Abstract
Background
The aim of this study was to evaluate whether Global Initiative for Chronic Obstructive Lung Disease (GOLD) classification could predict mortality risk factors and whether baseline treatment intensity would relate to mortality within each group, using data from TIOSPIR®, the largest randomized clinical trial in COPD performed to date.
Methods
A total of 17,135 patients from TIOSPIR® were pooled and grouped by GOLD grading (A–D) according to baseline Medical Research Council breathlessness score, exacerbation history, and spirometry. All-cause mortality and adjudicated cardiovascular (CV) and respiratory mortality were assessed.
Results
Of the 16,326 patients classified, 1,248 died on treatment. Group B patients received proportionally more CV treatment at baseline. CV mortality risk, but not all-cause mortality risk, was significantly higher in Group B than Group C patients (CV mortality – hazard ratio [HR] =1.74, P=0.004; all-cause mortality – HR =1.18, P=0.11). Group D patients had a higher incidence of all-cause mortality than Group B patients (10.9% vs 6.6%). Similar trends were observed regardless of respiratory or CV medication at baseline. In contrast, respiratory deaths increased consistently from Groups A–D (0.3%, 0.8%, 1.6%, and 4.2% of patients, respectively).
Conclusion
The data obtained from the TIOSPIR® trial, supporting earlier studies, suggest that proportionally more CV medication and CV deaths occur in GOLD Group B COPD patients, although deaths attributed to respiratory causes are more prevalent in Groups C and D.
Introduction
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) provides guidelines for current COPD management and is used by practicing clinicians worldwide.Citation1 In 2011, the GOLD grading system changed from the previous I–IV grading to the current A–D system (last updated in early 2015). This system distinguishes between four categories of patients with COPD based on the assessment of symptoms, severity of airflow limitation using spirometry, and exacerbation risk: A = low risk, fewer symptoms; B = low risk, more symptoms; C = high risk, fewer symptoms; and D = high risk, more symptoms; where risk is defined by forced expiratory volume in 1 second (FEV1) levels and/or exacerbation history and severity.Citation2 One of the goals of this clinical classification was to identify different populations of COPD patients with differing risks of future events and hence, stratify therapy appropriately.
Subsequent data from observational cohorts and population-based studies have suggested that despite having better spirometry, Group B patients have a higher risk of death and have more cardiovascular (CV) comorbidity than Group C patients.Citation3,Citation4 The authors suggested that the 2011 GOLD A–D classification provided a more enhanced prediction of exacerbations than the GOLD 2007 I–IV classification, and highlighted the role of dyspnea as a strong predictor of poor survival in patients with COPD. However, in these studies, the number of patients attributed to Group C was relatively modest compared to the overall population, and the cause of death was not reported or adjudicated – a problem in patients with COPD whose diagnosis is often omitted from routine death certification.Citation5 In COPD, the intensity of treatment is often considered as a marker of disease severity, but the relationship, if any, of treatment use to outcome within individual GOLD groups has so far not been explored.
In this report, we have used data from the TIOSPIR® study,Citation6,Citation7 the largest randomized clinical trial in COPD performed to date, to investigate the ability of different GOLD groups to predict important clinical outcomes in COPD. Specifically, we anticipated that there would be differences in the risk of death, the causes of death, and hospitalizations between the groups, and that baseline treatment intensity would relate to mortality within each group. As there were no differences in mortality or exacerbation rate between treatment arms in the trial, we have pooled the data in this analysis.
Methods
The TIOSPIR® methods have been described previously, and the complete study protocol is available online (http://www.nejm.org/action/showSupplements?doi=10.1056%2FNEJMoa1303342&viewType=Popup&viewClass=Suppl).Citation6,Citation7 This study’s protocol and procedures were approved by the Institutional Review Board (IRB), Independent Ethics Committee (IEC), and competent authority (CA) according to national and international regulations. The trial was conducted in compliance with the protocol, the principles laid down in the Declaration of Helsinki, in accordance with the ICH Harmonised Tripartite Guideline for Good Clinical Practice (GCP) and in accordance with applicable regulatory requirements. All patients provided written informed consent prior to participation.
