et alCitation8 recently reported that eosinophilia and T helper type 2 (Th2) inflammation might also play a significant role in a subset of patients with COPD and that approximately 20% of smokers with COPD have a Th2-high signature. This result is consistent with previous findings that airway eosinophilic inflammation is not uncommon, but is present in approximately 20%–40% of COPD patients.Citation9 Furthermore, several studies showed that an increased sputum eosinophil count is related to improvement in lung function following treatment with ICS in COPD.Citation10,Citation11 However, sputum induction is challenging in COPD, and the results are often difficult to interpret. A recent study suggested that blood eosinophils may be a good indicator for sputum eosinophils.Citation12 Another blood surrogate marker of eosinophilic airway inflammation may be serum periostin.Citation13 In this study, we determined whether increased levels of blood eosinophils and periostin are associated with a significant improvement in forced expiratory volume in 1 second (FEV1) after 3-month treatment with ICS/LABA in patients with stable COPD.
Patients and methods
Patients
All subjects were selected from the Korean Obstructive Lung Disease (KOLD) cohort, which prospectively recruited subjects with obstructive lung disease from the pulmonary clinics of 14 hospitals in Korea from June 2005 to December 2012. Details of the KOLD study have been published previously.Citation14 After enrollment in the KOLD cohort, some subjects underwent a 2-week washout period and then received treatment with a fixed-dose combination inhaler of ICS and LABA. However, subjects whose condition did not allow cessation of medications did not undergo a washout period and maintained their original treatment. This decision was made at the discretion of the treating physicians. Among the subjects with a washout period, a total of 189 fulfilled the four inclusion criteria: ≥40 years, post-bronchodilator FEV1/forced vital capacity (FVC) <0.7, more than 10 pack-years of smoking history, and no or minimal abnormality on chest radiography. Baseline clinical data were obtained after cessation of the following respiratory medications: an ICS for 2 weeks, an inhaled LABA, or long-acting muscarinic antagonist for 2 days, an inhaled short-acting β2-agonist or inhaled short-acting anticholinergic for 12 hours. For the following 3 months, COPD was treated with a fixed-dose combination inhaler of ICS and LABA (50 µg salmeterol/500 µg fluticasone or 9 µg formoterol/320 µg budesonide, twice daily). Spirometry data were evaluated in 182 subjects after 3-month treatment with combined inhalation ICS and LABA. Excluding 30 subjects with addition of tiotropium to ICS and LABA and 22 subjects for whom blood samples were not collected, 130 subjects remained eligible for analysis in this study (). The study protocol was approved by the Asan Medical Center Institutional Review Board (no 2005-0010). Written informed consent was obtained from all of the participating patients.
Blood eosinophils and plasma periostin measurements
Blood samples were collected after 2-week washout of ICS and before the 3-month treatment, separated into their various components, aliquoted, transferred to the KOLD Data Coordinating Center on dry ice, and kept in −80°C freezers until use in this study. Plasma samples were thawed for periostin and they were measured using a commercially available ELISA kit (Adipo Bioscience, Santa Clara, CA, USA) according to the manufacturer’s instructions. The lower limit of detection of these assays was 2 ng/mL, and values that were below this level of detection were recorded as 1 ng/mL. Peripheral blood eosinophil counts were routinely obtained from the standard complete blood counts after 2-week washout of ICS and before the 3-month treatment. The reference range of blood eosinophils is 1%–5%.
Pulmonary function test
Spirometry was performed according to the recommendations of the American Thoracic Society using a Vmax 22 (Sensor-Medics, Yorba Linda, CA, USA) and PFDX (MedGraphics, St Paul, MN, USA).Citation15 Absolute values of FVC and FEV1 were obtained, and the percentage of the predicted (pred) values (% pred) for FEV1 and FVC was calculated from equations obtained with a representative Korean sample.Citation16 A positive bronchodilator response (BDR) at baseline was defined as a post-bronchodilator increase in FEV1 of at least 12% and 200 mL from baseline values.Citation17
Spirometry was conducted at baseline and after 12 weeks of treatment. Subjects were classified as FEV1 responders or FEV1 non-responders based on FEV1 improvement of at least 12% and 200 mL from baseline after 3 months of combined treatment with ICS/LABA.
