Abstract
Background
Rosmarinic acid (RA) is a natural substance that may be useful for treating diabetes mellitus. The present study investigated the effects of RA on glucose homeostasis and insulin regulation in rats with streptozocin (STZ)-induced type 1 diabetes or high-fat diet (HFD)-induced type 2 diabetes.
Methods
Glucose homeostasis was determined using oral glucose tolerance tests and postprandial glucose tests, and insulin activity was evaluated using insulin tolerance tests and the homeostatic model assessment for insulin resistance. Additionally, the protein expression levels of PEPCK and GLUT4 were determined using Western blot analysis.
Results
RA administration exerted a marked hypoglycemic effect on STZ-induced diabetic rats and enhanced glucose utilization and insulin sensitivity in HFD-fed diabetic rats. These effects of RA were dose-dependent. Meanwhile, RA administration reversed the STZ- and HFD-induced increase in PEPCK expression in the liver and the STZ- and HFD-induced decrease in GLUT4 expression in skeletal muscle.
Conclusion
RA reduces hyperglycemia and ameliorates insulin sensitivity by decreasing PEPCK expression and increasing GLUT4 expression.
Introduction
Diabetes mellitus (DM) is one of the most common metabolic disorders. Its prevalence has grown as a result of the increased consumption of high-calorie foods and the increased adoption of a sedentary lifestyle. Pharmacological approaches to this disease frequently induce drug-related problems that are primarily seen in patients receiving multiple drugs to control their medical conditions. Complementary or alternative treatments, such as herbal compounds, are frequently used to treat diabetes.Citation1 In fact, one of the most commonly used drugs, metformin, is plant derived. Therefore, herbal medicine may be a promising source of compounds that are more effective and less harmful than existing drugs and that may constitute innovative remedies for treating DM.
Rosmarinic acid (RA) is a potent antioxidant that is present in many common culinary herbs, such as those that belong to Lamiaceae, Boraginaceae, and Anthoc-erotaceae families. RA was first isolated from rosemary (Rosmarinus officinalis), a widely used culinary plant.Citation2 Recently, studies examining the utility of RA to treat diabetic conditions have suggested that RA may reduce diabetes-induced disorders and complications. In diabetic animals, initial studies showed that RA may control plasma glucose by modulating SGLT1 trafficking to the intestinal brush-border membraneCitation3 to ameliorate hyperglycemia (HG).Citation4 Moreover, RA has been shown to protect endothelial functionsCitation5 and to prevent diabetes-induced sexual disorders by elevating serum testosterone levels,Citation6 primarily via its antioxidative effects. Furthermore, RA protects against diabetic glomerular deteriorationCitation7 and nephropathy,Citation7,Citation8 and it confers neuroprotective effects under ischemic conditionsCitation9 and in diabetic neuropathy.Citation10 Additionally, it mitigates oxidative diabetes-induced brain damage.Citation11 RA also protects pancreatic cells from glucolipotoxicity.Citation12 These findings indicate that RA may ameliorate DM-induced disorders and complications. However, the mechanism(s) potentially underlying the RA-induced amelioration of DM remains unclear.
To explore the role of RA in diabetes treatments, the present study investigated the effect of RA on glucose homeostasis and insulin regulation in two animal models of diabetes: streptozocin (STZ)-induced type 1-like diabetes in rats and high-fat diet (HFD)-induced type 2-like diabetes in rats. We performed oral glucose tolerance tests (OGTTs) and postprandial glucose tests (PGTs) to evaluate glucose utilization, and we used insulin tolerance tests (ITTs) to evaluate insulin sensitivity and the homeostatic model assessment (HOMA) to characterize insulin resistance. Additionally, the protein expression levels of PEPCK and GLUT4 were determined using Western blot analysis to investigate the potential mechanism(s) underlying the effects of RA on glucose homeostasis.
Materials and methods
Animals
Seven-week-old male Wistar rats weighing 170–210 g were obtained from Japan SLC, Inc. (Hamamatsu, Japan). These rats were housed individually in a pathogen-free, temperature-(25°C) and humidity-controlled room on a 12-hour light/12-hour dark cycle (lights on at 7 am) at the animal center of Kagoshima University (Kagoshima, Japan). The animals had free access to food and tap water. All experimental procedures were approved by the Ethics Committee for Animal Care and Use of Kagoshima University (institutional review board approval number MD15017) and conducted in accordance with the Japanese National Standardized Guidelines for Animal Experiments of Kagoshima University.
Induction of diabetes
After animals had fasted (48 hours), type 1-like diabetes was induced using a single intraperitoneal injection of STZ (65 mg/kg; Sigma-Aldrich, St Louis, MO, USA) that was freshly dissolved in a citrate buffer (10 mM, pH 4.5). On the seventh day after STZ administration, blood glucose concentrations were evaluated in samples obtained from tails using a blood glucose meter. Only rats with a basal blood glucose level >300 mg/dL were considered diabetic.