Study population
TIOSPIR® was an event-driven, randomized, double-blind, parallel-group trial of 17,135 patients with COPD. We recruited patients aged ≥40 years with a clinical diagnosis of COPD, ≥10 pack years of smoking history, a post-bronchodilator FEV1/forced vital capacity (FVC) ratio ≤0.70, and an FEV1 ≤70% predicted. Patients with concomitant cardiac disease were included except for those with unstable (requiring new treatment within last 12 months) or recent events (within last 6 months). Patients with other clinically significant lung diseases or a COPD exacerbation within the past month, moderate or severe renal impairment, or those with cancer requiring therapy within the past 5 years were excluded. All COPD medications, except other inhaled anticholinergics, were allowed. Patients were randomized to once-daily 2.5 μg of tiotropium Respimat® (Boehringer Ingelheim Pharma GmbH & Co. KG, Ingelheim am Rhein, Germany), 5 μg of tiotropium Respimat®, or 18 μg of tiotropium HandiHaler®. (Boehringer Ingelheim Pharma GmbH & Co. KG)
Study design
TIOSPIR® participants were free of exacerbation for ≥4 weeks before randomization and were seen every 12 weeks thereafter. At randomization, they recorded their degree of breathlessness (modified Medical Research Council [mMRC] score) and whether they had received antibiotics or systemic steroids in the preceding year. All regular medication use, including respiratory and CV therapy, was noted. Statin use was not assigned as a CV therapy. No record of prior COPD hospitalization was obtained. Pre- or post-bronchodilator spirometry was performed at this visit or a value recorded in the preceding 6 months was obtained from the case records. The trial was closed when at least 1,266 deaths were known to have occurred. Vital status was known in 99.7% of cases and the cause of death was adjudicated by an independent clinical endpoint committee (see details in Appendix 3 of Wise et alCitation6).
Patients across the treatment arms were pooled and divided into groups according to the GOLD 2011 guideline classification of A–D,Citation2 according to baseline mMRC dyspnea score (while on baseline therapy), exacerbations in year prior to the trial (based on antibiotic/systemic steroid use), and post-bronchodilator FEV1.Citation1 Patients were included in the analysis if all these data were available.
Statistical analysis
For patients classified into the GOLD Groups A–D, we examined patient baseline characteristics and all-cause mortality, including respiratory and CV deaths, major adverse CV events (MACE; defined as stroke, myocardial infarction [MI], sudden death, cardiac death, sudden cardiac death, or fatal event in system organ classes for cardiac and vascular disorders), fatal MACE, MI events (including fatal events), and hospitalized exacerbations occurring during the trial. Data by GOLD Stages I/II–IV were obtained for comparison.
Data are presented as mean (standard deviation [SD]) unless otherwise stated. Hazard ratios (HRs) and P-values were calculated based on Cox regression by GOLD 2011 groups (or GOLD Stage). No other covariates were included in the model. Only patients who had both GOLD 2011 group and GOLD Stage assigned were included, and patients with post-bronchodilator FEV1/FVC ≥70% were excluded. As this was an exploratory post hoc analysis, no nominal levels of significance were assessed between groups.
Results
Baseline characteristics
Data were available for 16,326 patients. They were classified into one of the 2011 GOLD groups: 3,594 (22.0%), 2,879 (17.6%), 3,534 (21.6%), and 6,319 (38.7%) patients in Groups A–D, respectively. The mean duration of follow-up in this population was similar between the groups (). Table S1 shows the shift of patients from GOLD Stages I–IV to GOLD Groups A–D, based on the GOLD 2011 classification.