Statistical analysis
Data are presented as medians and interquartile ranges (IQRs) for continuous variables and as frequencies (percentages) for categorical variables. Data were compared using the Mann–Whitney U-test for continuous variables because of non-normality and Pearson’s χ2 test or Fisher’s exact test for categorical variables. To determine the optimal cut-off value of blood eosinophils and plasma periostin for the prediction of FEV1 responders, the Youden’s index, defined as (sensitivity + specificity) −1, was calculated at each cut-off. The cut-off point showing the highest Youden’s index was considered the optimal cut-off value.Citation18 The optimal cut-off values of blood eosinophils and plasma periostin were 260/µL (sensitivity 60% and specificity 69% with Youden’s index of 0.288) and 23 ng/mL (sensitivity 43% and specificity 76% with Youden’s index of 0.190), respectively. A logistic regression model was used to assess the relationship between biomarkers and treatment response. Statistical adjustments were made for age, body mass index (BMI), smoking status, emphysema index, positive BDR, and baseline FEV1 <50% pred as covariates. Sex was not included as a covariate, because 97.4% of the population were male. We also determined the discriminatory properties of high blood eosinophils and plasma periostin in predicting FEV1 responders by constructing a receiver operator characteristic curve and calculating the area under the curve (AUC) or C-statistic. To create the best model, we first conducted univariate analyses of the salient risk factors listed previously and identified the variables that were significant. Among them, the variable with the highest AUC was selected as the baseline covariate. To this baseline covariate, we successively added the variable with the second highest AUC and then the variable with the third-highest AUC score, and so forth until all the significant variables on the univariate analysis were exhausted. To the penultimate model, we added high blood eosinophils or plasma periostin to evaluate the incremental effects of high blood eosinophils or plasma periostin on AUC. All tests were two-sided, and a P-value <0.05 was considered to indicate statistical significance. Data were analyzed using IBM SPSS Statistics for Windows, version 22.0 (Armonk, NY, USA) and Stata software, version 12 (StataCorp LP, College Station, TX, USA).
Results
Subjects
Baseline characteristics of the 130 COPD subjects are summarized in . There were 126 (97%) males and 4 (3%) females, with a median age of 67 years (IQR, 61–70 years). The median BMI was 23.4 kg/m2 (IQR, 21.5–25.1 kg/m2). There were 53 (41%) current smokers and 77 (59%) ex-smokers, with a median smoking history of 45 pack-years. Also, 50 (39%) subjects had dyspnea with a modified Medical Research Council grade ≥2. The most common comorbidity was chronic heart disease (42%), followed by diabetes mellitus (7%). The median FVC (L), FVC (% pred), FEV1 (L), and FEV1 (% pred) were 3.3 L (IQR, 2.6–3.7 L), 74.1% pred (IQR, 64.4%–87.1%), 1.5 L (IQR, 1.1–1.8 L), and 49.5% pred (IQR, 38.8%–60.8%), respectively. The median distance achieved on the 6-minute walk test was 445 m (IQR, 403–486 m), and a positive BDR was demonstrated in 47 (36%) subjects. Seventeen subjects reported 27 exacerbations during 3 months. Laboratory findings were as follows: median blood white blood cell count of 7,240/µL (IQR, 6,000–8,450/µL), median blood neutrophils 3,921/µL (IQR, 3,094–4,811/µL), median blood eosinophils 200.0/µL (IQR, 120.6–345.6/µL), and median C-reactive protein level 1.5 mg/L (IQR, 0.6–3.7 mg/L). The median plasma periostin level was 9.0 ng/mL (IQR, 1.0–41.3 ng/mL). There were 53 (41%) subjects with high blood eosinophilia (>260/µL) and 39 (30%) subjects with high plasma periostin level (>23 ng/mL). Fifteen (12%) subjects had both high blood eosinophilia and high plasma periostin level.
Table 1 Baseline characteristics of 130 COPD patients
Comparison between FEV1 responders and FEV1 non-responders
After 3 months of treatment with ICS and LABA, 42 (32%) subjects were classified as FEV1 responders. As shown in , there were no significant differences in sex, smoking history, BMI, dyspnea scale, distance on the 6-minute walk test, exacerbation, and emphysema index between FEV1 responders and FEV1 non-responders.
