Abstract
Background
Neurosteroid dehydroepiandrosterone sulfate (DHEAS) has been associated with important brain functions, including neuronal survival, memory, and behavior, showing therapeutic potential in various neuropsychiatric and cognitive disorders. However, the antagonistic effects of DHEAS on γ-amino-butyric acidA receptors and its facilitatory action on glutamatergic neurotransmission might lead to enhanced brain excitability and seizures and thus limit DHEAS therapeutic applications. The aim of this study was to investigate possible age and sex differences in the neuronal excitability of the mice following acute and chronic DHEAS administration.
Methods
DHEAS was administered intraperitoneally in male and female adult and old mice either acutely or repeatedly once daily for 4 weeks in a 10 mg/kg dose. To investigate the potential proconvulsant properties of DHEAS, we studied the effects of acute and chronic DHEAS treatment on picrotoxin-, pentylentetrazole-, and N-methyl-D-aspartate-induced seizures in mice. The effects of acute and chronic DHEAS administration on the locomotor activity, motor coordination, and body weight of the mice were also studied. We also investigated the effects of DHEAS treatment on [3H]flunitrazepam binding to the mouse brain membranes.
Results
DHEAS did not modify the locomotor activity, motor coordination, body weight, and brain [3H]flunitrazepam binding of male and female mice. The results failed to demonstrate significant effects of single- and long-term DHEAS treatment on the convulsive susceptibility in both adult and aged mice of both sexes. However, small but significant changes regarding sex differences in the susceptibility to seizures were observed following DHEAS administration to mice.
Conclusion
Although our findings suggest that DHEAS treatment might be safe for various potential therapeutic applications in adult as well as in old age, they also support subtle interaction of DHEAS with male and female hormonal status, which may underline observed sex differences in the relationship between DHEAS and various health outcomes.
Introduction
Dehydroepiandrosterone sulfate (DHEAS) is a neurosteroid associated with various important functions in the mammalian brain.Citation1–Citation4 Namely, DHEAS has been reported to modulate neuronal plasticity,Citation5–Citation9 cognition, and emotions,Citation10–Citation15 thus showing therapeutic potential in a variety of neuropsychiatric and cognitive disorders.Citation2,Citation16–Citation20 It appears that the effects of DHEAS in the central nervous system are mediated through its action on multiple signaling pathways and neurotransmitter systems, which are also involved in regulating the balance between excitation and inhibition in the brain.Citation3,Citation15,Citation21–Citation23 Perhaps, the most documented has been the ability of DHEAS to bind and allosterically modulate the γ-amino-butyric acidA (GABAA) receptor complex.Citation3,Citation15,Citation21,Citation24 In addition to its antagonistic effects on GABAA receptors,Citation25–Citation30 DHEAS has been shown to act as a positive modulator of N-methyl-D-aspartate (NMDA) receptors and facilitate NMDA-mediated glutamatergic neurotransmission through central sigma receptors.Citation31,Citation32
Some of these actions of DHEAS might lead to enhanced brain excitability and possible seizuresCitation33 and thus limit its potential therapeutic applications. Increase in the motor activity and body weight reduction may also appear consistently with potential proconvulsive, anxiogenic, and provoking effects of DHEAS.Citation34,Citation35 However, it is not yet fully elucidated to what extent DHEAS complex pharmacological profile contributes to its central actions, so the actual in vivo effects of DHEAS remain uncertain. Namely, in contrast to the reports suggesting that DHEAS can induce seizures when administered systemically or directly into the brain,Citation35,Citation36 other studies failed to confirm proconvulsant effects of DHEAS.Citation37–Citation41 Moreover, there is also a variety of data regarding the effects of DHEAS on the motor activity and body weight of the animals.Citation34,Citation37,Citation42–Citation45
Observed age-related decline in DHEAS levels suggests possible role of this neurosteroid in many disorders of aging.Citation2,Citation46,Citation47 Low DHEAS levels have been associated with age-related changes and decreased lifespan in various animal studies,Citation48 while exogenous DHEAS has been suggested to effectively ameliorate aging symptoms.Citation49 However, the findings of some studies are inconsistent or negative.
Although observed beneficial effects of DHEAS, such as proimmune, antidiabetic, antiobesity, antiatherosclerosis, antiosteoporosis, anticarcinogenic, antidementia, antiaging, and many other effects,Citation50,Citation51 probably require rather long-term therapeutic strategy, so far most studies have investigated the effects of acute DHEAS treatment.Citation15 Moreover, despite the knowledge that susceptibility to various mental disorders, including the sensitivity to seizures,Citation52 as well as association between DHEAS and different health outcomes,Citation53 often differ by sex, most preclinical studies of DHEAS were performed only in males.
Hence, given the chronic and age-related nature of many conditions for which DHEAS could be prescribed, as well as potential interaction of DHEAS with male and female hormonal status,Citation42,Citation54 we studied the effects of both acute and long-term DHEAS treatment in adult and aged mice of both sexes. Namely, in our study, we investigated possible age and sex differences in the susceptibility to seizures, as well as changes in locomotor activity, motor coordination, and body weight in mice following acute and chronic DHEAS administration.
In addition, sex differences in the seizure sensitivity might be due to the different modulation of the neurotransmitter receptor activity by DHEAS-derived sexual hormones and their metabolites. Therefore, we also studied the potential changes in the action of DHEAS at GABAA receptors using [3H]flunitrazepam binding on the brain membranes obtained from male and female mice following DHEAS treatment.
Materials and methods
Animals
Adult (~3 months old, weighing 20–25 g) and aged (~18 months old, weighing 30–45 g) male and female CBA mice, bred in Rudjer Boskovic Institute, Zagreb, Croatia, were used. The animals were housed at a constant temperature (22°C) and under a light cycle of 12-hour light/12-hour darkness (lights on at 7 am). Food and water were freely available. Mice were caged in groups of ten. As the female mice were caged next to the male mice, their estrus cycle was presumably synchronized due to the Whitten effect.Citation55 All animal care and experimental procedures were carried out in accordance with the Directive 2010/63/EU of European Parliament and Council of the European Union on the protection of animals used for scientific purposes and the Croatian law on animal welfare. The ethical approval was obtained from Rudjer Boskovic Bioethics Committee.
