Abstract
Background
Secondary hyperparathyroidism (SHPT) is one of the major risk factors of morbidity and mortality in end-stage renal disease. Cinacalcet effectively controls SHPT without causing hypercalcemia and hyperphosphatemia. However, there is significant inter-individual response variance to cinacalcet treatment. Therefore, we aimed to evaluate the genetic effects related with parathyroid hormone regulation as factors for cinacalcet response variance.
Methods
Patients with a diagnosis of SHPT based on intact parathyroid hormone (iPTH) >300 pg/mL on dialysis were included in this study. They were over 18 years and have been treated by cinacalcet for more than 3 months. Responders and nonresponders were grouped by the serum iPTH changes. Twenty-four single nucleotide polymorphisms of CASR, VDR, FGFR1, KL, ALPL, RGS14, NR4A2, and PTHLH genes were selected for the pharmacogenetic analysis.
Results
After adjusting for age, sex, and calcium level, CASR rs1042636 (odds ratio [OR]: 0.066, P=0.027) and rs1802757 (OR: 10.532, P=0.042) were associated with cinacalcet response. The association of haplotypes of CASR rs1042636, rs10190, and rs1802757; GCC (OR: 0.355, P=0.015); and ATT (OR: 2.769, P=0.014) with cinacalcet response was also significant.
Conclusion
We obtained supporting information of the associations between cinacalcet response and CASR polymorphisms. CASR single nucleotide polymorphisms (SNPs) rs1802757, rs1042636, and haplotypes of rs1042636, rs10190, and rs1802757 were significantly associated with cinacalcet response variance.
Introduction
Dysregulation of mineral homeostasis due to failing kidney function leads to decreased renal phosphate excretion, elevated fibroblast growth factor 23 level, and reduced synthesis of calcitriol. These changes lead to consistent secretion of parathyroid hormone (PTH) contributing to the development of parathyroid hyperplasia and secondary hyperparathyroidism (SHPT) in end-stage renal disease. SHPT with elevated calcium and phosphate levels contributes to the development of renal osteodystrophy, erythropoietin resistance, vascular calcification, and left ventricular hypertrophy. These clinical features are strongly associated with increased morbidity and mortality in dialytic end-stage renal disease population.Citation1–Citation4 It is therefore critical to maintain optimal level of PTH in these patients. However, because of the complicated problems of lowering PTH with simultaneously controlling imbalanced serum levels of calcium and phosphate, traditional therapies of phosphate binders and vitamin D analogues for managing SHPT have all too often led to clinical problems, including hypercalcemia, hyperphosphatemia, and refractory hyperparathyroidism or over-suppression of PTH.Citation5
One of the newer treatment options for SHPT is cinacalcet which increases the sensitivity of the calcium-sensing receptor (CaSR) on the parathyroid gland cells to extracellular calcium,Citation6 resulting in a decreased calcium set point and circulating PTH levels.Citation6–Citation8 The cinacalcet treatment resulted in 41% of SHPT patients achieving PTH and Ca × P levels recommended by the Kidney Disease Outcomes Quality Initiative (K/DOQI) guidelinesCitation9 whereas fewer than 10% of the patients treated with phosphate binders and vitamin D analogs reached optimal control.Citation10 Moreover, cinacalcet reduced the risk of parathyroidectomy by 91%.Citation11 Cinacalcet treatment is effective to the extent that it can be utilized as an alternative to surgical intervention in some patients.Citation12,Citation13 Despite the overall response to cinacalcet being promising in a number of clinical trials,Citation14–Citation16 the therapeutic efficacy has a significant inter-individual variability of 12.1%–46% PTH reduction, representing drug resistance.Citation17–Citation21 The factors associated with the drug resistance include delayed therapy, persistent hyperphosphatemia, nodular hyperplasia,Citation22 and reduced expression of CaSR and vitamin D receptor (VDR).Citation23–Citation25 In particular, genetic polymorphisms affecting CaSR and VDR expression have been considered as factors for drug resistance. The genetic polymorphism of CASR Arg990Gly (c.2968A > G, rs1042636) was associated with different cinacalcet response.Citation26 Several other studiesCitation27–Citation29 on CASR presented the possible association of CASR rs1042636 and rs2221266 polymorphisms with PTH level difference. VDR gene polymorphisms of rs7975232 (Apa 1), rs731236 (Taq 1), and rs1544410 (Bsm 1) were also associated with PTH regulation.Citation30–Citation32 These studies presented the clinical importance of genetic polymorphisms in PTH regulation. However, the results available so far show limited evidence for genetic polymorphisms associated with cinacalcet response involving a small number of patients and not including phosphate and bone regulation-related genes in PTH homeostasis. Thus, we aimed to evaluate the frequency of variants of study genes related to PTH regulation and the association between SNPs and haplotypes of genes and cinacalcet response in Korean SHPT patients on dialysis.