Table 2 Clinical findings of 130 COPD patients with and without ICS/LABA response
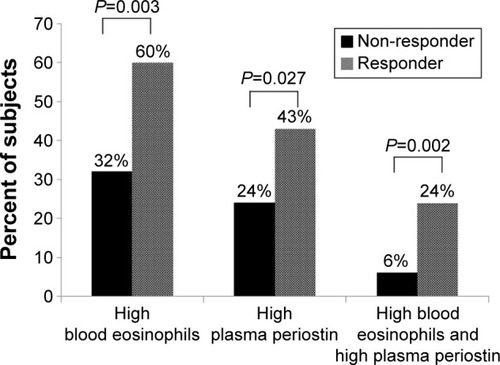
FEV1 responders were more likely to have baseline FEV1 <50% pred (P=0.045) and positive BDR at baseline (P=0.002), whereas FEV1 non-responders had a higher FEV1 (L) and (% pred) and FVC (L) and (% pred) than FEV1 responders (FEV1 (L), P=0.036; FEV1 [% pred], P=0.005; FVC (L), P=0.034; and FVC [% pred], P=0.004). The proportion of subjects with high blood eosinophils (>260/µL, 60% vs 32%; P=0.003), high plasma periostin (>23 ng/mL, 43% vs 24%; P=0.027), and with high blood eosinophils and high plasma periostin (24% vs 6%; P=0.002) were significantly higher in the FEV1 responders than the FEV1 non-responders ().
Blood eosinophils and plasma periostin levels in FEV1 responders
As shown in , blood eosinophils >260/µL, plasma periostin level >23 ng/mL, positive BDR at baseline, and baseline FEV1 <50% pred were positively associated with FEV1 responders. Adjustment of age, BMI, smoking status, exacerbation, emphysema index, positive BDR at baseline, and baseline FEV1 <50% pred showed that blood eosinophils >260/µL (adjusted odds ratio [aOR] =3.52; 95% confidence interval [CI] =1.37–8.99; P=0.009), plasma periostin level >23 ng/mL (aOR =3.52; 95% CI =1.31–9.49; P=0.013), and combined blood eosinophils >260/µL and plasma periostin level >23 ng/mL (aOR =6.40; 95% CI =1.60–25.55; P=0.009) were associated with FEV1 responders.
Table 3 Adjusted odds ratio of blood eosinophils >260/µL and plasma periostin >23 ng/mL for FEV1 responders
Among the clinical variables, the most predictive variable for FEV1 response after 3-month treatment with ICS and LABA was positive BDR at baseline, which had an AUC of 0.637 (95% CI =0.548–0.726). The next predictive variable was age (AUC =0.608; 95% CI =0.509–0.707), followed by baseline FEV1 <50% pred (AUC =0.594; 95% CI =0.504–0.684). The combination of positive BDR at baseline, age, and baseline FEV1 <50% pred yielded an AUC of 0.700 (95% CI =0.602–0.800). The addition of blood eosinophils >260/µL increased the AUC of this model significantly to 0.771 (P=0.045; ). However, addition of the variable of plasma periostin >23 ng/mL or combined high blood eosinophils >260/µL and high plasma periostin >23 ng/mL to the previous model did not yield a significant improvement in AUC (P=0.346 for plasma periostin >23 ng/mL and P=0.064 for high blood eosinophils >260/µL and high plasma periostin >23 ng/mL).
Table 4 Area under the curve values from receiver operator characteristics generated for variables with blood eosinophils >260/µL and plasma periostin >23 ng/mL for the prediction of FEV1 responders
Discussion
To the best of our knowledge, this is the first study that investigates the relationship of blood eosinophils and plasma periostin with lung function changes related to ICS and LABA combination treatment in stable COPD patients. We showed that high blood eosinophils (>260/µL) and high plasma periostin (>23 ng/mL) were significantly related to FEV1 improvement after 12 weeks of treatment with ICS and LABA in COPD patients and found that the addition of high blood eosinophils (>260/µL) to baseline positive BDR, age, and baseline FEV1 <50% pred significantly increased the prediction accuracy for treatment response.