Drugs
DHEAS (Aldrich Chemical Co, St Louis, Mo, USA), pentylenetetrazole (PTZ, Sigma-Aldrich Co., St Louis, MO, USA), picrotoxin (Sigma), and NMDA (Ascent Scientific, Bristol, UK) were dissolved in saline. Following the determination of animal body weight, DHEAS or saline was administered to mice intraperitoneally (ip) in a volume of 1 mL per 100 g body weight. In the acute experiments, 10 mg/kg DHEAS or saline was given 30 minutes prior to the animal testing. In the chronic experiments, mice were treated with DHEAS (10 mg/kg) or saline once daily for 4 consecutive weeks. The DHEAS dose was selected according to the literature data reporting that in mice, concentrations of dehydroepiandrosterone (DHEA/S) from 0.1 up to 1,200 mg/kg have been found to be active, while in the human studies, the administered doses range from 0.1 to 40 mg/kg/day.Citation56 The animal testing started 30 minutes following single (acute treatment) or the last (after 4 consecutive weeks of chronic treatment) DHEAS or saline administration. In addition to DHEAS, [3H]flunitrazepam (specific activity 96 Ci/mmol, Amersham Biosciences Ltd, Buckinghamshire, UK) and diazepam (Sigma) dissolved in 0.1 N HCl were used in radioligand binding studies on brain membranes, isolated from mice 30 minutes following DHEAS or saline administration.
Locomotor activity measurement
The locomotor activity measurements were performed between 8 am and 1 pm in a quiet room under normal laboratory lighting. The horizontal ambulatory activity in individual mice was registered by Ugo-Basile Activity Cage. The activity was recorded for 10 minutes, starting after placing the animal into the test cage, 30 minutes following the single (acute treatment) or the last (after 4 consecutive weeks of chronic treatment) administration of DHEAS or saline. The observer left the room after placing the animal in the apparatus. The movements the animal makes inside the cage interrupted one or more infrared beam/s. The beam interruptions were counted, recorded by the electronic unit, and expressed as counts/10 minutes.
Rota-rod test
The motor coordination testing was also performed between 8 am and 1 pm in a quiet room under normal laboratory lighting. Motor coordination was evaluated using a rota-rod test. Prior the experiment, all mice were tested in two 2-minute period sessions per 2 consecutive days on the rotating bar at a constant speed of 15 rpm. Only the animals with stable performance on the rotating rod were used in the experiment and tested again 30 minutes following the single (acute treatment) or the last (after 4 consecutive weeks of chronic treatment) administration of DHEAS or saline. Each mouse was placed on the rota-rod (Ugo-Basile Rota-rod Treadmill for mice, Ugo-Basile, Monvalle VA, Italy; speed of rod 15 rpm) and the number of falls and latency to fall were measured for up to 2 minutes.
Seizure threshold determination
All experiments regarding determination of proconvulsive activity were carried out between 8 am and 1 pm, 30 minutes following the single (acute treatment) or the last (after 4 consecutive weeks of chronic treatment) administration of DHEAS or saline. The animal was taken from its home cage and placed in a glass cylinder (20×7 cm2) with numerous holes for ventilation, while its tail was drawn through a hole of the plastic cover and warmed for 1 minute under a tensor lamp. A butterfly infusion needle (length 20 mm, gauge 27) was inserted into the tail vein, and correct placement was verified by the appearance of blood in the infusion tubing.
The convulsants acting on distinct ligand-gated ion channels (GABAA and NMDA glutamate receptors) – PTZ (4 mg/mL), picrotoxin (0.75 mg/mL), and NMDA (8 mg/mL) – were administered by constant intravenous (iv) infusion. The infusion rates, controlled by a microinfusion pump, were 1.1 mL/min for PTZ and picrotoxin and 0.55 mL/min for NMDA. During the convulsant infusion, the mouse was held lightly by the tip of the tail and observed, and the time between the start of infusion and the onset of seizures (latency) was recorded. Timed tail vein infusion provides qualitative assessments of several different convulsive responses, which occurred in progression and are divided into two categories: clonus indicates rapid rhythmic movements due to alternating contraction and relaxation of muscles, whereas tonus indicates rigidity due to contraction of muscles.Citation57,Citation58
For PTZ, the first convulsive sign was myoclonus (a sudden involuntary muscle jerk, usually accompanied by a head twitch), followed by running and bouncing clonus (violent whole-body clonus, including running and explosive jumps) and tonic hindlimb extension (characterized by extreme rigidity, with forelimbs and hindlimbs extended caudally). As myoclonic twitch was not observed consistently in all of the animals that were challenged with picrotoxin and NMDA, only two convulsive signs, running and bouncing clonus and tonic hindlimb extension, which reliably characterize picrotoxin- and NMDA-induced seizures, were reported.Citation57 For each animal, the threshold dose of convulsant (mg/kg of body weight) required to elicit particular convulsant sign was calculated from the time of infusion (latency), infusion rate, concentration of convulsant, and body weight.
Preparation of the brain membranes
The animals were sacrificed 30 minutes following the administration of DHEAS or saline. The mice forebrains were quickly isolated on ice, washed with ice-cold saline, and stored at −20°C. Brain membranes were prepared mainly as previously described.Citation59 The frozen brains were homogenized in ice-cold 50 mM Tris-citrate buffer (pH 7.4) and homogenates centrifuged at 47,000 rpm for 20 minutes at 4°C. The pellet was washed in ice-cold 50 mM Tris-citrate buffer (pH 7.4), resuspended, and centrifuged three more times, than resuspended again and stored at −20°C. On the day of assay, the suspension was thawed, centrifuged once more at 20,000× g for 20 minutes at 4°C and used in [3H]flunitrazepam binding studies.
[3H]Flunitrazepam binding assay
For the [3H]flunitrazepam binding assay, aliquots of the brain membrane preparation (~100 mg protein/mL) were incubated with [3H]flunitrazepam in 50 mM Tris-citrate buffer (pH 7.4) containing 150 mM NaCl at 4°C for 90 minutes. Concentrations of DHEAS in the range from 1 µM to 1 mM were added to 1 nM of [3H]flunitrazepam in inhibition studies. Nonspecific binding was determined in the presence of 100 µM diazepam. In all binding assays, the total assay volume was 0.5 mL. Each assay tube was run in duplicate. Radioactivity bound to membranes after vacuum filtration on Whatman GF/C filters was measured using β-scintillation counter (Wallace 1409 DSA, PerkinElmer Inc., Waltham, MA, USA).
Protein concentration determination
Protein content was determined in a 10 µL sample, according to the method of Lowry et alCitation60 using bovine serum albumin as a standard.
Data analysis
All results are expressed as mean values ± standard error of the mean (SEM). The analysis and graphic presentation of data were performed using GraphPad Prism Version 4.00 for Windows (GraphPad Software Inc., La Jolla, CA, USA). Percentage change in [3H]flunitrazepam binding produced by DHEAS was defined as (specific binding in the presence of drug/specific binding in the absence of drug) ×100. The inhibition curves were analyzed using the sigmoidal equation and the values of half-maximum and maximum inhibition of [3H]flunitrazepam binding produced by DHEAS were determined. Statistical analysis of the results was conducted by Student’s t-test (for the comparison of two mean values) or by one-way analysis of variance (ANOVA). ANOVA was followed by the Newman–Keuls multiple comparison test. In animal testing, there were seven to eight animals per group, while data in binding studies were from three independent experiments performed in duplicate. P-values <0.05 were considered significant.