Methods
Study subjects
Study patients were selected from a tertiary university hospital in Seoul, Korea, between June 2011 and July 2014. Patients with a diagnosis of SHPT based on intact parathyroid hormone (iPTH) >300 pg/mL were included in this study. The patients were over 18 years and have been treated by cinacalcet for more than 3 months. All these patients were on either hemodialysis or peritoneal dialysis, receiving optimal medical therapy in the form of dietary restriction, phosphate binders, or vitamin D sterols. The main exclusion criteria were serious concomitant hepatic disease, active cancers, or taking strong CYP3A4 inhibitors, such as itraconazole, clarithromycin, diltiazem, and verapamil.
The study complies with the Helsinki Declaration and was approved by the Ethics Committee of the Seoul National University Hospital (IRB #: H-1408-082-604). Written informed consents were obtained from all patients.
Data collection
Demographic information on the cause of chronic kidney disease (CKD), mode and duration of dialysis, concomitant drugs, and biochemical parameter levels of serum calcium, phosphorus, alkaline phosphatase, iPTH, creatinine, estimated glomerular filtration rate using Modification of Diet in Renal Disease equation, albumin, and hemoglobin at the start and during 3 months of cinacalcet treatment was obtained. Responders and nonresponders were grouped by their serum iPTH changes. Nonresponders were grouped as the patients whose iPTH levels increased even after 3 months of cinacalcet treatment.Citation33,Citation34 Otherwise, those patients whose iPTH values showed any reduction during the period of 3-month cinacalcet treatment were defined as responders.
Serum calcium and phosphate levels were measured by standard methods; iPTH was measured using a double-antibody immunoradiometric assay for the quantitative determination of intact biological chain of 84 amino acids of PTH in human serum (ELSA PTH, Cisbio Bioassays, Codolet, France). Serum calcium was reported as albumin corrected values throughout this research.
Genotype selection and analysis
Twenty-five SNPs in nine genes which are previously known as related to calcium, phosphate, and bone metabolism were selected from the results of previous literatures and databases. Of these genes, CASRCitation26–Citation29,Citation35–Citation37 and VDRCitation30–Citation32,Citation38,Citation39 involved in calcium regulation; FGFR1,Citation40,Citation41 KL,Citation42 RGS14,Citation43,Citation44 and SLC34A1Citation45 related to phosphate serum concentration; and ALPL,Citation46,Citation47 NR4A2,Citation48,Citation49 and PTHLHCitation50–Citation53 related to bone formation were evaluated. Peripheral blood samples were collected in ethylenediaminetetraacetic acid (EDTA) containing tubes and stored at −80°C before DNA isolation. The SNaPshot assay was performed according to the manufacturer’s instructions (ABI PRISM SNaPShot Multiplex kit, Thermo Fischer Scientific, Waltham, MA, USA). shows the included SNPs and the primer sets and melting temperature used for the SNaPshot assay. Twenty-four studied SNPs of CASR, VDR, FGFR1, KL, ALPL, RGS14, NR4A2, and PTHLH were in Hardy–Weinberg Equilibrium, but SLC34A1 rs3812035 was not. Thus, association analysis of SLC34A1 was excluded.
Statistical analysis
The Hardy–Weinberg Equilibrium of each SNP was tested using the goodness-of-fit chi-square test to compare the expected frequencies of genotypes in controls; SNPs with P>0.05 were considered to be in Hardy–Weinberg Equilibrium. The independent-samples t-test and Mann–Whitney test were used to determine the differences between parametric and nonparametric characteristics of patients and biochemical parameter changes. The chi-square or Fisher’s exact test was performed to assess the difference between categorical variables of the patient characteristics. Unconditional logistic regression analysis and chi-square or Fisher’s exact test were used to evaluate the frequency analysis of polymorphisms of the genes and the relationships between different genotypes and cinacalcet response. Statistical analysis was carried out using IBM SPSS Statistics 21.0 for Windows (SPSS Inc., IBM Corporation, Armonk, NY, USA). Haplotypes and haplotype frequencies were calculated using Haploview software (version 4.2, Massachusetts Institute of Technology, Cambridge, MA, USA). The haplotype with P<0.05 was considered statistically significant.
Results
Patient data
A total of 70 dialysis patients with SHPT were included in the biochemical parameter analysis. Baseline characteristics were well balanced between the responders and nonresponders except for baseline calcium levels (P=0.001). The baseline iPTH for 68 patients was 622 (300–1,493) pg/mL versus 601 (316–1,183) pg/mL (P=0.375) for responders versus nonresponders, respectively. The parathyroid gland hyperplasia or adenomatous state did not differ between two groups (P=0.726). The amount of vitamin D (calcitriol or paricalcitol) taken by the patients (P=0.116) and the number of patients on phosphate binders and vitamin D analogues were not different between the two groups (P=0.116 and P=1.000, respectively) (). The maintenance dose of cinacalcet ranged from 12.5 to 100 mg per day, depending on patient’s status. The dose was higher in nonresponders: responders 25 (12.5–75) mg/day versus nonresponders 50 (25–100) mg/day (P<0.001).