An insulin-resistant model was induced by feeding the rats with a HFD (60% kcal fat; D12492; Research Diets Inc., New Brunswick, NJ, USA) ad libitum for 8 weeks. The altered animals were then validated: HFD rats showed significant gains in body weight, accumulated body fat, and impaired glucose tolerance (fasting glucose ≥130 mg/dL).
Experimental procedure and drug administration
Once HG was achieved (DM1 ≥300 mg/dL, HFD ≥130 mg/dL), the animals were assigned to a working group (n=8), and each group was treated with RA at the indicated dose for 7 days. RA (≥98% purified by high-performance liquid chromatography; Sigma-Aldrich) was dissolved in saline and administrated via intraperitoneal injection once a day for 7 days. The control group was administered saline. Additionally, to investigate the chronic effects of RA, additional STZ and HFD groups that displayed HG were treated with RA at the indicated dose for 28 days. Then, changes in body weight and variations in daily food and water intake were evaluated on the 28th day and compared to the values that were obtained on the first day prior to treatment with RA.
Once treatment was complete, the animals were fasted for 8 hours and then anesthetized using an intraperitoneal injection of pentobarbital (150 mg/kg). After blood samples were obtained via heart puncture, the animals were sacrificed using cervical dislocation. Serum was collected in heparin-ized tubes containing heparin and maintained on ice. The samples were centrifuged at 3,000× g for 15 minutes at 4°C, and then the top yellow plasma layer was carefully pipetted and placed in a new tube. Liver samples were also collected from the right ventral lobe, and skeletal muscle samples were taken from soleus muscle. All samples were frozen in isopentane, cooled in liquid nitrogen, and stored at -80°C until further processing. To investigate its chronic effects, RA was administered to rats for 28 days after a confirmation of diabetes, and food and water intake was measured during the RA administration period. No infusion-related side effects were observed during RA treatment.
Measurements of biochemical parameters
To study the effectiveness of RA at different doses in modulating hypoglycemic activity, postprandial blood glucose (PGT) was estimated daily during the RA treatment period in the STZ rats. Fasting animals (8 hours) were fed a bolus of 180 mg/dL of glucose, and 2 hours later, their blood was drained and tested to determine the concentration of glucose.
To understand the acute effects of RA on glucose modulation, OGTTs were performed in the normal and STZ groups. The rats were fasted for 8 hours, and D-glucose (2 g/kg) was then administered via intragastric gavage to each rat. Blood samples were drawn from the tail vein of each rat at 0, 30, 60, 120, and 180 minutes after the administration of glucose, and glucose levels were measured. The OGTT was performed after 7 days of treatment with RA. The area-under-the-curve (AUC) value of glucose was determined using the total AUC from the sampling period from 0 to 120 minutes.
Plasma glucose levels were measured using a blood glucose meter (NIPRO, Osaka, Japan), and insulin levels were determined using enzyme-linked immunosorbent assay (ELISA) with a rat insulin ELISA kit (Mercodia AB, Uppsala, Sweden) according to the manufacturer’s instructions. To determine whether RA treatment improved insulin resistance, we assessed the results of the ITT and the HOMA for insulin resistance (HOMA-IR) on the first and last days of RA treatment. In the ITT experiment, after the rats were fasted for 8 hours, they were intraperitoneally injected with insulin at 0.5 IU/kg body weight, and blood samples were collected at 0, 15, 30, and 60 minutes to measure glucose levels. The HOMA index was calculated as the fasting glucose concentration (mmol/L) × the fasting insulin concentration (U/mL)/22.5, with the insulin resistance of normal subjects assumed to be 1, as previously described.Citation13
Western blot analysis
The expression of PEPCK and GLUT4 was characterized using Western blot analysis as previously described.Citation14 Frozen muscle samples (100 mg) were homogenized in 1 mL of lysis buffer. The homogenate was centrifuged at 1,000× g for 5 minutes to pellet the nuclei and insoluble material. The supernatant was moved to a new tube and further centrifuged at 100,000× g for 1 hour to pellet the membranes. The supernatant from this step was collected as the cytosolic fraction that was used in the assay. Protein concentrations were measured using bicinchoninic acid protein assay methods (Thermo Fisher Scientific, Waltham, MA, USA). Protein samples were filtered and transferred to membranes via sodium dodecyl sulfate-polyacrylamide gel electrophoresis (10% acrylamide gels) in a Bio-Rad Trans-Blot system (Bio-Rad Laboratories, Inc., Tokyo, Japan). The membranes were submerged in 5% nonfat milk in Tris-buffered saline containing 0.1% Tween 20 (TBS-T) for ~1 hour. They were then washed in TBS-T and hybridized with primary antibodies (diluted in Tris-buffered saline) for 16 hours. The following specific antibodies were used to characterize protein expression: PEPCK (anti-rabbit, 1:1,000 dilution; Santa Cruz Biotechnology Inc., Dallas, TX, USA) and GLUT4 (anti-mouse, 1:1,000 dilution; Abcam plc, Cambridge, UK). Additionally, the membranes were incubated with an antibody for β-actin (Sigma-Aldrich) (1:5,000 dilution) as the internal control. Incubation with secondary antibodies and antigen–antibody complex detection were performed using an electrochemiluminescence kit (Thermo Fisher Scientific). The immunoblots for PEPCK (62 kDa), GLUT4 (30 kDa), and β-actin (42 kDa) were compared to those for anti-β-actin (1:1,000; Santa Cruz Biotechnology), which was used as an internal control.