Results
The effects of DHEAS treatment on the seizure threshold
To investigate the possible proconvulsant activity of DHEAS in mice, we examined the effects of acute and chronic DHEAS administration on seizures induced by iv infusions of three different convulsants: pentylentetrazole, picrotoxin, and NMDA.
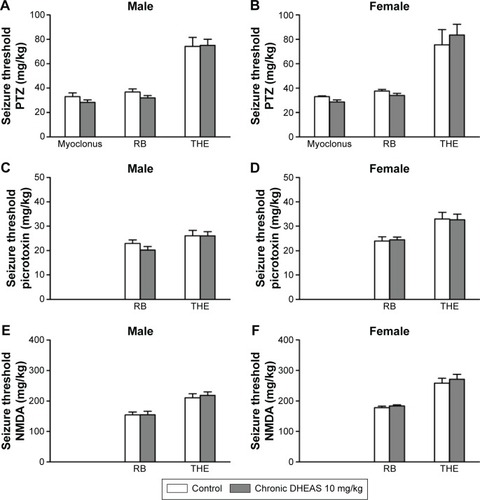
As shown in , 10 mg/kg DHEAS administered acutely 30 minutes prior to iv infusion of convulsants failed to significantly affect the doses of noncompetitive GABAA receptor antagonists pentylentetrazole () and picrotoxin (), as well as the doses of NMDA, an agonist of NMDA glutamate receptors (), needed to produce convulsive signs in the male as well as in the female adult mice. Even considerably higher doses of DHEAS (25 and 50 mg/kg), administered acutely in animals of both sexes, have not produced any significant effects on the threshold of seizures induced by pentylentet-razole, picrotoxin, and NMDA ().
Table 1 The effects of higher DHEAS doses (25 and 50 mg/kg) on the seizure thresholds for PTZ, picrotoxin, and NMDA in adult male and female mice
Figure 1 The effects of acute DHEAS treatment on the seizure thresholds for (A and B) PTZ, (C and D) picrotoxin, and (E and F) NMDA in adult male and female mice.
Abbreviations: DHEAS, dehydroepiandrosterone sulfate; NMDA, N-methyl-D-aspartate; PTZ, pentylenetetrazole; RB, running and bouncing clonus; SEM, standard error of the mean; THE, tonic hindlimb extension.
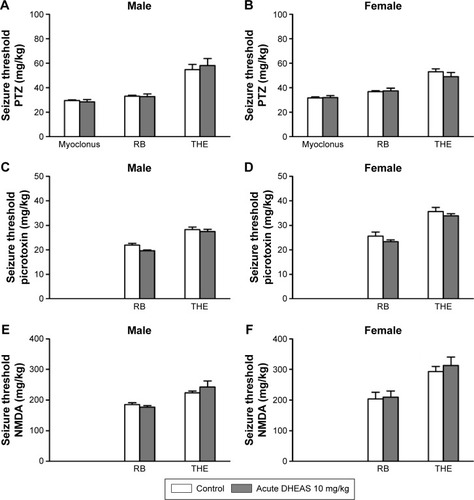
Because of the limited number of aged animals, we tested only the effects of acute DHEAS treatment on the picrotoxin-induced seizures. DHEAS applied acutely in the dose of 10 mg/kg has not significantly modified the seizure reactivity of aged male () as well as aged female mice to picrotoxin (), when compared to control group administered with saline.
Figure 2 The effects of acute DHEAS treatment on the seizure thresholds for picrotoxin in aged (A) male and (B) female mice.
Abbreviations: DHEAS, dehydroepiandrosterone sulfate; RB, running and bouncing clonus; SEM, standard error of the mean; THE, tonic hindlimb extension.
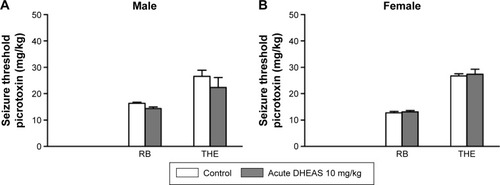
Moreover, following chronic DHEAS (10 mg/kg) treatment, we observed no significant differences compared to control group in the doses of pentylentetrazole (), picrotoxin (), and NMDA () needed to induce clonic and tonic seizures in adult mice of both sexes.
Figure 3 The effects of chronic DHEAS treatment on the seizure thresholds for (A and B) PTZ, (C and D) picrotoxin, and (E and F) NMDA in adult male and female mice.
Abbreviations: DHEAS, dehydroepiandrosterone sulfate; NMDA, N-methyl-D-aspartate; PTZ, pentylenetetrazole; RB, running and bouncing clonus; SEM, standard error of the mean; THE, tonic hindlimb extension.
Sex and age differences in the seizure susceptibility following DHEAS treatment
When control adult male and female mice were compared, significant differences were revealed in the doses of all three convulsants, required to elicit particular convulsant signs (). Namely, in saline-treated animals, we observed significant sex differences in the clonic (P<0.04, Student’s t-test) PTZ-induced seizures (), as well as in the tonic convulsions produced by picrotoxin (P<0.002, Student’s t-test) () and NMDA (P<0.001, Student’s t-test) ().
Figure 4 The sex differences in the effects of acute and chronic DHEAS treatment on the seizure thresholds for (A and C) PTZ, (D and E) picrotoxin, and (F and G) NMDA in adult mice.
Abbreviations: DHEAS, dehydroepiandrosterone sulfate; NMDA, N-methyl-D-aspartate; PTZ, pentylenetetrazole; RB, running and bouncing clonus; SEM, standard error of the mean; THE, tonic hindlimb extension.