Table 1 Demographic characteristics at baseline for included subjects (n=70)
Biochemical parameter changes
The biochemical parameter changes between baseline and 3-month treatment are summarized in . Cinacalcet significantly reduced serum iPTH levels in 53 responders. The percent iPTH changes between baseline and 3-month treatment were −42.29 (−97.41 to 9.23) % versus 6.16 (0.90 to 45.25) % in responders versus nonresponders, respectively (P<0.001). At 3-month treatment, 39.6% (21/53) of responders had an iPTH level below 300 pg/mL, the level recommended by the K/DOQI guidelines.Citation9 The percent change of serum calcium concentration at 3-month treatment was −5.75 (−25.67 to 15.81) % in responders compared to −7.13 (−19.09 to 1.67) % in nonresponders (P=0.182). The percent change of serum phosphorus reduction in responders was −9.84 (−69.23 to 69.43) % compared to −8.42 (−33.33 to72.43) % in nonresponders (P=0.170).
Table 2 Comparison of biochemical parameter percent changes between baseline and 3-month treatment with cinacalcet (n=68)
Frequency analysis of genetic polymorphisms
The loci, allele, and minor allele frequency (MAF) results are summarized in . The observed MAFs in CASR rs1042636 and rs1802757 were 45.8% and 35.8%, respectively. VDR rs7975232 (Apa 1) and rs1544410 (bsm 1) were 16.0% and 6.3%, respectively, whereas rs2228570 (Fok 1) was 38.2%.
Table 3 The loci and minor allele frequency (MAF) information of the 24 SNPs in CASR, VDR, FGFR1, KL, RGS14, SLC34A1, ALPL, NR4A2, and PTHLH genes
Association with cinacalcet response
Association of 24 SNPs with iPTH
The association of 24 SNPs with cinacalcet response was evaluated (). The chi-square test showed that CASR rs1042636 had a significant difference in genotype frequencies between responder and nonresponders (P=0.036) but the MAF was not significant (P=0.075). The chi-square test also proved that CASR rs1042636 had a significant association with cinacalcet response (odds ratio [OR]: 0.267, P=0.035) in the dominant genetic model (). Unconditional logistic regression analysis showed that CASR rs1042636 (OR: 0.074, P=0.025) (95% confidence interval [CI]: 0.008–0.721) and rs1802757 (OR: 8.625, P=0.042) (95% CI: 1.077–69.075) had an association with the cinacalcet response in the genotype model, indicating that the A to G substitution of rs1042636 decreased the risk of nonresponse by 93%, whereas the C to T substitution of rs1802757 increased the risk of nonresponse by approximately eight times. After adjusting for age, sex, and baseline calcium level, CASR rs1042636 (OR: 0.066, P=0.027) and rs1802757 (OR: 10.532, P=0.042) were associated with cinacalcet response.
Table 4 Comparisons of genotypes and allele frequencies between responders versus nonresponders group
Table 5 Association of PTH regulation by cinacalcet with SNPs of CASR, VDR, FGFR1, KL, ALPL, RGS14, NR4A2, and PTHLH
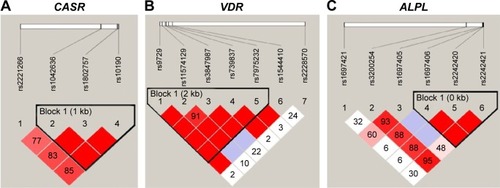
Association of CASR haplotypes with iPTH
The distribution of haplotypes was constructed for three genes and assessed for the association with the cinacalcet response. Linkage disequilibrium structures were designated by the D’ value shown in . Each of CASR, VDR, and ALPL genes included one haplotype block. The correlation coefficient (r2) between rs1042636 and rs1802757, rs1042636 and rs10190, and rs1802757 and rs10190 was 0.68, 0.77, and 0.87, respectively, and showed high correlations. The CASR haplotypes of GCC (OR: 0.355, P=0.015) and ATT (OR: 2.769, P=0.014) were associated with cinacalcet response. With regard to the haplotypes of VDR and ALPL, no significant association with cinacalcet response was found ().
Table 6 Association of PTH regulation by cinacalcet with CASR, VDR, and ALPL haplotypes
Figure 1 The haplotype blocks of the three studied genes: (A) CASR, (B) VDR, and (C) ALPL.
Abbreviation: SNPs, single nucleotide polymorphisms.