Statistical analysis
Statistical analyses were performed using SPSS software (SPSS Inc., Chicago, IL, USA). Between-group differences were analyzed using one-way analysis of variance followed by Dunnett or Tukey–Kramer multiple comparisons tests. A post hoc least significant difference test was used to analyze insulin and HOMA-IR. OGTT responses were analyzed using repeated measures analysis of variance. The results are reported as the mean ± standard error of the mean and were considered significant at P<0.05.
Results
RA improved glucose homeostasis in the diabetic groups
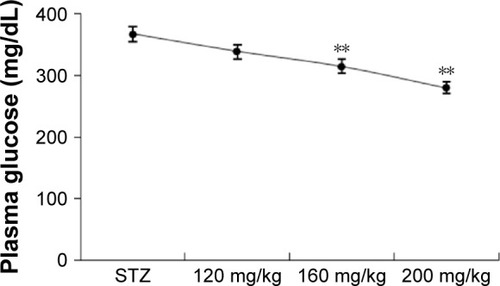
demonstrates that PGT was performed via an intraperitoneal injection of a 20% glucose solution (2 mg/g of body weight) at 1 week after STZ administration. Plasma glucose levels decreased and reached a minimum value at 120 minutes. The higher dose of RA (200 mg/kg) resulted in a lower glucose level than the 160 mg/kg dose. It therefore appears that 200 mg/kg of RA is the most effective dose for affecting postprandial blood glucose in STZ rats. Thus, we found that RA had a pronounced dose-dependent hypoglycemic effect.
Figure 1 Dose-response effects of RA on plasma glucose level in STZ-rats.
Abbreviations: RA, rosmarinic acid; STZ, streptozocin; SEM, standard error of the mean.
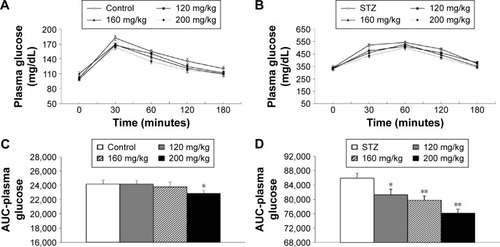
In pharmacology studies, OGTT was performed after the STZ rats and normal rats were treated with RA () to demonstrate the therapeutic efficiency of RA. The OGTT showed that the maximal blood glucose levels at 30 minutes after glucose administration were 183±5.12 mg/dL in normal rats and 522±11.99 mg/dL in diabetic rats. Pretreatment with RA improved glucose tolerance in a dose-dependent manner () and significantly reduced the calculated relative area under the glucose concentration curve (AUC) () in both normal and diabetic rats.
Figure 2 Effect of RA on the OGTT in normal and STZ rats.
Abbreviations: RA, rosmarinic acid; OGTT, oral glucose tolerance test; STZ, streptozocin; AUC, area under the curve; SEM, standard error of the mean.
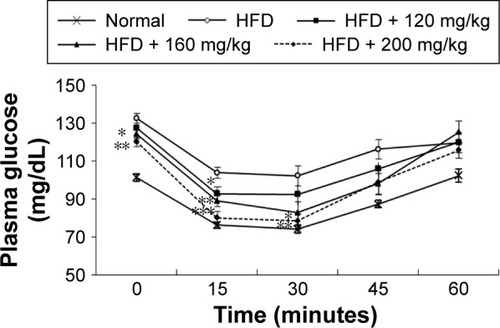
In the HFD group, ITT was conducted once a hyperglycemic condition was confirmed to determine the effect of RA on insulin sensitivity. The blood glucose level in the RA-administered group (160 and 200 mg/kg) was significantly different and remained lower after insulin loading than the level in the diabetic rats at the 15- and 30-minute time points (). These results indicate that RA enhanced insulin sensitivity in diabetic rats. The effect of RA on insulin sensitivity was greater at higher doses.
Figure 3 Insulin tolerance test in HFD-fed diabetic rats after 7 days of RA treatment at the indicated doses.
Abbreviations: HFD, high-fat diet; RA, rosmarinic acid; SEM, standard error of the mean.