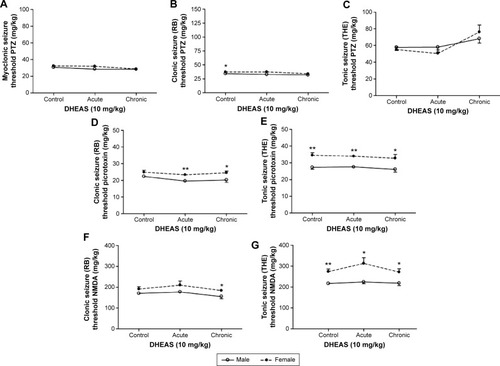
However, following DHEAS administration, we determined some discrete but significant changes regarding sex differences in the susceptibility to clonic seizures in comparison to control groups (). Namely, as opposed to the control group, significant sex differences in the clonic PTZ-induced seizures were not observed in the adult mice treated acutely or chronically with 10 mg/kg of DHEAS (). These sex differences were not observed in the myoclonus () or tonic seizures (), produced by PTZ, in saline- as well as in DHEAS-treated mice. On the other hand, significant differences (P<0.04, Student’s t-test) between adult male and female mice were found in the dose of NMDA needed to produce clonic convulsions () following chronic DHEAS (10 mg/kg) treatment, although these sex differences were not observed in control animals or mice treated with DHEAS acutely. In the case of threshold for tonic NMDA-induced seizures, significant differences between male and female mice were present in control, as well as in animals treated acutely and chronically with DHEAS (). In contrast to the saline-treated animals, after both acute (P<0.002, Student’s t-test) and chronic (P<0.05, Student’s t-test) DHEAS treatment, significantly lower clonic picrotoxin-induced seizure thresholds were also found in adult male when compared to adult female mice (). However, as shown in , significant sex differences in the threshold of tonic seizures produced by picrotoxin were observed in both saline- and DHEAS-treated groups (acute and chronic treatment).
In aged mice, the findings regarding sex differences in the picrotoxin-induced seizure thresholds were opposite form the results obtained on adult mice (). Namely, in contrast to adult animals, when control aged male and female mice were compared, significant differences were revealed in the doses of picrotoxin required to elicit clonic convulsive signs. On the other hand, in aged mice acutely treated with 10 mg/kg DHEAS, no significant sex differences were observed in clonic seizures produced by picrotoxin. In the case of picrotoxin-induced tonic convulsions, no sex differences were observed in aged mice treated acutely with saline or 10 mg/kg DHEAS, as opposed to the adult animals.
Table 2 The sex and age differences in the effects of acute DHEAS treatment on the seizure thresholds for picrotoxin in mice
As shown in , the aged mice of both sexes demonstrated higher seizure sensitivity to picrotoxin when compared to adult mice, regardless of whether they were acutely treated with 10 mg/kg DHEAS or not. However, in the case of threshold for tonic convulsions, the age-related differences in male mice have not reached a level of significance.
The effects of DHEAS treatment on the locomotor activity
The results of ANOVA demonstrated that acute as well as chronic administration of 10 mg/kg DHEAS has not affected the number of counts measuring the movements produced by adult male or female mice during 10 minutes stay in the activity cage, suggesting unchanged locomotor activity of the adult animals of both sexes ().
Table 3 The effects of acute and chronic DHEAS treatment on locomotor activity of adult male and female mice
The effects of DHEAS treatment on the motor coordination
As shown in , in experiments addressing the effect of acute and chronic 10 mg/kg DHEAS treatment on motor coordination, we observed no significant differences (ANOVA) in the number of falls and the latency to fall from the rota-rod treadmill between control- and DHEAS-treated adult male and female mice.
Table 4 The effects of acute and chronic DHEAS treatment on motor coordination of adult male and female CBA mice
The effects of DHEAS treatment on the body weight
Following determination of animal body weight, saline or 10 mg/kg DHEAS was administered ip to male and female mice once daily for 4 consecutive weeks. As expected, the male mice had a higher body weight than female mice both in control- and DHEAS-treated groups (P<0.001, ANOVA followed by Newman–Keuls test), on the first day as well as on the last day of the chronic treatment (). Animals in all groups gained body weight after 4 weeks of treatment, but only the body weight of DHEAS-treated female mice was significantly higher on the last day when compared to the first day of the treatment (P<0.05, ANOVA followed by Newman–Keuls test). However, as shown in , there were no differences in the body weight between control- and DHEAS-treated groups of both male and female mice, on the first day as well as on the last day of treatment.
Table 5 The effects of chronic DHEAS treatment on the body weight of adult male and female mice
The effects of DHEAS treatment on the [3H]flunitrazepam binding to the brain membranes
DHEAS inhibited the binding of [3H]flunitrazepam to the brain membranes isolated from both saline- and DHEAS-treated adult male and female mice in a concentration-dependent manner (). The inhibitory potency of DHEAS (the half-maximum inhibition value) was in the micromolar range, maximum inhibition obtained with DHEAS was approximately 70%, and slope factor was near unity in all investigated groups. As shown in , there were no significant differences in the maximum inhibition and half-maximum inhibition of [3H]flunitrazepam binding by DHEAS between saline- and DHEAS-treated adult male and female mice.
Table 6 The effects of acute DHEAS treatment on the potency of DHEAS for inhibiting [3H]flunitrazepam binding to the brain membranes of adult male and female mice
Figure 5 The effects of acute DHEAS treatment on DHEAS-produced inhibition of [3H]flunitrazepam binding to the brain membranes of adult male and female mice.
Abbreviations: DHEAS, dehydroepiandrosterone sulfate; SEM, standard error of the mean.
![Figure 5 The effects of acute DHEAS treatment on DHEAS-produced inhibition of [3H]flunitrazepam binding to the brain membranes of adult male and female mice.](/cms/asset/74f5fe04-4e15-46fb-9fb9-ce50f2e0468e/dddt_a_12163071_f0005_b.jpg)
Discussion
This study demonstrated no differences in the body weight between control- and DHEAS-treated groups of both male and female mice. Our findings are in line with some previous animal studies reporting that acute and chronic administration of DHEAS has not affected body weight gain and food intake.Citation43,Citation44 On the other hand, our results are in contrast with the decrease in the body weight of mice observed following chronic DHEAS treatment, which the authors found to be consistent with reported proconvulsive, anxiogenic, provoking, as well as hypophagic effects of DHEAS.Citation34,Citation35,Citation61,Citation62 The same authors have also shown that DHEAS (1 and 2 mg/kg) increased the motor activity in mice.Citation34 However, in the present study and some other studies, locomotor activity, general ambulation, and motor coordination of animals were not affected by DHEAS treatment.Citation37,Citation42,Citation45 Hence, as the present study has not provided experimental evidence on the provoking and proconvulsant effects of long-term DHEAS administration, perhaps it is not surprising that no changes in animal weight were observed as well.