Association of CASR genotypes with biochemical parameters
The associations between CASR genotypes and biochemical parameter changes were evaluated in 70 patients. The parameters showed that 3-month cinacalcet treatment lowered overall levels of serum calcium, −6.64 (−19.09 to 15.81) mg/dL, phosphate, −8.13 (−43.69 to 69.43) mg/dL, and Ca × P, −12.22 (−44.87 to 68.70) mg2/dL2 but the level of alkaline phosphatase, 2.36 (−28.48 to 146.58) mg/dL was increased. None of the genotypes of CASR was associated with the biochemical parameter changes ().
Discussion
In this study, the genotype frequencies of the SNPs related to PTH regulation and the association with cinacalcet response were evaluated. The observed MAFs in CASR rs1042636 and rs1802757 were double the reported frequency in HapMapCitation54 whereas VDR rs7975232 (Apa I) and rs1544410 (bsm I) showed a lower frequency than that of HapMap. The rs2228570 (Fok 1) showed a similar frequency. Furthermore, the novel findings of our study are that genetic polymorphisms of CASR rs1802757 besides rs1042636 and haplotypes of rs1042636, rs10190, and rs1802757 were significantly associated with cinacalcet response. The gain of function nonsynonymous SNP of rs1042636, which is frequently found in the Asian population (MAF: Han Chinese 52.3%, Japanese 55.8%)Citation54 results in different functionalityCitation55 and showed the consistent result with the previous study by Rothe et al.Citation26 The study suggested that an Asian dialysis patient carrying a homozygous (G/G) variant of CASR rs1042636 showed significant iPTH reduction after 2-month cinacalcet treatment compared to another six patients having different genotypes. Several other studiesCitation27–Citation29 on CASR also presented the possible association of CASR rs1042636 with PTH level difference. However, the functionality of rs1802757 which is located in 3′ untranslated region (UTR) has not been well documented. We can assume that the rs1802757 influences the microRNAs which have the binding sites within 3′UTR, and can decrease gene expression of various mRNAs.Citation56,Citation57 Other studies presented that CaSR activated by calcimimetics decreased PTH–mRNA stability through the posttranslational modification of the PTH–mRNA binding protein AUF1.Citation58 The rs1802757 can be investigated further via similar mechanism. In particular, the SNPs of rs1042636 and rs1802757 and haplotypes of GCC and ATT occurred at high frequencies of 45.8%, 35.9%, 52.9%, and 37.5%, respectively; in our result, the impact of these variants should not be negligible among Asians.
The regulation of PTH secretion and synthesis involves a multitude of pathways.Citation59 However, in our study, the SNPs of genes other than CASR seemed not to correlate with cinacalcet response. Studies of Casr and Vdr knockout mice can explain part of our results. VDR-deficient mice which developed severe SHPT could be corrected by stabilizing serum calcium concentrations,Citation60 whereas CaSR-deficient mice with SHPT were not corrected by elevated serum calcium and vitamin D levels and normal serum phosphorus,Citation61 which demonstrates that CaSR is the major regulator of PTH secretion and bone abnormalities.Citation62 Thus, other genes related to phosphate and bone metabolism do not have much effect on the PTH regulation by cinacalcet.
The maximum dose of cinacalcet approved in Korea is 100 mg/day and the higher titration dose did not control the PTH level in nonresponders. Previous studies suggested that early response to cinacalcet determined the effectiveness of cinacalcet treatment.Citation63,Citation64
Therefore, our study can suggest that CKD patients who carry T allele of rs1802757 and/or ATT haplotype of rs1042646, rs10190 and rs1802757 have high risk of cinacalcet treatment failure and might be candidates for the parathyroidectomy. There have been pharmacoeconomic issues about the indefinite long-term cinacalcet treatment versus one-time surgery. The research in the 5 European countries by Iannazzo et alCitation65 proved that cinacalcet treatment was beneficial when compared to the standard therapy of vitamin D analogues in cost-effectiveness and cost utility model compared to standard therapy of vitamin D analogs. Komaba et alCitation66 presented that cinacalcet was cost-effective in 99.9% of the Monte Carlo simulations in parathyroidectomy ineligible patients, but only 0.9% of the simulations in parathyroidectomy eligible patients. Thus, nonresponders to cinacalcet treatment can have minimal cost-effectiveness compared to surgery in economic issues.
SHPT patients are highly vulnerable to cardiovascular morbidity and mortality and neither parathyroidectomyCitation67 nor cinacalcetCitation68 reduces the risk of death or major cardiovascular events in SHPT patients undergoing dialysis. Therefore, the timely management of SHPT at the initial stage may offer long-term survival in CKD patients. Several studies have proved the efficacy and safety of cinacalcet in stage 3 and 4 CKD patients with SHPT;Citation69–Citation72 however, the noticeable PTH decrease followed by hypocalcaemia and hyperphosphatemia brought about the avoidance of cinacalcet prescription in CKD predialysis patients.Citation73–Citation75 Since our findings in this study provided a potential target for the investigation of cinacalcet nonresponder group who have high possibility of parathyroidectomy or refractory SHPT, CASR SNPs may be useful biomarkers to administer cinacalcet in early stage of CKD patients to attain the individualized medical treatment of SHPT.