Table 1 Patient baseline characteristics by GOLD group
Patient baseline demographics by GOLD group (A–D) are shown in . Mean age and body mass index were similar across GOLD groups, although numerically higher in Group B patients. Overall, 71.5% of patients were male, with Group B having a lower ratio of male to female patients (64.8% male) compared with Group C (74.0% male). Race distribution was similar across GOLD Groups A, B, and D; Group C had a higher ratio of Asian to white patients. COPD duration increased from 6.3 to 8.3 years for Groups A–D, while smoking history was longest in Group B and shortest in Group C. Previous history of CV disease was highest in Groups B and D. The percentage of male patients increased from GOLD Stage I/II (67.6%) to IV (81.7%), as did the proportion of Asian patients (10.5% in Stage I/II; 24.0% in Stage IV), while CV history was similar across stages (Table S2).
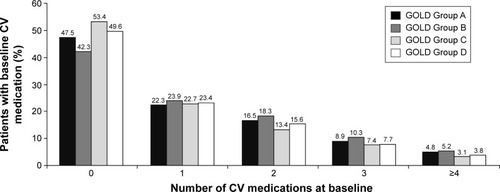
The percentage of patients using pulmonary medication combinations at baseline increased from GOLD Groups A–D (). Proportionally more patients from Groups C and D used double therapy with long-acting β2-agonists (LABAs) and inhaled corticosteroids (ICSs) or triple therapy (long-acting muscarinic antagonists [LAMAs], LABA, and ICS) at baseline than those from Groups A and B. Furthermore, a higher proportion of Group A patients used LAMAs only (without LABA and ICS) compared to the other GOLD groups ().
Table 2 Type of baseline pulmonary and CV medication use categorized by GOLD group
A wide range of CV medication was taken at baseline, with over 50% of each group using one or more CV therapy, and did not increase proportionally from GOLD Groups A–D (). Compared to any other GOLD group, Group B had proportionally more patients using one, two, three, four, or more CV medications at baseline while Group C patients used the least (). These medications included more calcium channel blockers, angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, acetylsalicylic acid, and nitrates than in any other group (). GOLD Group A, however, used the most β-blockers at baseline. Using the old GOLD Stage I–IV classification, it was found that GOLD Stage I/II patients received more CV medication at baseline than Stage III or IV patients (Table S3).
Cardiovascular and respiratory events
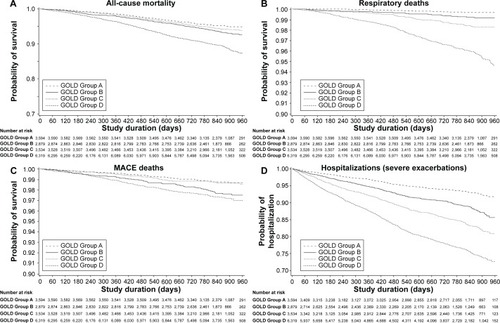
Compared to Group A, there was no increased risk of MACE during the trial for patients in Group B or C, but the risk was significantly higher in Group D (HR =1.23, P=0.10 for Group B; HR =0.91, P=0.45 for Group C; HR =1.30, P=0.02 for Group D) (). Compared to Group C, Groups B and D both experienced significantly more MACE (HR =1.36, P=0.02 for Group B; HR =1.43, P=0.002 for Group D). MI occurrence in Groups B–D was proportionally similar to that of patients in Group A, although Group D patients had a significantly higher risk of MI than Group C patients (HR =1.56, P=0.04). These data are supported by Kaplan–Meier curves showing that patients in GOLD Groups A and C had a lower risk of MACE than those in Groups B and D (). Patients from the old GOLD Stage III and IV did not have increased risks of MACE or MI compared to GOLD Stage I/II patients (Table S4, Figure S1). The number of patients with hospitalized exacerbations during the trial increased proportionally from GOLD Groups A–D and from GOLD Stages I/II–IV ( and Table S4; and Figure S1).