Namely, our results demonstrated that both acute and chronic DHEAS treatments have not modified the seizure reactivity of mice to PTZ, picrotoxin, or NMDA, widely used convulsants in animal seizure models. These results are in agreement with studies in which DHEAS displayed neither proconvulsant nor anticonvulsant effects. For instance, DHEAS administered to mice in the doses from 1 to 40 mg/kg did not have any effects against acutely PTZ-induced or kindled convulsions or on the development of PTZ seizures.Citation39 DHEAS (25 and 50 mg/kg) also demonstrated no effect on kainate-induced convulsions, but elevated the kainate-induced lethality in mice.Citation41 In line with our results, ip administration of DHEAS in doses of 12.5, 50, and 100 mg/kg showed no effect on the NMDA-induced seizures.Citation37 On the other hand, 25 mg/kg of DHEAS significantly increased the dose of NMDA necessary to induce clonic convulsions in 50% of the tested mice, suggesting protective effect of DHEAS against NMDA-induced seizures.Citation37 Previously, DHEAS was also shown to protect, both in vitro and in vivo, hippocampal neurons against excitatory amino acids-induced toxicity.Citation63 Moreover, DHEAS (20 mg/kg) administered daily with 45 mg/kg of cocaine for 12 days decreased the number of mice exhibiting cocaine-induced convulsions.Citation40
In contrast to these results are the reports suggesting that DHEAS can induce seizures when administered systemically or directly into the brain.Citation35,Citation36 Namely, it has been shown that intracerebroventricular injection of DHEAS-induced clonic-tonic convulsions in mice in a dose-dependent manner.Citation36 Moreover, Reddy and KulkarniCitation35 reported that although acute DHEAS treatment did not demonstrate proconvulsant effects, mice treated for 4 weeks with 10 mg/kg DHEAS exhibited increased seizure sensitivity to PTZ. The results are also contradictory in human studies. While some studies demonstrated high levels of DHEAS in female patients with epilepsy,Citation64 other authors reported that women with more frequent seizures had an increase of cortisol and a decrease of DHEAS levels.Citation38
The reasons for such discrepancies are not clear, but are usually explained by differences in the experimental approach or protocol, such as the solvent applied, seizure model, route of drug administration, administered dose, time of neurosteroid administration, as well as species or even animal strains used. For instance, unlike Reddy and KulkarniCitation35 who induced PTZ seizures in mice by a single ip injection, in our study, convulsions were induced by iv infusion of PTZ. PTZ infusion test is described as a sensitive model that allows determination of drug effects on separate components of seizure behavior and has been suggested for laboratory evaluation of anticonvulsant drugs.Citation65,Citation66 It is generally accepted that both PTZ and picrotoxin act as noncompetitive antagonists via picrotoxin site of the GABAA receptor,Citation67,Citation68 although Huang et alCitation69 suggested that these two convulsants interact with overlapping but distinct domains of the GABAA receptor.
As DHEAS has been also reported as an allosteric antagonist of GABAA receptor,Citation26,Citation27,Citation30 which binds at or near the picrotoxin/t–butylbicyclophosphorothionate site on the GABAA receptor complex,Citation70 it is possible that DHEAS competes with PTZ and picrotoxin for the same binding site or displaces their binding by steric hindrance. Such an interaction would explain why proconvulsive effects of DHEAS were not observed in PTZ- and picrotoxin-induced seizures. The interaction of DHEAS with excitatory amino acid transmission also seems rather complex.Citation37 In line with our results, demonstrating that NMDA-induced seizures were not affected by chronic DHEAS treatment, some authors failed to detect significant effects of DHEAS on glutamate binding sites and glutamate uptake.Citation71
The presence of sex differences in the sensitivity to GABA-related convulsants also seems to depend on the convulsive drug and methodology used to inject it.Citation52 However, various results have been reported even when using convulsants that share the same receptor binding site. In contrast to the study demonstrating that PTZ, which acts via picrotoxin binding site, affects more female than male mice,Citation52 Pericic et al observed higher picrotoxin sensitivity in male than in female mice.Citation72 Even the same route of drug administration has not presented consistent results. For instance, intravenously injected bicuculline has been reported to affect more femalesCitation73 or malesCitation72,Citation74 or to have the same effect on both sexes.Citation75 Moreover, regarding intravenously administered PTZ, Kokka et alCitation76 demonstrated that male rats were more susceptible than females, while Finn and GeeCitation73 found no seizure differences between sexes.
The discrepancy in the results might also be due to the differences in the stages of the female estrous cycle. Although the estrus cycle in our study was presumably synchronized due to the Whitten effect, we have not controlled the stage of the estrous cycle between experiments. As the estrous cycle is short, a variation of the estrous stages between different experiments is possible and might influence the effect of DHEAS on neuronal excitability of female mice. Different stages in the estrus cycle might be the reason why in our study sex differences were not always significant. However, male adult mice generally demonstrated lower thresholds for seizures induced by all three convulsants, when compared to female adult mice, regardless of whether they were acutely or chronically treated with DHEAS or treated with saline.
In contrast, in aged mice, we observed significant sex differences only in the control group, with female mice showing lower threshold for clonic picrotoxin-induced seizures. In line with our results, Zhang et alCitation77 comparing adult and aged male and female mice following kainic acid treatment found that aged female mice demonstrated more severe seizure activity. On the other hand, our findings demonstrating sex differences in the susceptibility to tonic picrotoxin-induced convulsions only in adult but not in aged CBA mice are in line with a study reporting that unlike adult mice, 2-year- old CBA mice fail to display sex differences following picrotoxin administration.Citation78 Nevertheless, since aged mice were not submitted to all experimental procedures as adult mice, our limited results regarding differences in neuronal excitability of aged male and female mice should be taken with caution.
In our study, the aged mice of both sexes demonstrated higher seizure sensitivity to picrotoxin when compared to adult mice, regardless of whether they were acutely treated with DHEAS or not. These results are in line with various findings demonstrating that aging is associated with an increased risk of seizures/epilepsy.Citation79,Citation80 It has been shown that aged rats exhibit altered EEG activity and clinical manifestations during kainate-induced status epilepticus.Citation81 In addition, increased hippocampal excitability has been found in aged mice suggesting its relevance to the increased seizure susceptibility observed in aged subjects.Citation82 However, different studies have reported both increased or decreased seizure susceptibility associated with advanced age, depending on the model and animal strain used.Citation83
Following DHEAS administration, we determined some discrete but significant changes regarding sex differences in the susceptibility to clonic seizures in comparison to control groups. Because DHEA and its sulfate ester DHEAS serve as precursors for both androgenic and estrogenic steroids,Citation1,Citation84,Citation85 some effects of DHEAS may be attributable to its conversion into these sexual hormones.Citation53 Baulieu et alCitation86 reported that following long-term DHEA administration to elderly men and women, in addition to the higher concentration of DHEAS, a small increase of testosterone and estradiol was noted, particularly in women, which may be involved in the observed beneficial physiological–clinical manifestations. Moreover, it has been suggested that both DHEA and DHEAS might have either estrogen- or androgen-like effects depending on the hormonal environment and thus influence the receptor type with which these neurosteroids interact.Citation87 The suggestion of such interaction between DHEAS and male or female hormonal milieu is in line with previously reported sex-related differences in the DHEAS serum/plasma levels.Citation54,Citation88–Citation90
Hence, the small changes regarding sex differences in the seizure sensitivity, observed following DHEAS treatment, could be explained by different modulation of the neurotransmitter receptor activity by DHEAS-derived sexual hormones and their metabolites. However, our findings showing that acute DHEAS treatment has not modified the potency of DHEAS to inhibit [3H]flunitrazepam binding on the brain membranes of adult male and female mice do not support this hypothesis, although they confirm previous results, which demonstrated that DHEAS behaves as an allosteric antagonist of the GABAA receptor.Citation25–Citation30 Further studies are needed in order to elucidate the role of GABAergic and other neurotransmitter systems in the complex effects of DHEAS on neuronal excitability.