The main factors of cinacalcet resistance after several months or years of therapy are severely enlarged parathyroid gland (>1 cm), nodular hyperplasia,Citation22,Citation76,Citation77 and the downregulation of CaSR and VDR by uremic status which is resulted by inadequate dialysis.Citation78 However, the parathyroid hyperplasia did not affect the cinacalcet nonresponsiveness in our short-term study as in previous studies.Citation79,Citation80 The dose of vitamin D was not different between two groups and findings from the study by Block et alCitation81 presented that cinacalcet reduced PTH levels regardless of whether the vitamin D doses were increased, decreased, or unchanged. We performed multiple testing by Bonferroni correction and false discovery rate test. The result turned out that the association of rs1042636 or rs1802757 with PTH change was not significant. However, neither of these methods take into account the correlation of SNPs due to linkage disequilibrium, which tags the genetic variation across gene regions. Our result showed that the three SNPs of CASR, five SNPs of VDR, and three SNPs of ALPL comprise one haploblock each, representing that the SNPs are not independent and highly correlated. Thus, we rather not reflect the multiple testing in the results.
Our study has several limitations as follows. The first, 70 Korean patients are relatively small for genetic association study and diverse ethnic factors could not be considered. This limitation should warrant further large-scale studies to replicate and confirm our findings. The second, the included patients were on either hemodialysis or peritoneal dialysis; the Kt/V(Kurea ×Td/Vurea) value which indicates the dialysis adequacy is not recirpocally conversed between two modalities, so the influence of dialysis could not be evaluated in our study. The third, we focused on the 3-month treatment of cinacalcet, and the result of long-term follow-up study on the mineral bone disease and cardiovascular disease could not be assessed. The last, most patients were on either phosphate binders or vitamin D analogs and the proportion of patients on either drug was not different between two groups, but the possible impact of different amount of concomitant calcium intake cannot be ruled out.
Conclusion
We obtained supporting evidence for an association between cinacalcet response and CASR polymorphisms. We performed a comprehensive search for SNPs within genes associated with PTH regulations to find the precise frequencies and association with cinacalcet response in 70 dialytic SHPT patients for the first time in Korea. CASR SNPs rs1802757, rs1042636, and haplotypes of rs1042636, rs10190, and rs1802757 were significantly associated with cinacalcet response variance.
Acknowledgments
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2014R1A1A2055734) and BK21 Plus Program in 2015.
Supplementary materials
Table S1 The primer sets and melting temperature (Tm) used for the SNaPshot assay
Table S2 Association between biochemical parameters and the genotypesTable Footnote* of four SNPs of CASR
Disclosure
The authors report no conflicts of interest in this work.
References
- DelmezJASlatopolskyEHyperphosphatemia: its consequences and treatment in patients with chronic renal diseaseAm J Kidney Dis1992193033171562018
- WinchesterJFRotellarCGogginsMRobinoDRakowskiTAArgyWPCalcium and phosphate balance in dialysis patientsKidney Int Suppl199341S174S1788320914
- LevinAThompsonCREthierJLeft ventricular mass index increase in early renal disease: impact of decline in hemoglobinAm J Kidney Dis19993412513410401026
- HarnettJDKentGMBarrePETaylorRParfreyPSRisk factors for the development of left ventricular hypertrophy in a prospectively followed cohort of dialysis patientsJ Am Soc Nephrol1994148614908161730
- LocatelliFCannata-AndiaJDruekeTManagement of disturbances of calcium and phosphate metabolism and chronic renal insufficiency with emphasis on the control of hyperphosphatemiaNephrol Dial Transplant20021772373111981055
- PadhiDHarrisRClinical pharmacokinetic and pharmacodynamic profile of cinacalcet hydrochlorideClin Pharmacokinet200948530331119566113
- DruekeTBModulation and action of the calcium sensing receptorNephrol Dial Transplant200419suppl 5V20V2615284356
- NaganoNNemethEFFunctional proteins involved in regulation of intracellular Ca(21) for drug development: the extracellular calcium receptor and an innovative medical approach to control secondary hyperparathyroidism by calcimimeticsJ Pharmacol Sci20059735536015781990