RA reversed insulin resistance in the insulin-resistant group
Plasma glucose and insulin levels were measured to determine the effect of RA on insulin sensitivity in HFD-fed rats. Before the administration of RA, the rats in all groups were confirmed to be markedly hyperglycemic, with fasting plasma glucose levels >130 mg/dL. The insulin and plasma glucose levels after 7 days of RA treatment were significantly lower than the levels in the control group (insulin 537.76±20.71 vs 409.79±26.49 pmol/L and fasting plasma glucose 133±3.61 vs 120±2.41 mg/dL, both shown before RA treatment vs after 7 days of RA treatment; P<0.05). ITT was conducted once a hyperglycemic condition was confirmed to determine the effect of RA on insulin sensitivity. Compared to the chow diet group, the HFD-fed rats were insulin resistant. Within the HFD group, the blood glucose level in the RA-administered group (160 and 200 mg/kg) was significantly different after insulin loading and remained lower than the level in the diabetic rats at the 15- and 30-minute time points (). The mean values of the analyzed parameters (insulin, glycemia, HOMA-IR) in the study groups are shown in . These data indicate that RA enhanced insulin sensitivity in diabetic rats. The effect of RA on insulin sensitivity was greater at higher doses.
Table 1 Effect of rosmarinic acid on blood glucose, plasma insulin, and HOMA-IR levels in the experimental groups of rats after a 7-day experimental period
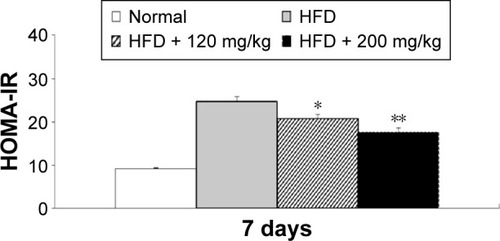
In addition, a comparison of the effects of lowest and highest dosage (120 and 200 mg/kg) of RA revealed a dose-related effect on plasma insulin and glucose levels (data not shown). Furthermore, in the HFD-fed diabetic rats, RA decreased the HOMA-IR index in a dose-related manner (). Therefore, RA treatment for 7 days reversed insulin resistance in HFD-fed rats.
Figure 4 HOMA-IR analysis in HFD-fed diabetic rats after the 7-day experimental period.
Abbreviations: HOMA-IR, homeostatic model assessment for insulin resistance; HFD, high-fat diet; SEM, standard error of the mean.
Effects of RA on hepatic gluconeogenesis activity via PEPCK expression and on insulin sensitivity via GLUT4 expression
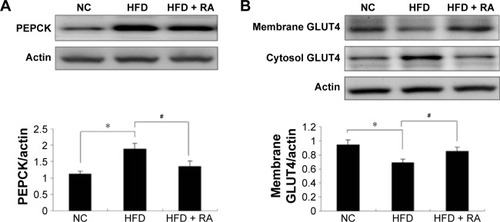
The protein expression levels of PEPCK and GLUT4 were analyzed in STZ-induced diabetic rats and HFD-fed diabetic rats using Western blot analysis. PEPCK expression was markedly increased in isolated liver cells ( and ) in both the STZ-induced diabetic rats and the HFD-fed diabetic rats. RA treatment reduced hepatic PEPCK expression in both groups, suggesting that RA increased gluconeogenesis in the livers of diabetic rats. However, the decrease in PEPCK levels in the STZ group was more significant than the decrease in the HFD group. Additionally, we examined GLUT4 protein expression in muscle tissues to explore changes in glucose uptake (), and we found that GLUT4 expression was significantly lower in both the STZ-induced diabetic rats and the HFD-fed diabetic rats. RA treatment markedly increased GLUT4 expression in both groups. Therefore, RA increased GLUT4 expression in skeletal muscle.
Figure 5 Effect of RA on the PEPCK protein levels in the liver and GLUT4 protein levels in the skeletal muscle of STZ-induced diabetic rats.
Abbreviations: RA, rosmarinic acid; STZ, streptozocin; NC, normal control; SEM, standard error of the mean.
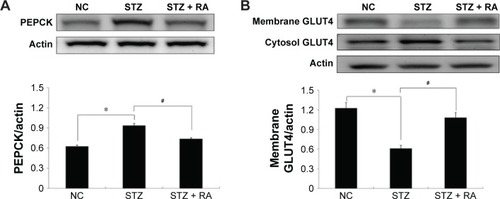
Figure 6 Effect of RA on PEPCK protein levels in the liver and GLUT4 protein levels in the skeletal muscle in HFD rats.
Abbreviations: RA, rosmarinic acid; HFD, high-fat diet; NC, normal control; SEM, standard error of the mean.
Effect of RA on body weight, water intake, and food intake
To investigate the long-term effects of RA in diabetic rats, changes in body weight and daily food and water intake were measured in both the STZ and the HFD groups, and the results are shown in . The administration of RA (120, 160, or 200 mg/kg) for 28 days caused a significant decrease in body weight in the HFD group, but there was no significant difference in daily food or water intake. Furthermore, RA had no significant effect on body weight or daily food or water intake in the normal (control) and STZ groups.