Eosinophilic airway inflammation is increased during acute exacerbations of COPD, and peripheral blood eosinophil count has been reported as a promising biomarker to predict response to corticosteroid therapy during COPD exacerbations.Citation19 Stable COPD is classically thought to be driven by neutrophils and the T helper type 1 immune response;Citation20 however, COPD patients have elevated eosinophils in airway wall biopsies and increased activity of eosinophils in bronchoalveolar lavage fluid.Citation21,Citation22 Moreover, Christenson et al showed a significant overlap of airway epithelial gene expression alterations in both asthma and COPD subset, and demonstrated that asthma-derived gene expression signatures of Th2 inflammation are associated with increased eosinophil counts and a favorable ICS response in COPD patients.Citation8 Our data extend these findings by demonstrating that high blood eosinophils were associated with treatment response (defined as an increase in FEV1 of at least 12% and 200 mL from baseline) following ICS and LABA treatment. Furthermore, combining high blood eosinophils with age and lung function parameters at baseline (baseline positive BDR and baseline FEV1 <50 [% pred]) increased AUC significantly, providing additional discrimination beyond that achieved by clinical variables. Thus, high blood eosinophils, together with lung function parameters at baseline, may identify an ICS-responsive phenotype in COPD.
Periostin is a disulfide-linked 90-kDa heparin-binding N-glycosylated protein containing four tandem fasciclin domains.Citation23 Periostin is secreted from bronchial epithelial cells in response to interleukin (IL)-13, a central mediator of allergic asthma.Citation24,Citation25 Jia et al showed that the circulating levels of periostin are significantly higher in asthmatic patients with evidence of eosinophilic airway inflammation relative to those with minimal eosinophilic airway inflammation, suggesting that periostin may serve as a good systemic indicator of eosinophilic airway inflammation.Citation13 Because ICS treatment substantially suppresses Th2-driven eosinophilic inflammation and reduces IL-13 levels,Citation26 periostin has received attention as a potential biomarker of treatment response in asthmatic patients. Indeed, lung function improvement following anti-IL-13 therapy in severe asthma was greater in patients with high periostin than in those with lower levels.Citation27 Our study analyzed the plasma periostin as another biomarker related to the Th2-related signature and showed that high plasma periostin levels were independently associated with treatment response after 3 months of treatment with ICS and LABA. However, in contrast to high blood eosinophils, high plasma periostin levels alone or combined with high blood eosinophils did not have an additive role to increase prediction accuracy for treatment response, suggesting limited utility as a biomarker of ICS-responsive phenotype in COPD. Our data agree with those of a previous study, which showed that the periostin gene (POSTN), one of the IL-13 inducible genes, was repressed by smoking, thus may not be a good Th2 inflammatory marker in smokers with COPD.Citation8
In our study, a positive BDR at baseline and baseline FEV1 were related to FEV1 improvement after 3-month treatment with ICS and LABA in COPD patients, whereas the emphysema index was not significantly different between FEV1 non-responders and FEV1 responders. There have been conflicting data regarding the relationship between airway reversibility and emphysema severity. Kitaguchi et alCitation28 reported that COPD patients without emphysema on chest high-resolution computed tomography were more likely to have bronchodilator responsiveness and the reversibility response to treatment with ICS, compared with those with emphysema. However, other studies found no significant differences in bronchodilator responsiveness among groups classified according to emphysema severity or presence of emphysema.Citation29,Citation30 Our results are concordant with studies showing a lack of association between bronchodilator responsiveness and emphysema severity. However, a prospective study with treatment response according to emphysema severity is necessary to resolve conflicting data.