- National Kidney Foundation:K/DOQI Clinical Practice Guidelines for Bone Metabolism and Disease in Chronic Kidney DiseaseAm J Kidney Dis200342suppl 3S1S20114520607
- QuarlesLDCinacalcet HCl: a novel treatment for secondary hyperparathyroidism in stage 5 chronic kidney diseaseKidney Int Suppl200568Supplement 96s24s2815954947
- CunninghamJDaneseMOlsonKKlassenPChertowGEffects of the calcimimetic cinacalcet HCl on cardiovascular disease, fracture, and health-related quality of life in secondary hyperparathyroidismKidney Int2005684179316164656
- KruseAEisenbergerUFreyFMohauptMThe calcimimetic cinacalcet normalizes serum calcium in renal transplant patients with persistent hyperparathyroidismNephrol Dial Transplant2005201311131415941846
- YangRFreemanKReinkeCTertiary hyperparathyroidism in kidney transplant recipients: characteristics of patients selected for different treatment strategiesTransplantation201294707622706321
- GoodmanWHladikGTurnerSThe calcimimetic agent AMG 073 lowers plasma parathyroid hormone levels in hemodialysis patients with secondary hyperparathyroidismJ Am Soc Nephrol2002131017102411912261
- WüthrichRMartinDBilezikianJThe role of calcimimetics in the treatment of hyperparathyroidismEur J Clin Invest20073791592218036025
- BallingerAEPalmerSCNistorICraigJCStrippoliGFCalcimimetics for secondary hyperparathyroidism in chronic kidney disease patientsCochrane Database of Syst Rev201412182
- KuczeraPAdamczakMWięcekASafety and efficiency of treatment with cinacalcet of haemodialysed patients with chronic kidney disease and secondary hyperparathyroidismEndokrynol Pol201364317618123873419
- LiDShaoLZhouHJiangWZhangWXuYThe efficacy of cinacalcet combined with conventional therapy on bone and mineral metabolism in dialysis patients with secondary hyperparathyroidism: a meta-analysisEndocrine2013431687722669774
- QuarlesLDSherrardDJAdlerSThe calcimimetic AMG 073 as a potential treatment for secondary hyperparathyroidism of end-stage renal diseaseJ Am Soc Nephrol20031457558312595492
- LafranceJCardinalHLeblancMEffect of cinacalcet availability and formulary listing on parathyroidectomy rate trendsBMC Nephrol20131410023642012
- WetmoreJGurevichKSpragueSA randomized trial of cinacalcet versus vitamin D analogs as monotherapy in secondary hyperparathyroidism (PARADIGM)Clin J Am Soc Nephrol20151061031104025904755
- OkadaMTominagaYIzumiKTertiary hyperparathyroidism resistant to cinacalcet treatmentTher Apher Dial201115Suppl 1333721595850
- GogusevJDuchambonPHoryBDepressed expression of calcium receptor in parathyroid gland tissue of patients with hyperparathyroidismKidney Int19975113283368995751
- FukudaNTanakaHTominagaYFukagawaMKurokawaKSeinoYDecreased 1,25-dihydroxyvitamin D3 receptor density is associated with a more severe form of parathyroid hyperplasia in chronic uremic patientsJ Clin Invest199392143614428397225
- KiforOMooreFJWangPReduced immunostaining for the extracellular Ca2+-sensing receptor in primary and uremic secondary hyperparathyroidismJ Clin Endocrinol Metab199681159816068636374
- RotheHMShapiroWBSunWYChouSYCalcium-sensing receptor gene polymorphism Arg990Gly and its possible effect on response to cinacalcet HClPharmacogenet Genomics200515293415864123
- YanoSSugimotoTKanzawaMAssociation of polymorphic alleles of the calcium-sensing receptor gene with parathyroid hormone secretion in hemodialysis patientsNephron200085431732310940742
- YokoyamaKShigematsuTTsukadaTCalcium-sensing receptor gene polymorphism affects the parathyroid response to moderate hypercalcemic suppression in patients with end-stage renal diseaseClin Nephrol200257213113511863123
- ErenPTuranKBerberIThe clinical significance of parathyroid tissue calcium sensing receptor gene polymorphisms and expression levels in end-stage renal disease patientsClin Nephrol200972211412119640368
- ChudekJKarkoszkaHSchmidt-GaykHRitzEKokotFPlasma parathyroid hormone, phosphatemia and vitamin D receptor genotype: are they interrelated?J Nephrol2000131545810720215
- YokoyamaKShigematsuTTsukadaTApa I polymorphism in the vitamin D receptor gene may affect the parathyroid response in Japanese with end-stage renal diseaseKidney Int19985324544589461106
- OzdemirFNSezerSAtacBVitamin D receptor BsmI and TagI gene polymorphisms in a Turkish ESRD population: influences on parathyroid hormone responseTransplant Proc20053772922292416213262