The limitations of this study are a lack of lower doses of DHEAS (1 and 2 mg/kg), which have been also shown to be active in mice, a lack of control for the stage of the mice estrous cycle between experiments, as well as the fact that aged mice were not submitted to all experimental procedures as adult mice.
Conclusion
Our findings demonstrating that DHEAS does not modify the locomotor activity, motor coordination, seizure susceptibility, brain [3H]flunitrazepam binding, and body weight in the mice of both sexes suggest that DHEAS treatment might be safe for various potential therapeutic applications in both adult and aged population. The results of our study also suggest discrete interaction of DHEAS with male and female hormonal status, which may underline observed sex-related differences in the association of DHEAS with various indicators of health and morbidity, although the underlying molecular mechanisms are not clear.
Acknowledgments
This work was supported by Croatian Ministry of Science, Education and Sport and International Brain Research Organization (IBRO). The skillful technical assistance of Zlatica Tonsetic, Jasminka Golubic Talic, and Goran Goles is gratefully acknowledged. The abstract of this paper was presented at the 7th Croatian Congress of Pharmacology Zagreb, Croatia, September 18–21, 2013, as a poster presentation with interim findings. The abstract of the poster was published in “Poster Abstracts” in Periodicum Biologorum Vol 115, Suppl 3. Vitale, Branko (ed.), Zagreb, Croatian Society for Natural Sciences, Rudjer Boskovic Institute, LASERplus, 2013. 96–96.
Disclosure
The authors report no conflicts of interest in this work.
References
- FriessESchiffelholzTStecklerTSteigerADehydroepiandrosterone – a neurosteroidEur J Clin Invest200030465011281367
- ManingerNWolkowitzOMReusVIEpelESMellonSHNeurobiological and neuropsychiatric effects of dehydroepiandrosterone (DHEA) and DHEA sulfate (DHEAS)Front Neuroendocrinol200930659119063914
- Pérez-NeriIMontesSOjeda-LópezCRamírez-BermúdezJRíosCModulation of neurotransmitter systems by dehydroepiandrosterone and dehydroepiandrosterone sulfate: mechanism of action and relevance to psychiatric disordersProg Neuropsychopharmacol Biol Psychiatry2008321118113018280022
- DongYZhengPDehydroepiandrosterone sulphate: action and mechanism in the brainJ Neuroendocrinol20122421522422145821
- BologaLSharmaJRobertsEDehydroepiandrosterone and its sulfated derivative reduce neuronal death and enhance astrocytic differentiation in brain cell culturesJ Neurosci Res1987172252342955123
- KurataKTakebayashiMMorinobuSYamawakiSBeta-estradiol, dehydroepiandrosterone, and dehydroepiandrosterone sulfate protect against N-methyl-D-aspartate-induced neurotoxicity in rat hippocampal neurons by different mechanismsJ Pharmacol Exp Ther200431123724515175425
- LapchakPAChapmanDFNunezSYZivinJADehydroepiandrosterone sulfate is neuroprotective in a reversible spinal cord ischemia model: possible involvement of GABA(A) receptorsStroke2000311953195610926963
- ZhangLLiBSMaWDehydroepiandrosterone (DHEA) and its sulfated derivative (DHEAS) regulate apoptosis during neurogenesis by triggering the Akt signaling pathway in opposing waysBrain Res Mol Brain Res200298586611834296
- TaylorMKStoneMLaurentHKRauhMJGrangerDANeuroprotective-neurotrophic effect of endogenous dehydroepiandrosterone sulfate during intense stress exposureSteroids201487545824887210
- de BruinVMVieiraMCRochaMNVianaGSCortisol and dehydroepiandosterone sulfate plasma levels and their relationship to aging, cognitive function, and dementiaBrain Cogn20025031632312464198
- FloodJFMorleyJERobertsEPregnenolone sulfate enhances post-training memory processes when injected in very low doses into limbic system structures: the amygdala is by far the most sensitiveProc Natl Acad Sci U S A19959210806108107479888
- MauriceTLockhartBPNeuroprotective and anti-amnesic potentials of sigma (sigma) receptor ligandsProg Neuropsychopharmacol Biol Psychiatry199721691029075259
- MilmanAZoharOMaayanRWeizmanRPickCGDHEAS repeated treatment improves cognitive and behavioral deficits after mild traumatic brain injuryEur Neuropsychopharmacol20081818118717669633
- NicolasLBPinoteauWPapotSRoutierSGuillaumetGMortaudSAggressive behavior induced by the steroid sulfatase inhibitor COUMATE and by DHEAS in CBA/H miceBrain Res200192221622211743952
- WolfOTKirschbaumCActions of dehydroepiandrosterone and its sulfate in the central nervous system: effects on cognition and emotion in animals and humansBrain Res Brain Res Rev19993026428810567728
- AssiesJVisserINicolsonNAElevated salivary dehydroepiandrosterone-sulfate but normal cortisol levels in medicated depressed patients: preliminary findingsPsychiatry Res200412811712215488954
- Buydens-BrancheyLBrancheyMHudsonJMajewskaDMPerturbations of plasma cortisol and DHEA-S following discontinuation of cocaine use in cocaine addictsPsychoneuroendocrinology200227839711750771
- HillenTLunAReischiesFMBorcheltMSteinhagen-ThiessenESchaubRTDHEA-S plasma levels and incidence of Alzheimer’s diseaseBiol Psychiatry20004716116310664834
- SöndergaardHPHanssonLOTheorellTElevated blood levels of dehydroepiandrosterone sulphate vary with symptom load in posttraumatic stress disorder: findings from a longitudinal study of refugees in SwedenPsychother Psychosom20027129830312207110
- StrousRDMaayanRLapidusRIncreased circulatory dehydroepiandrosterone and dehydroepiandrosterone-sulphate in first-episode schizophrenia: relationship to gender, aggression and symptomatologySchizophr Res20047142743415474914
- RupprechtRNeuroactive steroids: mechanisms of action and neuropsychopharmacological propertiesPsychoneuroendocrinology20032813916812510009