Table 3 CV outcomes and patient mortality by GOLD group
Figure 2 Kaplan–Meier curves of patient events by GOLD group (Groups A–D).
Abbreviations: GOLD, Global Initiative for Chronic Obstructive Lung Disease; MACE, major adverse cardiovascular event.
Mortality by GOLD classification
All-cause mortality
All-cause mortality did not increase consistently from GOLD Groups A–D (). There were proportionally more deaths from any cause in Group B (6.6%) than in Groups A (4.8%) and C (5.6%) (HR =1.39, P=0.002 vs Group A; HR =1.18, P=0.11 vs Group C), although Group D had the highest death rate in total (10.9%) (, ). In the old GOLD stage classification, patients in Stage I/II had a lower risk of all-cause death than patients in Stages III and IV (Table S4, Figure S1).
Respiratory deaths
The proportion of respiratory deaths increased consistently from Groups A–D, with more respiratory deaths in Groups D (4.2%) and C (1.6%) than in Groups B (0.8%) and A (0.3%) (, ). Group B patients had nearly 50% lower risk of death from respiratory causes than Group C patients (HR =0.49, P=0.005), and a 2.5-fold higher risk than Group A patients (HR =2.52, P=0.01).
CV deaths
Similarly to the results observed for all-cause mortality, the risk of death from MACE in Group B was 83% higher than in Group A (HR =1.83, P=0.002) and 74% higher than in Group C (HR =1.74, P=0.004) (, ). Patients in Group D also had a higher risk of death from MACE than patients in Groups A and C (, ). The proportion of patients who died from MI was very small (0.2% in Groups B and D, 0.1% in Group A, and 0 patients in Group C) (). Using the old GOLD stage classification, it was found that the risk of death from MACE was significantly higher in GOLD Stage IV patients than in GOLD Stage I/II patients (Table S4, Figure S1).
Mortality by GOLD classification and by pulmonary and cardiac baseline therapy
The proportionally higher all-cause mortality observed for Group B patients persisted regardless of the baseline pulmonary or CV medication received (). In Group B, 6.5% of patients with LAMA only and 7.6% with triple therapy at baseline died compared to 5.6% (LAMA only) and 4.4% (triple therapy) for Group A. Similarly, CV therapy at baseline did not affect the proportionally higher mortality in Group B patients (7.8% [with any CV therapy at baseline] and 5.0% [without CV therapy at baseline] of patients in Group B died, compared to 5.7% [with any CV therapy at baseline] and 3.8% [without CV therapy at baseline] in Group A). These differences reached statistical significance in patients with triple therapy and those with CV therapy at baseline ().
Table 4 All-cause mortality by GOLD group and by pulmonary and cardiac therapy at baseline
In the old GOLD stage classification, the risk of all-cause death increased from Stage I/II–IV, irrespective of baseline pulmonary or CV therapy (Table S5).
Discussion
The data obtained from the TIOSPIR® trial support results from earlier studies indicating that there are differences in CV medication use and proportionately more all-cause deaths, driven by CV deaths, in COPD patients from GOLD Group B than Group C.Citation3 This trend persisted regardless of the kind of therapy (pulmonary or CV) received at baseline. Of the 16,326 patients from TIOSPIR® classified according to the 2011 GOLD A–D groups, 1,248 died during the study. As expected, due to disease severity levels (high risk = more symptoms), Group D had the highest mortality, followed by Group B, with the highest proportion of CV death in Group D. Patients classified under Group B were using more CV treatments at baseline than any other group, and their risk of CV death was significantly higher than in Groups C or A. Group A patients, however, received proportionally more β-blockers than other groups (in particular Groups C and D), possibly due to more widespread prescription owing to their mild obstruction and low dyspnea. A significant number of Group A patients received baseline medication, and 25% were treated with triple therapy at baseline.