Our study had several limitations. First, as the KOLD prospective cohort was not originally designed to identify blood biomarkers for COPD, our study design and analysis had to be retrospective in nature. A prospective study with a larger sample size is needed to validate these findings. Second, we did not have serial measurements of blood eosinophil number or plasma periostin levels over 3 months. Third, airway inflammatory markers, such as eosinophils in sputum or bronchoalveolar lavage fluid, were not evaluated, thus mutual relationships between blood eosinophils and plasma periostin with eosinophil levels in the lung could not be investigated. Moreover, the cut-off values optimized for our study need to be replicated in other studies. Fourth, because ICS monotherapy is not recommended and a long-acting bronchodilator is a key treatment of COPD, a combination of ICS and LABA was administered to all subjects in this study. Thus, the FEV1 function improvement after inhaled treatment cannot be explained by only the ICS effect on eosinophilic airway inflammation. Nevertheless, some COPD patients showed remarkable improvement in lung function following treatment with ICS and LABA. The American Thoracic Society/European Respiratory Society Task Force suggested a change of 100–140 mL or 5%–10% in FEV1 from baseline as clinically important,Citation31 and a previous study reported an improvement in FEV1 of approximately 200 mL with 26-week treatment of combination of long-acting muscarinic antagonist and LABA.Citation32 Thus, we employed the Global Initiative for Chronic Obstructive Lung Disease criteria, ie, an increase in FEV1 of at least 12% and 200 mL from baseline after 3-month treatment, to define treatment response. Finally, further investigation is needed to determine whether high blood eosinophils are associated with long-term outcomes, such as lung function decline or mortality, in COPD.
In summary, the findings of this study showed that high blood eosinophils and high plasma periostin are significantly related to improved lung function with ICS and LABA treatment. However, only elevated blood eosinophils significantly improved prediction of treatment response beyond that achieved with age and traditional lung function parameters. These data raise the possibility of using blood eosinophils in concert with lung function parameters (baseline positive BDR and baseline FEV1 <50% pred) to identify COPD patients who will benefit from ICS and LABA treatment. Additional studies are required to validate these preliminary findings.
Author contributions
Substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data: HYP, HL, WK, SK, IJ, HK, TK, JWK, WJK, YO, DDS, SYL, and SL. Drafting the article or revising it critically for important intellectual content: HYP, HL, WK, SK, IJ, HK, TK, JWK, WJK, YO, DDS, SYL, and SL. Final approval of the version to be published: HYP, HL, WK, SK, IJ, HK, TK, JWK, WJK, YO, DDS, SYL, and SL. Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved: HYP, HL, WK, SK, IJ, HK, TK, JWK, WJK, YO, DDS, SYL, and SL.
Acknowledgments
We thank Su-Young Kim and the following members of the Korean Obstructive Lung Disease (KOLD) Study Group for the generous provision of data and samples: Tae Rim Shin, Sang Yeub Lee, Ho Il Yoon, Seung Soo Sheen, Jin Hwa Lee, Joo Hun Park, Yong Bum Park, Chin Kook Rhee, Changhwan Kim, Yong Il Hwang, Kwang Ha Yoo, Young Sam Kim, Ji Ye Jung, Yoon ki Hong, Seung Won Ra, Joon Beom Seo, Sang Min Lee, Chang Hoon Lee, Sei Won Lee, Jae Seung Lee, Ji-Hyun Lee, Eun Kyung Kim, Jin Won Huh, Ji Yong Moon, Hyoung Kyu Yoon, Hye Kyeong Park, Jin Woo Kim, Kang Hyeon Choi, Joo Ock Na, Doh Hyung Kim, Hye Sook Choi, Kwang Ha Lee, Myung Jae Park, and Sung Soon Lee. The KOLD Study was supported by a grant from the Korean Health 21 R&D Project, Ministry of Health and Welfare, Republic of Korea (HI10C2020 and A102065). This study was supported by Samsung Medical Center Foundation for Medical Research (SMO1140211).
Disclosure
The authors report no conflicts of interest in this work.