- StárkaLDuškováMHillMDehydroepiandrosterone: a neuroactive steroidJ Steroid Biochem Mol Biol201514525426024704258
- HillMDuškováMStárkaLDehydroepiandrosterone, its metabolites and ion channelsJ Steroid Biochem Mol Biol201514529331424846830
- MajewskaMDNeurosteroids: endogenous bimodal modulators of the GABAA receptor. Mechanism of action and physiological significanceProg Neurobiol1992383793951349441
- BaulieuEERobelPDehydroepiandrosterone (DHEA) and dehydroepiandrosterone sulfate (DHEAS) as neuroactive neurosteroidsProc Natl Acad Sci U S A199895408940919539693
- DemirgörenSMajewskaMDSpivakCELondonEDReceptor binding and electrophysiological effects of dehydroepiandrosterone sulfate, an antagonist of the GABAA receptorNeuroscience1991451271351661387
- MajewskaMDDemirgörenSSpivakCELondonEDThe neurosteroid dehydroepiandrosterone sulfate is an allosteric antagonist of the GABAA receptorBrain Res19905261431461964106
- LambertJJBelelliDPedenDRVardyAWPetersJANeurosteroid modulation of GABAA receptorsProg Neurobiol200371678014611869
- ShenWMennerickSZorumskiECCoveyDFZorumskiCFPregnenolone sulfate and dehydroepiandrosterone sulfate inhibit GABA-gated chloride currents in Xenopus oocytes expressing picrotoxin-insensitive GABA(A) receptorsNeuropharmacology19993826727110218867
- Svob StracDJazvinscak JembrekMErhardtJMirkovic KosKPericicDModulation of recombinant GABA(A) receptors by neurosteroid dehydroepiandrosterone sulfatePharmacology20128916317122433179
- IrwinRPLinSZRogawskiMAPurdyRHPaulSMSteroid potentiation and inhibition of N-methyl-D-aspartate receptor-mediated intracellular Ca++ responses: structure-activity studiesJ Pharmacol Exp Ther19942716776827965782
- MauriceTRomanFJPrivatAModulation by neurosteroids of the in vivo (+)-[3H]SKF-10,047 binding to sigma 1 receptors in the mouse forebrainJ Neurosci Res1996467347438978508
- CaretteBPoulainPExcitatory effect of dehydroepiandrosterone, its sulphate ester and pregnenolone sulphate, applied by iontophoresis and pressure, on single neurones in the septo-preoptic area of the guinea pigNeurosci Lett1984452052106328376
- ReddyDSKulkarniSKDifferential anxiolytic effects of neurosteroids in the mirrored chamber behavior test in miceBrain Res199775261719106441
- ReddyDSKulkarniSKProconvulsant effects of neurosteroids pregnenolone sulfate and dehydroepiandrosterone sulfate in miceEur J Pharmacol199834555599593594
- CzłonkowskaAIKrzaścikPSienkiewicz-JaroszHThe effects of neurosteroids on picrotoxin-, bicuculline- and NMDA-induced seizures, and a hypnotic effect of ethanolPharmacol Biochem Behav20006734535311124400
- BudziszewskaBSiwanowiczJLeśkiewiczMJaworska-FeilLLasońWProtective effects of neurosteroids against NMDA-induced seizures and lethality in miceEur Neuropsychopharmacol199887129452934
- GalimbertiCAMagriFCopelloFSeizure frequency and cortisol and dehydroepiandrosterone sulfate (DHEAS) levels in women with epilepsy receiving antiepileptic drug treatmentEpilepsia20054651752315816945
- HansenSLSperlingBBSánchezCAnticonvulsant and antiepileptogenic effects of GABAA receptor ligands in pentylenetetrazole-kindled miceProg Neuropsychopharmacol Biol Psychiatry20042810511314687864
- LeśkiewiczMBudziszewskaBJaworska-FeilLKuberaMBasta-KaimALasońWInhibitory effect of some neuroactive steroids on cocaine-induced kindling in micePol J Pharmacol2003551131113614730111
- LeśkiewiczMBudziszewskaBJaworska-FeilLLasońWEffects of neurosteroids on kainate-induced seizures, neurotoxicity and lethality in micePol J Pharmacol1997494114179566044
- AndradeSSilveiraSLGomezRBarrosHMRibeiroMFGender differences of acute and chronic administration of dehydroepiandrosterone in rats submitted to the forced swimming testProg Neuropsychopharmacol Biol Psychiatry20073161362117223242
- Lea-CurrieYRWenPMcIntoshMKDehydroepiandrosterone-sulfate (DHEAS) reduces adipocyte hyperplasia associated with feeding rats a high-fat dietInt J Obes Relat Metab Disord199721105810649368831
- Lea-CurrieYRWuSMMcIntoshMKEffects of acute administration of dehydroepiandrosterone-sulfate on adipose tissue mass and cellularity in male ratsInt J Obes Relat Metab Disord1997211471549043970
- ZajdaMEKrzascikPHillMMajewskaMDPsychomotor and rewarding properties of the neurosteroids dehydroepiandrosterone sulphate and androsterone: effects on monoamine and steroid metabolismActa Neurobiol Exp (Wars)201272657922508085
- DhatariyaKKNairKSDehydroepiandrosterone: is there a role for replacement?Mayo Clin Proc2003781257127314531485
- RaineyWENakamuraYRegulation of the adrenal androgen biosynthesisJ Steroid Biochem Mol Biol200810828128617945481
- RutkowskiKSowaPRutkowska-TalipskaJKuryliszyn-MoskalARutkowskiRDehydroepiandrosterone (DHEA): hypes and hopesDrugs2014741195120725022952
- ChenJRTsengGFWangYJWangTJExogenous dehydroisoan-drosterone sulfate reverses the dendritic changes of the central neurons in aging male ratsExp Gerontol20145719120224929010
- NawataHYanaseTGotoKOkabeTAshidaKMechanism of action of anti-aging DHEA-S and the replacement of DHEA-SMech Ageing Dev20021231101110612044959
- LeowattanaWDHEAS as a new diagnostic toolClin Chim Acta200434111514967152
- MedinaAEManhãesACSchmidtSLSex differences in sensitivity to seizures elicited by pentylenetetrazol in micePharmacol Biochem Behav20016859159611325416
- GleiDAGoldmanNWeinsteinMLiuIWDehydroepiandrosterone sulfate (DHEAS) and health: does the relationship differ by sex?Exp Gerontol20043932133115036391
- LaughlinGABarrett-ConnorESexual dimorphism in the influence of advanced aging on adrenal hormone levels: the Rancho Bernardo StudyJ Clin Endocrinol Metab2000853561356811061502
- WhittenWKBronsonFHGreensteinJAEstrus-inducing pheromone of male mice: transport by movement of airScience19681615845855690897