Table 2 Changes in body weight, food intake, and water intake in the experimental groups
Discussion
Diabetic disorders are metabolic diseases that are characterized by prolonged HG, mainly as a result of the dysfunctional secretion and action of insulin. Glucose-stimulated insulin secretion occurs mainly in β-cells in the islets of Langerhans of the pancreas, and this process enhances glucose uptake.Citation15 Therefore, the main diabetes treatment strategies are designed to maintain blood glucose levels, enhance β-cell function, and reduce insulin resistance to prevent complications. Meanwhile, medications used to treat diabetic disorders must not only lower blood glucose levels but also improve glucose homeostasis and insulin sensitivity.Citation16
Chemically, RA (alpha-O-caffeoyl-3,4-dihydroxyphenyl lactic acid) is an ester of caffeic acid (CA) and 3,4-dihydroxyphenyl lactic acid.Citation17 It is a common CA ester often found in plants that are the members of the Lamiaceae and Boraginaceae families.Citation2 RA was originally isolated from rosemary (R. officinalis) by ML Scarpatti and G Oriente in 1958. The chemical structure of RA is a dimer of CA and 3,4-dihydroxyphenyl lactic acid, with phenylpropanoid as the ester linkage. Biologically, it is synthesized from 4-coumaroyl-4′-hydroxyphenyllactate. In plants, RA constitutes an essential preformed defense compound against pathogens and herbivores.Citation2,Citation17 RA can be directly absorbed from the gastrointestinal tract. In rats, oral administration of RA produces seven metabolites in urine, including the intact form of RA and other metabolites, such as trans-CA 4-O-sulfate, trans-ferulic acid 4-O-sulfate, trans-m-coumaric acid 3-O-sulfate, trans-CA, trans-m-coumaric acid, and m-hydroxyphenylpropionic acid.Citation18 These metabolites account for ~31.8% of total excretions in urine at 48 hours after oral administration of RA. Surprisingly, these metabolites are not found in bile, suggesting that RA is mainly excreted in urine.Citation19
Antioxidant supplementation has been shown to improve the overall treatment outcome of diabetes, particularly chronic diabetes. Excessive oxidative stress worsens diabetic conditions and their complications.Citation20 Persistent HG and elevated levels of free fatty acids contribute to oxidative stress and constitute a proximate cause of the onset and progression of diabetes and its complications.Citation13 Therefore, in clinical practice, antioxidants are frequently included in complementary therapeutics.Citation21
RA has been identified as a potent antioxidant.Citation22,Citation23 Extensive studies of RA have demonstrated its therapeutic properties, which mainly stem from its potent antioxidative effects.Citation23 Those therapeutic properties include analgesic and anti-inflammatory effects,Citation24 anticancer effects,Citation25 and antidepressant, anxiolytic, and neuroprotective effects.Citation26,Citation27 RA can also disrupt the amyloid-β conglomerates that are characteristic of Alzheimer’s disease,Citation28 and it has demonstrated antiviralCitation29 as well as antibacterial and nematicidal activities.Citation30 Additionally, no reports, including studies of hepatic and renal function and extensive blood cell counts, have identified any adverse effects of RA.Citation31,Citation32 Taken together, these data suggest that RA is a potential treatment for diabetes, particularly chronic diabetes.
In the present study, we focused on the efficacy of RA in treating diabetes. A previous paper showed that RA had little impact on glucose levels in normal rats.Citation4 Therefore, experiments were conducted to explore the effects of RA in animal models of both type 1 and type 2 diabetes. Injecting STZ at a high dose (>55 mg/kg) can induce type 1-like diabetesCitation33 as a result of damage to pancreatic β-cells, which subsequently causes HG. Therefore, this procedure is frequently implemented to create animal models of type 1 DM.Citation34,Citation35 Furthermore, HFD feeding produces insulin resistanceCitation36 and can be implemented to create animal models of type 2 DM.Citation33,Citation34,Citation37 In the present study, RA was shown to be effective at improving glucose homeostasis and insulin sensitivity in a dose-dependent manner.
The results from the PGTs, OGTTs, and ITTs showed that RA significantly improved glucose utilization and insulin sensitivity in normal, HFD-fed, and STZ-induced diabetic rats, all in a dose-dependent manner. The HOMA-IR provides an accurate measurement of insulin sensitivity in rats.Citation38 The HOMA-IR evolved from the first HOMA, which was introduced in 1985, and included modifications to formulate a model of steady-state insulin and glucose domains by determining the physiological dose responses of insulin secretion and glucose uptake. In this assay, plasma glucose and insulin concentrations were used to estimate peripheral and hepatic glucose uptake and efflux. Thus, cell function defects could be estimated by altering the cell response to plasma glucose levels.Citation39 In the present study, we adopted this model to estimate insulin resistance based on fasting glucose and insulin levels. The results of the HOMA-IR demonstrated that the RA-treated rats showed a significantly higher dose-dependent reduction than the rats in the control group, suggesting that RA has the potential to improve insulin resistance.