- Management of secondary hyperparathyroidism and mineral metabolism abnormalities in dialysis patientsWolters Kluwer2015 Available from: www.uptodate.com/contents/management-of-secondary-hyperparathyroidism-and-mineral-metabolism-abnormalities-in-dialysis-patients?source=search_result&search=treatment+failure+secondary+hyperparathyroidism&selectedTitle=1~150Accessed December 9
- CunninghamJLocatelliFRodriguezMSecondary hyperparathyroidism: pathogenesis, disease progression, and therapeutic optionsClin J Am Soc Nephrol2011691392121454719
- YamauchiMSugimotoTYamaguchiTAssociation of polymorphic alleles of the calcium-sensing receptor gene with the clinical severity of primary hyperparathyroidismClin Endocrinol (Oxf)20015537337911589681
- FilopantiMVergaUErmeticiFMEN1-related hyperparathyroidism: response to cinacalcet and its relationship with the calcium-sensing receptor gene variant Arg990GlyEur J Endocrinol2012167215716422577108
- HanGWangONieMClinical phenotypes of Chinese primary hyperparathyroidism patients are associated with the calcium-sensing receptor gene R990G polymorphismEur J Endocrinol2013169562963823946278
- YokoyamaKShigematsuTKagamiSVitamin D receptor gene polymorphism detected by digestion with Apa I influences the parathyroid response to extracellular calcium in Japanese chronic dialysis patientsNephron200189331532011598396
- KarkoszkaHChudekJStrzelczykPVitamin D receptor gene polymorphism and the rate of bone loss of the femur neck and lumbar spine in hemodialized patients with chronic renal failurePol Merkur Lekarski19982819920210101442
- JunJKKimSMAssociation study of fibroblast growth factor 2 and fibroblast growth factor receptors gene polymorphism in Korean ossification of the posterior longitudinal ligament patientsJ Korean Neurosurg Soc20125271322993671
- GuimarãesJMGuimarãesICDuarteMEPolymorphisms in BMP4 and FGFR1 genes are associated with fracture non-unionJ Ortho Res2013311219711979
- SakanHNakataniKAsaiOReduced renal α-Klotho expression in CKD patients and its effect on renal phosphate handling and vitamin D metabolismPLoS One201491e8630124466013
- KestenbaumBGlazerNLKöttgenACommon genetic variants associate with serum phosphorus concentrationJ Am Soc Nephrol20102171223123220558539
- YasuiTOkadaAUrabeYA replication study for three nephrolithiasis loci at 5q35.3, 7p14.3 and 13q14.1 in the Japanese populationJ Hum Genet20135858859323719187
- BoronWFBoulpaepELMedical Physiology: A Cellular and Molecular ApproachPhiladelphiaSaunders2012
- TsuiHWInmanRDReveilleJDTsuFWLAssociation of a TNAP haplotype with ankylosing spondylitisArthritis Rheum200756123424317195227
- ChengNCaiQFangMNo significant association between genetic polymorphisms in the TNAP gene and ankylosing spondylitis in the Chinese Han populationRheumatol Int200829330531018769922
- MeirTDurlacherKPanZParathyroid hormone activates the orphan nuclear receptor Nurr1 to induce FGF23 transcriptionKidney Int2014861106111524940803
- TetradisSBezouglaiaOTsingotjidouAParathyroid hormone induces expression of the nuclear orphan receptor Nurr1 in bone cellsEndocrinology2001142266367011159837
- NakajimaKTamaiMOkaniwaSHumoral hypercalcemia associated with gastric carcinoma secreting parathyroid hormone: a case report and review of the literatureEndocr J201360555756223303131
- TenneMMcGuiganFJanssonLGenetic variation in the PTH pathway and bone phenotypes in elderly women: evaluation of PTH, PTHLH, PTHR1 and PTHR2 genesBone200842471972718280230
- TruongNUde B EdwardesMDPapavasiliouVGoltzmanDKremerRParathyroid hormone-related peptide and survival of patients with cancer and hypercalcemiaAm J Med2003115211512112893397
- IwaseMTakemiTManabeMNagumoMHypercalcemia upon recurrence of renal cell carcinoma producing parathyroid hormone-related proteinInt J Oral Maxillofac Surg200332217418012729778
- dbSNP. National Library of Medicine Available from: http://www.ncbi.nlm.nih.gov/snp/Accessed December 9, 2015
- MiyataTMiyazawaSYasunagaTTwo types of amino acid substitutions in protein evolutionJ Mol Evol1979123219236439147
- BarrettLWFletcherSWiltonSDRegulation of eukaryotic gene expression by the untranslated gene regions and other non-coding elementsCell Mol Life Sci201269213613363422538991
- PichonXWilsonLAStoneleyMRNA binding protein/RNA element interactions and the control of translationCurr Protein Pept Sci201213429430422708490