- SvecFPorterJRThe actions of exogenous dehydroepiandrosterone in experimental animals and humansProc Soc Exp Biol Med19982181741919648935
- FinnDACrabbeJCChronic ethanol differentially alters susceptibility to chemically induced convulsions in withdrawal seizure-prone and -resistant miceJ Pharmacol Exp Ther19992887827909918589
- KosobudAECrabbeJCGenetic correlations among inbred strain sensitivities to convulsions induced by 9 convulsant drugsBrain Res19905268162078820
- PericicDSvobDJazvinscakMMirkovicKAnticonvulsive effect of swim stress in micePharmacol Biochem Behav20006687988610973529
- LowryOHRosebroughNJFarrALRandallRJProtein measurement with the folin phenol reagentJ Biol Chem195119326527514907713
- ReddyDSKulkarniSKThe role of GABA-A and mitochondrial diazepam-binding inhibitor receptors on the effects of neurosteroids on food intake in micePsychopharmacology (Berl)19981373914009676900
- ReddyDSKulkarniSKSex and estrous cycle-dependent changes in neurosteroid and benzodiazepine effects on food consumption and plus-maze learning behaviors in ratsPharmacol Biochem Behav19996253609972845
- KimonidesVGKhatibiNHSvendsenCNSofroniewMVHerbertJDehydroepiandrosterone (DHEA) and DHEA-sulfate (DHEAS) protect hippocampal neurons against excitatory amino acid-induced neurotoxicityProc Natl Acad Sci U S A199895185218579465106
- El-KhayatHAAbd El-BassetFZTomoumHYPhysical growth and endocrinal disorders during pubertal maturation in girls with epilepsyEpilepsia2004451106111515329076
- LoscherWNoltingBThe role of technical, biological and pharmacological factors in the laboratory evaluation of anticonvulsant drugs. IV. Protective indicesEpilepsy Res199191101884714
- MandhaneSNAavulaKRajamannarTTimed pentylenetetrazol infusion test: a comparative analysis with s.c. PTZ and MES models of anticonvulsant screening in miceSeizure20071663664417570689
- RamanjaneyuluRTickuMKInteractions of pentamethylenetetrazole and tetrazole analogues with the picrotoxinin site of the benzodiazepine-GABA receptor-ionophore complexEur J Pharmacol1984983373456327331
- SquiresRFSaederupECrawleyJNSkolnickPPaulSMConvulsant potencies of tetrazoles are highly correlated with actions on GABA/benzodiazepine/picrotoxin receptor complexes in brainLife Sci198435143914446090836
- HuangRQBell-HornerCLDibasMICoveyDFDreweJADillonGHPentylenetetrazole-induced inhibition of recombinant gamma-aminobutyric acid type A (GABA(A)) receptors: mechanism and site of actionJ Pharmacol Exp Ther200129898699511504794
- SousaATickuMKInteractions of the neurosteroid dehydroepiandrosterone sulfate with the GABA(A) receptor complex reveals that it may act via the picrotoxin siteJ Pharmacol Exp Ther19972828278339262347
- LeśkiewiczMBudziszewskaBJaworska-FeilLKajtaMLasońWEffect of neurosteroids on glutamate binding sites and glutamate uptake in rat hippocampusPol J Pharmacol19985035536010091721
- PericicDManevHGeberJSex related differences in the response of mice, rats and cats to administration of picrotoxinLife Sci1986389059133951316
- FinnDAGeeKWThe estrus cycle, sensitivity to convulsants and the anticonvulsant effect of a neuroactive steroidJ Pharmacol Exp Ther19942711641707965710
- GuilletRDunhamLNeonatal caffeine exposure and seizure susceptibility in adult ratsEpilepsia1995367437497635092
- DevaudLLPurdyRHMorrowALThe neurosteroid, 3 alpha-hydroxy-5 alpha-pregnan-20-one, protects against bicuculline-induced seizures during ethanol withdrawal in ratsAlcohol Clin Exp Res1995193503557625568
- KokkaNSappDWWitteUOlsenRWSex differences in sensitivity to pentylenetetrazole but not in GABAA receptor bindingPharmacol Biochem Behav1992434414471332080
- ZhangXMZhuSWDuanRSMohammedAHWinbladBZhuJGender differences in susceptibility to kainic acid-induced neurodegeneration in aged C57BL/6 miceNeurotoxicology20082940641218342945
- ManevHPericicDAnic-StojiljkovicSSex differences in the sensitivity of CBA mice to convulsions induced by GABA antagonists are age-dependentPsychopharmacology (Berl)1987912262293107037
- JetterGMCavazosJEEpilepsy in the elderlySemin Neurol20082833634118777480
- BrodieMJElderATKwanPEpilepsy in later lifeLancet Neurol200981019103019800848
- DarbinONaritokuDPatryloPRAging alters electroencephalographic and clinical manifestations of kainate-induced status epilepticusEpilepsia2004451219122715461676
- El-HayekYHWuCYeHWangJCarlenPLZhangLHippocampal excitability is increased in aged miceExp Neurol201324771071923510762
- KellyKMAging models of acute seizures and epilepsyEpilepsy Curr201010152020126334
- ArltWCalliesFAllolioBDHEA replacement in women with adrenal insufficiency – pharmacokinetics, bioconversion and clinical effects on well-being, sexuality and cognitionEndocr Res20002650551111196420
- LabrieFLuu-TheVLabrieCSimardJDHEA and its transformation into androgens and estrogens in peripheral target tissues: intracrinologyFront Neuroendocrinol20012218521211456468
- BaulieuEEThomasGLegrainSDehydroepiandrosterone (DHEA), DHEA sulfate, and aging: contribution of the DHEAge Study to a socio-biomedical issueProc Natl Acad Sci U S A2000974279428410760294
- EbelingPKoivistoVAPhysiological importance of dehydroepiandrosteroneLancet1994343147914817911183
- SulcováJHillMHamplRStárkaLAge and sex related differences in serum levels of unconjugated dehydroepiandrosterone and its sulphate in normal subjectsJ Endocrinol199715457629246938
- YoungDGSkibinskiGMasonJIJamesKThe influence of age and gender on serum dehydroepiandrosterone sulphate (DHEA-S), IL-6, IL-6 soluble receptor (IL-6 sR) and transforming growth factor beta 1 (TGF-beta1) levels in normal healthy blood donorsClin Exp Immunol199911747648110469050
- ZumoffBVBradlowHLSex difference in the metabolism of dehydroisoandrosterone sulfateJ Clin Endocrinol Metab1980513343366447162