The liver is a primary organ that is involved in the regulation of blood glucose levels, and it does this by balancing glucose uptake and storage (glycogenesis) with glucose release (glycogenolysis and gluconeogenesis). Hepatic glucose production (HGP) occurs via the breakdown of glycogen and the de novo synthesis of glucose from noncarbohydrate precursors via gluconeogenesis.Citation40–Citation42 In contrast to acute HG, in which HGP and gluconeogenic gene expression are suppressed, chronic HG is characterized by a progressive increase in basal HGP and is the main form of HG observed in diabetes.Citation42 The rate of gluconeogenesis is modulated by enzymes, including PEPCK, fructose-1,6-biphosphatase, and glucose-6-phosphatase.Citation40–Citation42 However, the initial step in hepatic gluconeogenesis is catalyzed by PEPCK,Citation43 which is upregulated during chronic HG, including when this condition occurs in diabetic patients. Additionally, PEPCK is insulin-independentCitation44 and can therefore be analyzed to investigate the amelioration of HG separately from increased insulin sensitivity. In the present study, the increase in the level of PEPCK expression in the liver was significantly lower after RA treatment. This effect of RA contributed to a reduction in HG in diabetic rats.
Skeletal muscle is a crucial site for glucose disposal activities. Glucose uptake is increased by insulin in skeletal muscles via the triggering of the translocation of GLUT4 from an intracellular deposit site to the membrane. Therefore, impaired GLUT4 translocation increases insulin resistance.Citation45 Acute glucose-induced insulin resistance in rat skeletal muscle results in part from the consequential rapid decrease in GLUT4 in the plasma membrane. Glucose induces a decrease in plasma membrane levels of GLUT4 as a protective mechanism against excessive glucose uptake during HG accompanied by insulin resistance.Citation46 Insulin sensitivity is enhanced by an increase in GLUT4 expression in skeletal muscle.Citation47 In the present study, RA increased GLUT4 expression in skeletal muscle, and this process reversed the development of insulin resistance.
The limitations of the present study are mainly associated with the inclusion of data from only in vivo experiments. The cellular actions of RA were not analyzed in this report. Therefore, the mechanisms underlying the RA-induced increases of PEPCK and GLUT4 expression and the amelioration of HG, glucose levels, and insulin tolerance by upregulation of these proteins require additional studies for clarification. Additionally, many enzymes and pathways contribute to the regulation of HGP. Other key enzymes in the glucose phosphorylation/dephosphorylation, glycogen synthesis/degradation, and glycolysis and gluconeogenesis pathways must be analyzed in future studies. RA possesses antioxidant-like activity, and antioxidants are associated with decreased HG in animals.Citation48,Citation49 Therefore, the attenuation of HG by RA is likely to stem, in part, from its antioxidant-like activity. However, this hypothesis was not addressed in the present study.
Conclusion
The obtained data suggest that RA dose-dependently ameliorates HG and insulin resistance by decreasing PEPCK expression in the liver and increasing GLUT4 expression in muscles. For comparison, the clinical dose of RA in humans is 200 mg/kg,Citation50 and this level has been found to be conducive to most of its favorable biological properties, including anti-inflammatory and antiallergic effects.Citation51 In the present study, a 200 mg/kg dose by body weight in rats was found to be the most effective dose for attaining RA’s antidiabetic properties. This dose is similar to that used in previous studies of RA in ratsCitation52–Citation54 but may not be appropriate for humans. Therefore, RA should be further investigated in clinical trials to evaluate its effectiveness as a new therapeutic agent for treating DM in humans.
Authors contributions
All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Acknowledgments
The authors thank all the staff members of the Institute of Laboratory Animal Sciences at Kagoshima University (Frontier Science Research Center) for maintaining the animals in good condition. This study was supported by the Core Projects of the Diet and Obesity Translational Research of Kagoshima University Graduate School of Medical and Dental Sciences.
Disclosure
The authors report no conflicts of interest in this work.