There were more respiratory deaths in Groups C and D than B, even though patients in Group B were still significantly more likely to die from a respiratory cause than patients in Group A. In TIOSPIR®, the imbalance of CV adverse event outcomes seen in patients from GOLD Group B was therefore not reflected in respiratory mortality; however, when patients were categorized by the old GOLD stages, the risk of death from respiratory causes significantly increased with increasing stage level.
Data from TIOSPIR® have supported the hypothesis that GOLD Group B, which includes patients with more symptoms but better lung function, reflects a group of patients with underlying CV comorbidities, as was seen in a Danish study.Citation3 Lange et al reported that in their patient cohort, patients classified as GOLD Group B had a poorer survival than those in Group C, in particular due to CV causes (2.9% vs 0.5%; 3-year mortality rate; P<0.001).Citation3 Groups B and D, the more symptomatic groups, had more CV deaths than Groups A and C, the less symptomatic groups. Our results have highlighted the importance of symptoms, including dyspnea, as an indicator for increased CV risk, suggesting that these patients should be monitored closely for CV disease and accordingly receive appropriate management. Defining patients by the 2014 GOLD classification adds a new dimension to the risks and causes of mortality, and our data suggest that the mMRC dyspnea scale may be a good indicator of CV risk in patients with a contrast in intensity of dyspnea and severity of airway obstruction based on FEV1.
Our data have also confirmed the previous reports which indicate that using dyspnea as a measure of disease severity in the staging of COPD is a better predictor of 5-year survival than airway obstruction (FEV1).Citation8,Citation9 Therefore, particular attention should be paid to patients classified as GOLD B according to the 2011 guidelines, who have a high mMRC dyspnea score, as these patients represent a relatively high risk of underlying CV comorbidity. CV diseases are the most frequent, as well as the most important, COPD comorbidity,Citation1 but often remain undiagnosed.Citation10 CV comorbidities, such as atrial fibrillation, heart failure, and MI, result in breathlessness, making diagnosis and treatment particularly challenging for the physician.Citation1 Furthermore, heart failure (HR =1.9; 95% confidence interval [CI] =1.3–2.9), ischemic heart disease (HR =1.5; 95% CI =1.1–2.0), and heart disease (HR =1.5; 95% CI =1.2–2.0) all have also been shown to increase the odds of mortality when coexistent with COPD,Citation11 which may explain why Group B patients exhibited a higher risk of CV mortality in TIOSPIR®.
Data from TIOSPIR® have shown that all-cause mortality was higher in Group B patients than the patients from Groups A and C, regardless of whether the patients were receiving LAMA only versus triple therapy at baseline and whether or not they were treated with CV medication at baseline. On the other hand, a pooled analysis of data from Spanish patient cohortsCitation12 showed that in their patient population, there was a better long-term survival of GOLD Group B patients compared to Group C patients and that this followed the trend seen with the old GOLD Stages I–IV. Both the Spanish and Danish studies did observe, however, that the 2011 GOLD grading classification produced an uneven split of patients with COPD, with more patients in the extreme (low risk, fewer symptoms, or high risk, more symptoms) Groups A and D than the mixed Groups B and C. This uneven split was also observed in TIOSPIR®, with more patients in Groups D and A than B and C. In the UPLIFT® trial, mortality also increased from Groups A–D, but there was no difference in the overall mortality of Groups B and C.Citation13
The TIOSPIR® trial has several strengths. It was a large trial with >34,000 patient-years’ exposure to tiotropium, powered to precisely estimate the rates of death and exacerbations: Tiotropium Respimat® was not inferior to tiotropium HandiHaler® in terms of all-cause mortality, and the risk of CV mortality or MACE did not significantly differ between the two devices.Citation6 The high vital status follow-up rates (99.7%) allow little leeway for bias owing to differential follow-up. In addition, patients in TIOSPIR® were permitted to use all respiratory medications except other inhaled anticholinergics. Therefore, the trial results reflect those likely to be achieved in routine clinical practice. Although this is a prospective study, the statistical power is high due to the large study population, covering all GOLD groups and – with more than 3,500 patients in GOLD Group C and over 6,000 patients in Group D – including patients worldwide.