References
- Global Initiative for Chronic Obstructive Lung DiseaseGlobal Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease2015 [cited March 1, 2015]. Available from: www.goldcopd.org
- LozanoRNaghaviMForemanKGlobal and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010Lancet201238098592095212823245604
- PriceDWestDBrusselleGManagement of COPD in the UK primary-care setting: an analysis of real-life prescribing patternsInt J Chron Obstruct Pulmon Dis2014988990425210450
- VestboJVogelmeierCSmallMHigginsVUnderstanding the GOLD 2011 strategy as applied to a real-world COPD populationRespir Med2014108572973624675239
- LapperreTSSnoeck-StrobandJBGosmanMMEffect of fluticasone with and without salmeterol on pulmonary outcomes in chronic obstructive pulmonary disease: a randomized trialAnn Intern Med2009151851752719841453
- MiravitllesMSoler-CatalunaJJCalleMSorianoJBTreatment of COPD by clinical phenotypes: putting old evidence into clinical practiceEur Respir J20134161252125623060631
- BarnesPJMediators of chronic obstructive pulmonary diseasePharmacol Rev200456451554815602009
- ChristensonSASteilingKvan den BergeMAsthma-COPD overlapClinical relevance of genomic signatures of type 2 inflammation in chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2015191775876625611785
- SahaSBrightlingCEEosinophilic airway inflammation in COPDInt J Chron Obstruct Pulmon Dis200611394718046901
- PapiARomagnoliMBaraldoSPartial reversibility of airflow limitation and increased exhaled NO and sputum eosinophilia in chronic obstructive pulmonary diseaseAm J Respir Crit Care Med200016251773177711069811
- BrightlingCEMcKennaSHargadonBSputum eosinophilia and the short term response to inhaled mometasone in chronic obstructive pulmonary diseaseThorax200560319319815741434
- WagenerAHde NijsSBLutterRExternal validation of blood eosinophils, FE(NO) and serum periostin as surrogates for sputum eosinophils in asthmaThorax201570211512025422384
- JiaGEricksonRWChoyDFPeriostin is a systemic biomarker of eosinophilic airway inflammation in asthmatic patientsJ Allergy Clin Immunol20121303647654.e1022857879
- ParkTSLeeJSSeoJBStudy design and outcomes of Korean obstructive lung disease (KOLD) cohort StudyTuberc Respir Dis (Seoul)201476416917424851130
- Standardization of spirometry, 1994 update. American Thoracic SocietyAm J Respir Crit Care Med19951523110711367663792
- ChoiJKPaekDLeeJONormal predictive values of spirometry in Korean populationTuberc Respir Dis (Seoul)2005583230242
- PellegrinoRViegiGBrusascoVInterpretative strategies for lung function testsEur Respir J200526594896816264058
- BewickVCheekLBallJStatistics review 13: receiver operating characteristic curvesCrit Care20048650851215566624
- BafadhelMMcKennaSTerrySAcute exacerbations of chronic obstructive pulmonary disease: identification of biologic clusters and their biomarkersAm J Respir Crit Care Med2011184666267121680942
- BarnesPJThe cytokine network in asthma and chronic obstructive pulmonary diseaseJ Clin Invest2008118113546355618982161
- PanznerPLafitteJJTsicopoulosAHamidQTulicMKMarked up-regulation of T lymphocytes and expression of interleukin-9 in bronchial biopsies from patients With chronic bronchitis with obstructionChest200312451909191514605067
- RiiseGCAhlstedtSLarssonSBronchial inflammation in chronic bronchitis assessed by measurement of cell products in bronchial lavage fluidThorax19955043603657785007
- TakayamaGArimaKKanajiTPeriostin: a novel component of subepithelial fibrosis of bronchial asthma downstream of IL-4 and IL-13 signalsJ Allergy Clin Immunol200611819810416815144
- Wills-KarpMLuyimbaziJXuXInterleukin-13: central mediator of allergic asthmaScience19982825397225822619856949
- GrunigGWarnockMWakilAERequirement for IL-13 independently of IL-4 in experimental asthmaScience19982825397226122639856950
- WoodruffPGBousheyHADolganovGMGenome-wide profiling identifies epithelial cell genes associated with asthma and with treatment response to corticosteroidsProc Natl Acad Sci U S A200710440158581586317898169
- CorrenJLemanskeRFHananiaNALebrikizumab treatment in adults with asthmaN Engl J Med2011365121088109821812663
- KitaguchiYFujimotoKKuboKHondaTCharacteristics of COPD phenotypes classified according to the findings of HRCTRespir Med2006100101742175216549342
- BoschettoPMiniatiMMiottoDPredominant emphysema phenotype in chronic obstructive pulmonaryEur Respir J200321345045412662000
- MakitaHNasuharaYNagaiKCharacterisation of phenotypes based on severity of emphysema in chronic obstructive pulmonary diseaseThorax2007621193293717573447
- CazzolaMMacNeeWMartinezFJOutcomes for COPD pharmacological trials: from lung function to biomarkersEur Respir J200831241646918238951
- BatemanEDFergusonGTBarnesNDual bronchodilation with QVA149 versus single bronchodilator therapy: the SHINE studyEur Respir J20134261484149423722616