References
- YehGYEisenbergDMKaptchukTJPhillipsRSSystematic review of herbs and dietary supplements for glycemic control in diabetesDiabetes Care20032641277129412663610
- PetersenMRosmarinic acid: new aspectsPhytochem Rev2013121207227
- AzevedoMFLimaCFFernandes-FerreiraMAlmeidaMJWilsonJMPereira-WilsonCRosmarinic acid, major phenolic constituent of Greek sage herbal tea, modulates rat intestinal SGLT1 levels with effects on blood glucoseMol Nutr Food Res201155Suppl 1S15S2521433280
- JayanthyGSubramanianSRosmarinic acid, a polyphenol, ameliorates hyperglycemia by regulating the key enzymes of carbohydrate metabolism in high fat diet – STZ induced experimental diabetes mellitusBiomed Prev Nutr201443431437
- SotnikovaROkruhlicovaLVlkovicovaJRosmarinic acid administration attenuates diabetes-induced vascular dysfunction of the rat aortaJ Pharm Pharmacol201365571372323600389
- FarzadiLKhakiAGhasemzadehAOuladsahebmadarekEGhadamkheirEEffect of rosmarinic acid on sexual behavior in diabetic male ratsAfr J Pharm Pharmacol201151619061910
- TavafiMAhmadvandHKhalatbariATamjidipoorARosmarinic acid ameliorates diabetic nephropathy in uninephrectomized diabetic ratsIran J Basic Med Sci2011143275283
- JiangWLXuYZhangSPHouJZhuHBEffect of rosmarinic acid on experimental diabetic nephropathyBasic Clin Pharmacol Toxicol2012110439039522053730
- LuanHKanZXuYLvCJiangWRosmarinic acid protects against experimental diabetes with cerebral ischemia: relation to inflammation responseJ Neuroinflammation2013102823414442
- HasaneinPMohammad ZaheriLEffects of rosmarinic acid on an experimental model of painful diabetic neuropathy in ratsPharm Biol201452111398140225026351
- MushtaqNSchmatzRPereiraLBRosmarinic acid prevents lipid peroxidation and increase in acetylcholinesterase activity in brain of streptozotocin-induced diabetic ratsCell Biochem Funct201432328729324301255
- GovindarajJSorimuthu PillaiSRosmarinic acid modulates the antioxidant status and protects pancreatic tissues from glucolipotoxicity mediated oxidative stress in high-fat diet: streptozotocin-induced diabetic ratsMol Cell Biochem20154041–214315925735949
- MatthewsDRHoskerJPRudenskiASNaylorBATreacherDFTurnerRCHomeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in manDiabetologia19852874124193899825
- RuntuweneJAmitaniHAmitaniMAsakawaAChengKCInuiAHydrogen-water enhances 5-fluorouracil-induced inhibition of colon cancerPeer J20153e85925870767
- BeardsallKYuenKWilliamsRDungerDApplied physiology of glucose controlCurr Paediatr2006166434438
- WilcoxGInsulin and insulin resistanceClin Biochem Rev2005262193916278749
- PetersenMSimmondsMSRosmarinic acidPhytochemistry200362212112512482446
- LaiXJZhangLLiJSComparative pharmacokinetic and bioavailability studies of three salvianolic acids after the administration of Salviae miltiorrhizae alone or with synthetical borneol in ratsFitoterapia201182688388821575691
- NakazawaTOhsawaKMetabolism of rosmarinic acid in ratsJ Nat Prod19986189939969722482
- GiaccoFBrownleeMOxidative stress and diabetic complicationsCirc Res201010791058107021030723
- BradleyRObergEBCalabreseCStandishLJAlgorithm for complementary and alternative medicine practice and research in type 2 diabetesJ Altern Complement Med200713115917517309390
- FadelOEl KiratKMorandatSThe natural antioxidant rosmarinic acid spontaneously penetrates membranes to inhibit lipid peroxidation in situBiochim Biophys Acta20111808122973298021864504
- ZhuFAsadaTSatoAKoiYNishiwakiHTamuraHRosmarinic acid extract for antioxidant, antiallergic, and alpha-glucosidase inhibitory activities, isolated by supramolecular technique and solvent extraction from perilla leavesJ Agric Food Chem201462488589224400891
- LucariniRBernardesWAFerreiraDSIn vivo analgesic and anti-inflammatory activities of Rosmarinus officinalis aqueous extracts, rosmarinic acid and its acetyl ester derivativePharm Biol20135191087109023738822
- KarthikkumarVSivagamiGVinothkumarRRajkumarDNaliniNModulatory efficacy of rosmarinic acid on premalignant lesions and antioxidant status in 1,2-dimethylhydrazine induced rat colon carcinogenesisEnviron Toxicol Pharmacol201234394995822960260
- PereiraPTyscaDOliveiraPda Silva BrumLFPicadaJNArdenghiPNeurobehavioral and genotoxic aspects of rosmarinic acidPharmacol Res200552319920316026713
- TicliFKHageLICambraiaRSRosmarinic acid, a new snake venom phospholipase A2 inhibitor from Cordia verbenacea (Boraginaceae): antiserum action potentiation and molecular interactionToxicon200546331832715992846