According to the current GOLD guidelines, the severity assessment of COPD disease involves calculation of the risk of exacerbation with one of the three methods: 1) airflow limitation GOLD grade (GOLD 1/2 [FEV1 ≥50% predicted]: low risk; GOLD 3/4 [FEV1 <50% predicted]: high risk); 2) number of exacerbations within the previous year (0 or 1: low risk; ≥2: high risk); and 3) one or more hospitalizations (≥1 severe exacerbation) within the previous year.Citation1 The guidelines stipulate that the method which provides the highest risk should be used. A limitation of this study was that severe exacerbation history (resulting in hospitalizations) was not collected at baseline, and patients were classified according to their history of exacerbations requiring treatment with antibiotics and/or systemic steroids, in line with the GOLD 2011 categorization system.Citation2 However, exacerbation history remains the single best predictor of future exacerbations, thus validating the use of actual exacerbation rate in assessing exacerbation risk.Citation14
Pretrial mMRC score was also used, which – though it can be modified by therapy – reflects decisions made upon selecting treatments. The COPD assessment test (CAT) score was not collected in the TIOSPIR® trial, and it was previously shown that the CAT and mMRC symptom cut-points cannot be used interchangeably,Citation15 making it difficult for clinicians and researchers alike to evaluate the GOLD system. We believe that the mMRC cut-point is more readily available to clinicians, allowing for a wider applicability of the data presented in this analysis.
Another limitation of the TIOSPIR® trial is that patients with unstable CV conditions or moderate or severe renal impairment were excluded, so the results cannot be extended to such patients. However, ~10% of patients in TIOSPIR® had prior cardiac arrhythmias and ~20% had prior MI, ischemic heart disease, or coronary artery disease.Citation6
Conclusion
The TIOSPIR® data support the observation that GOLD Group B patients, with more symptoms and better lung function, have more underlying CV comorbidities as well as an increased CV mortality compared to Groups A and C, and suggest that this is the case regardless of CV and pulmonary therapy at baseline. Although respiratory deaths increased according to GOLD group (A–D), particular attention should be given to patients with high mMRC dyspnea scores who may be at increased risk of CV events and mortality.
Acknowledgments
This study was funded by Boehringer Ingelheim. The authors wish to thank Achim Mueller, of Boehringer Ingelheim, for statistical support. Writing assistance was provided by Sarah J Petit of PAREXEL, funded by Boehringer Ingelheim.
Supplementary materials
Figure S1 Kaplan–Meier curves of patient events by GOLD Stage (I/II–IV).
Notes: (A) All-cause mortality, (B) respiratory deaths, (C) MACE deaths, and (D) hospitalizations (severe exacerbations).
Abbreviations: GOLD, Global Initiative for Chronic Obstructive Lung Disease; MACE, major adverse cardiovascular event.