- LeviRBen DovILavi-MoshayoffVIncreased parathyroid hormone gene expression in secondary hyperparathyroidism of experimental uremia is reversed by calcimimetics: correlation with posttranslational modification of the trans acting factor AUF1J Am Soc Nephrol20061710711216291838
- GilbertSJWeinerDEGipsonDSPerazellaMATonelliMNational Kidney Foundation’s Primer on Kidney Disease6th edPhiladelphiaElsevier2014
- LiYAmlingMPirroANormalization of mineral ion homeostasis by dietary means prevents hyperparathyroidism, rickets and osteomalacia, but not alopecia in vitamin D receptor ablated miceEndocrinology1998139439143969751523
- GarnerSPiMTuQQuarlesLRickets in cation sensing receptor deficient mice: an unexpected skeletal phenotypeEndocrinology200114293996400511517179
- BlockGAThe impact of calcimimetics on mineral metabolism and secondary hyperparathyroidism in end-stage renal diseaseKidney Int Suppl200364Suppl 87S131S13614531786
- TorresPSUtielFJBPeralesMCSCortésMJGBaldánMMBBañascoVPAnalysis of efficacy and factors that impact the response of secondary hyperparathyroidism to cinacalcet in haemodialysis patientsNefrologia201030444345120651886
- KimJKKwonYJChoiKHRapid decrease of intact parathyroid hormone could be a predictor of better response to cinacalcet in hemodialysis patientsYonsei Med J201354245346323364981
- IannazzoSCarsiMChiroliSA cost-utility analysis of cinacalcet in secondary hyperparathyroidism in five European countriesAppl Health Econ Health Policy201210212713822268372
- KomabaHMoriwakiKGotoSCost-effectiveness of cinacalcet hydrochloride for hemodialysis patients with severe secondary hyperparathyroidism in JapanAm J Kidney Dis201260226227122445709
- ConzoGPernaAFSavicaVImpact of parathyroidectomy on cardiovascular outcomes and survival in chronic hemodialysis patients with secondary hyperparathyroidism. A retrospective study of 50 cases prior to the calcimimetics eraBMC Surg201313Suppl 2S2S424268048
- EVOLVE InvestigatorsChertowGMBlockGAEffect of cinacalcet on cardiovascular disease in patients undergoing dialysisN Engl J Med2012367262482249423121374
- CharytanCCoburnJWChoncholMCinacalcet hydrochloride is an effective treatment for secondary hyperparathyroidism in patients with CKD not receiving dialysisAm J Kidney Dis200546586715983958
- ChoncholMLocatelliFAbboudHEA randomized, double-blind, placebo-controlled study to assess the efficacy and safety of cinacalcet HCl in participants with CKD not receiving dialysisAm J Kidney Dis20095319720719110359
- MontenegroJCornagoIGallardoIEfficacy and safety of cinacalcet for the treatment of secondary hyperparathyroidism in patients with advanced chronic kidney disease before initiation of regular dialysisNephrology201217263122022934
- de FranciscoALPiñeraCPalomarRCinacalcet should be used to treat secondary hyperparathyroidism in stage 3–4 chronic kidney diseaseNat Clin Pract Nephrol2008436636718506170
- CoyneDWCinacalcet should not be used to treat secondary hyperparathyroidism in stage 3–4 chronic kidney diseaseNat Clin Pract Nephrol2008436436518477990
- Cannata-AndíaJBFernández-MartínJLMineral metabolism: should cinacalcet be used in patients who are not on dialysis?Nat Rev Nephrol2009530730819474823
- Kalantar-ZadehKKovesdyCPIs it worth correcting hyperparathyroidism if hyperphosphatemia and hypocalcemia worsen? A cinacalcet storyAm J Kidney Dis20095318318819166796
- ShobackDBilezikianJBinderTGravesTTurnerSPeacockMCalcimimetic AMG 073 normalizes total serum calcium in patients with primary hyperparathyroidism [abstract]J Bone Miner Res200015210
- IndridasonOSHeathHKhoslaSYohayDAQuarlesLDNon- suppressible parathyroid hormone secretion is related to gland size in uremic secondary hyperparathyroidismKidney Int199650166316718914034
- LewinEGarfiaBRecioFRodriguezMOlgaardKPersistent downregulation of calcium-sensing receptor mRNA in rat parathyroids when severe secondary hyperparathyroidism is reversed by an isogenic kidney transplantationJ Am Soc Nephrol20021382110211612138143
- KomabaHNakanishiSFujimoriACinacalcet effectively reduces parathyroid hormone secretion and gland volume regardless of pretreatment gland size in patients with secondary hyperparathyroidismClin J Am Soc Nephrol201052305231420798251
- MeolaMPetrucciIBarsottiGLong-term treatment with cinacalcet and conventional therapy reduces parathyroid hyperplasia in severe secondary hyperparathyroidismNephrol Dial Transplant20092498298919181759
- BlockGMartinKde FranciscoACinacalcet for secondary hyperparathyroidism in patients receiving hemodialysisN Engl J Med2004350151516152515071126