- AiroldiCSironiEDiasCNatural compounds against Alzheimer’s disease: molecular recognition of Aβ1-42 peptide by Salvia sclareoides extract and its major component, rosmarinic acid, as investigated by NMRChem Asian J20138359660223303581
- SwarupVGhoshJGhoshSSaxenaABasuAAntiviral and anti-inflammatory effects of rosmarinic acid in an experimental murine model of Japanese encephalitisAntimicrob Agents Chemother20075193367337017576830
- WangJPanXHanYGuoDGuoQLiRRosmarinic acid from eelgrass shows nematicidal and antibacterial activities against pine wood nematode and its carrying bacteriaMar Drugs201210122729274023201594
- TakanoHOsakabeNSanbongiCExtract of perilla frutescens enriched for rosmarinic acid, a polyphenolic phytochemical, inhibits seasonal allergic rhinoconjunctivitis in humansExp Biol Med (Maywood)2004229324725414988517
- HuGWallsRSBassDThe Chinese herbal formulation biminne in management of perennial allergic rhinitis: a randomized, double-blind, placebo-controlled, 12-week clinical trialAnn Allergy Asthma Immunol200288547848712027069
- SrinivasanKViswanadBAsratLKaulCLRamaraoPCombination of high-fat diet-fed and low-dose streptozotocin-treated rat: a model for type 2 diabetes and pharmacological screeningPharmacol Res200552431332015979893
- KingAJThe use of animal models in diabetes researchBr J Pharmacol2012166387789422352879
- SrinivasanKRamaraoPAnimal models in type 2 diabetes research: an overviewIndian J Med Res2007125345147217496368
- WinzellMSAhrénBThe high-fat diet-fed mouse: a model for studying mechanisms and treatment of impaired glucose tolerance and type 2 diabetesDiabetes200453Suppl 3S215S21915561913
- CachoJSevillanoJde CastroJHerreraERamosMPValidation of simple indexes to assess insulin sensitivity during pregnancy in Wistar and Sprague-Dawley ratsAm J Physiol Endocrinol Metab20082955E1269E127618796548
- WallaceTMLevyJCMatthewsDRUse and abuse of HOMA modelingDiabetes Care20042761487149515161807
- NordlieRCFosterJDLangeAJRegulation of glucose production by the liverAnnu Rev Nutr19991937940610448530
- SurwitRSKuhnCMCochraneCMcCubbinJAFeinglosMNDiet-induced type II diabetes in C57BL/6J miceDiabetes1988379116311673044882
- Vidal-PuigAO’RahillySMetabolism. Controlling the glucose factoryNature2001413685212512611557965
- ShaoJQiaoLJanssenRCPagliassottiMFriedmanJEChronic hyperglycemia enhances PEPCK gene expression and hepatocellular glucose production via elevated liver activating protein/liver inhibitory protein ratioDiabetes200554497698415793235
- QuinnPGYeagleyDInsulin regulation of PEPCK gene expression: a model for rapid and reversible modulationCurr Drug Targets Immune Endocr Metabol Disord20055442343716375695
- ScottDKO’DohertyRMStaffordJMNewgardCBGrannerDKThe repression of hormone-activated PEPCK gene expression by glucose is insulin-independent but requires glucose metabolismJ Biol Chem19982733724145241519727036
- TremblayFLavigneCJacquesHMaretteADefective insulin-induced GLUT4 translocation in skeletal muscle of high fat-fed rats is associated with alterations in both Akt/protein kinase B and atypical protein kinase C (zeta/lambda) activitiesDiabetes20015081901191011473054
- MaretteADimitrakoudisDShiQRodgersCDKlipAVranicMGlucose rapidly decreases plasma membrane GLUT4 content in rat skeletal muscleEndocrine1999101131810403566
- RichterEAHargreavesMExercise, GLUT4, and skeletal muscle glucose uptakePhysiol Rev2013933993101723899560
- GulatiVHardingIHPalomboEAEnzyme inhibitory and antioxidant activities of traditional medicinal plants: potential application in the management of hyperglycemiaBMC Complement Altern Med2012127722713130
- ShoriABScreening of antidiabetic and antioxidant activities of medicinal plantsJ Integr Med201513529730526343100
- BabaSOsakabeNNatsumeMAbsorption, metabolism, degradation and urinary excretion of rosmarinic acid after intake of perilla frutescens extract in humansEur J Nutr20054411915309457
- OsakabeNTakanoHSanbongiCAnti-inflammatory and anti-allergic effect of rosmarinic acid (RA); inhibition of seasonal allergic rhinoconjunctivitis (SAR) and its mechanismBiofactors2004211–412713115630183
- OsakabeNYasudaANatsumeMRosmarinic acid, a major polyphenolic component of perilla frutescens, reduces lipopolysaccharide (LPS)-induced liver injury in d-galactosamine (d-GalN)-sensitized miceFree Radic Biol Med200233679880612208367
- ZouZWXuLNTianJYAntithrombotic and antiplatelet effects of rosmarinic acid, a water-soluble component isolated from radix Salviae miltiorrhizae (danshen)Yao Xue Xue Bao1993284241245 Chinese8213164
- MakinoTOnoTLiuNNakamuraTMusoEHondaGSuppressive effects of rosmarinic acid on mesangioproliferative glomerulonephritis in ratsNephron200292489890412399637