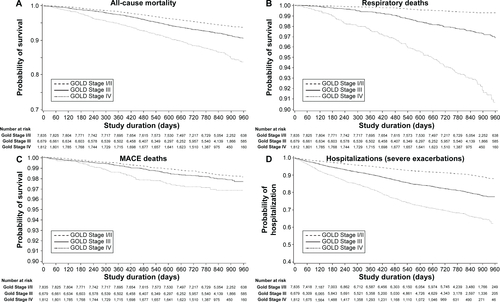
Table S1 Number of patients with GOLD Stage I/II–IV at baseline by GOLD Group A–D at baseline
Table S2 Patient baseline characteristics by GOLD stage
Table S3 Type of baseline pulmonary and CV medication use categorized by GOLD stage
Table S4 CV outcomes and patient mortality by GOLD stage
Table S5 All-cause mortality by GOLD stage and by pulmonary and cardiac therapy at baseline
Disclosure
Daniel Dusser reports receiving consulting fees, lecture fees, and payment for the development of educational activities from Boehringer Ingelheim, Pfizer, Novartis, Chiesi, Nycomed, and Dey Pharma. Robert Wise reports receiving consulting fees from Boehringer Ingelheim, Bristol-Myers Squibb, GlaxoSmithKline, Janssen, Mylan, Novartis, Pfizer, Sunovion, Pulmonx, Spiration, Roche, Grifols, and AstraZeneca, and grant support from Boehringer Ingelheim, GlaxoSmithKline, Pearl Therapeutics, and Forest Laboratories. Ronald Dahl reports receiving consulting fees, lecture fees, and grant support from Boehringer Ingelheim and Novartis. Antonio Anzueto reports receiving consulting fees, lecture fees, and travel support from AstraZeneca, Boehringer Ingelheim, Forest Laboratories, GlaxoSmithKline, and Novartis, and grant support from GlaxoSmithKline. Kerstine Carter and Andy Fowler report being employees of Boehringer Ingelheim. Peter Calverley reports receiving consulting fees, lecture fees, and travel support from Novartis, GlaxoSmithKline, Boehringer Ingelheim, and Takeda. The authors report no other conflicts of interest in this work.
References
- Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD)2014 Available from: http://www.goldcopd.org/Accessed March 17, 2015
- The Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD)2011 Available from: http://www.goldcopd.orgAccessed March 17, 2015
- LangePMarottJLVestboJPrediction of the clinical course of chronic obstructive pulmonary disease, using the new GOLD classification: a study of the general populationAm J Respir Crit Care Med20121861097598122997207
- AgustiAEdwardsLDCelliBCharacteristics, stability and outcomes of the 2011 GOLD COPD groups in the ECLIPSE cohortEur Respir J201342363664623766334
- JensenHHGodtfredsenNSLangePVestboJPotential misclassification of causes of death from COPDEur Respir J200628478178516807258
- WiseRAAnzuetoACottonDTiotropium Respimat inhaler and the risk of death in COPDN Engl J Med2013369161491150123992515
- WiseRAAnzuetoACalverleyPThe Tiotropium Safety and Performance in Respimat Trial (TIOSPIR), a large scale, randomized, controlled, parallel-group trial-design and rationaleRespir Res2013144023547660
- NishimuraKIzumiTTsukinoMOgaTDyspnea is a better predictor of 5-year survival than airway obstruction in patients with COPDChest200212151434144012006425
- CelliBRCoteCGMarinJMThe body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary diseaseN Engl J Med2004350101005101214999112
- BrekkePHOmlandTSmithPSoysethVUnderdiagnosis of myocardial infarction in COPD – Cardiac Infarction Injury Score (CIIS) in patients hospitalised for COPD exacerbationRespir Med200810291243124718595681
- MillerJEdwardsLDAgustiAComorbidity, systemic inflammation and outcomes in the ECLIPSE cohortRespir Med201310791376138423791463
- SorianoJBAlfagemeIAlmagroPDistribution and prognostic validity of the new Global Initiative for Chronic Obstructive Lung Disease grading classificationChest2013143369470223187891
- GoossensLMLeimerIMetzdorfNBeckerKRutten-van MolkenMPDoes the 2013 GOLD classification improve the ability to predict lung function decline, exacerbations and mortality: a post-hoc analysis of the 4-year UPLIFT trialBMC Pulm Med20141416325326750
- HurstJRVestboJAnzuetoASusceptibility to exacerbation in chronic obstructive pulmonary diseaseN Engl J Med2010363121128113820843247
- JonesPWAdamekLNadeauGBanikNComparisons of health status scores with MRC grades in COPD: implications for the GOLD 2011 classificationEur Respir J201342364765423258783