Abstract
The novel (1-(4-aryl)-1H-1,2,3-triazol-4-yl)methyl, substituted phenyl-6-methyl-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate derivatives were synthesized by the click reaction of the dihydropyrimidinones, bearing a terminal alkynyl group, with various substituted aryl azides at room temperature using a catalytic amount of Cu(OAc)2 and sodium ascorbate in a 1:2 ratio of acetone and water as a solvent. The newly synthesized compounds were characterized by a number of spectroscopic techniques, such as infrared, liquid chromatography-mass spectrometry, 1H, and 13C nuclear magnetic resonance along with single crystal X-ray diffraction. The current procedure for the synthesis of 1,2,3-triazole hybrids with dihydropyrimidinones is appropriate for the synthesis of a library of analogs 7a-l and the method accessible here is operationally simple and has excellent yields. The title compounds 7a-l were evaluated for their in vitro antitubercular activity against H37RV and multidrug-resistant strains of Mycobacterium tuberculosis by resazurin microplate assay plate method and it was found that compound 7d was promising against H37RV and multidrug-resistant strains of M. tuberculosis at 10 and 15 μg/mL, respectively.
Introduction
The pyrimidine system is an important pharmacophore with abundant occurrence in nature. Natural and synthetic dihydropyrimidine derivatives have a wide range of pharmacological actions, such as anticancer,Citation1 antiviral,Citation2,Citation3 antihypertensive,Citation4 calcium channel blocking,Citation5 antitubercular,Citation6 antimicrobial,Citation7,Citation8 anti-inflammatory,Citation9,Citation10 and larvicidal and insecticide actions.Citation11,Citation12 1,2,3-Triazoles, as a vital class of N-heterocyclic compounds, due to their unique chemical and structural properties, have received a great deal of attention over the past few decades and found broad application in medicinal chemistryCitation13 and particularly as anticancer,Citation14 antimicrobial,Citation15 antitubercular,Citation16 anti-HIV,Citation17 and antifungal agents.Citation18 On the other hand, this special class of scaffolds has also found relevance in objective oriented synthesis,Citation19 bioconjugation,Citation20 materials and surface science,Citation21 combinatorial chemistry,Citation22 and medicinal chemistry.Citation23 Moreover, 1,2,3-triazole can mimic natural peptides and heterocycles in geometrical shape and interaction function.Citation24 1,2,3-Triazoles could be easily constructed by click chemistry reactionCitation25 and which yielded small molecules with special properties, such as moderate dipole character, hydrogen bonding capability, rigidity, and stability.Citation26 HeterocyclicCitation27–Citation29 fluorine-containing compounds have been shown to exhibit promising anti-tuberculosis (anti-TB) activity,Citation30 including 1,2,3-triazole analogs, for their promising anti-TB activity.Citation31 Keeping this in mind and considering the pharmacological significance of dihydropyrimidine and 1,2,3-triazole pharmacophores, in the present investigation it was decided to design and synthesize a series of novel 1,2,3-triazole hybrid with dihydropyrimidinone (DHPM) scaffolds in accordance with Lipinski rule except compound 7d.Citation32 The title compounds, (1-(4-aryl)-1-H-1,2,3-triazol-4-yl)methyl, substitutedphenyl-6-methyl-2-o xo-1,2,3,4-tetrahydropyrimidine-5-carboxylates 7a-l, have been tested for safety studies by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assayCitation33 following in vitro antitubercular activity against H37RV and multidrug-resistant strains of Mycobacterium tuberculosis (MDR-MTB) by resazurin microplate assay plate method.
In this communication and in continuation of our work on the development of pharmacologically active heterocyclic compoundsCitation6,Citation34,Citation35 and screening of heterocyclic compounds for properties of polymorphism,Citation36–Citation38 we have synthesized 1,2,3-triazole hybrid with DHPMs using aryl azide as well as DHPMs having a terminal alkynyl group, which was synthesized by the four component Biginelli-like cyclocondensation reaction (tert-butyl β-ketoester, propargyl alcohol, aryl aldehyde, and urea) along with catalytic amount of Cu(OAc)2 and sodium ascorbate in a 1:2 ratio of acetone and water as a solvent at room temperature as shown in .
Materials and methods
Chemistry
All the chemicals were purchased from Sigma-Aldrich Corporation (St Louis, MO, USA; analytical grade) and used without further purification. Fourier transform infrared (FTIR) spectra were registered on a Bruker Corporation (Billerica, MA, USA) IFS 55 equinox Fourier transform IR spectrophotometer as KBr discs. 1H- and 13C-nuclear magnetic resonance (NMR) spectra were recorded using a Bruker 400 or 500 MHz spectrometer in the solvents indicated (referenced to the residual 1H signals in the deuterated solvents) using tetramethylsilane (TMS) as an internal standard. Chemical shifts are reported in ppm (δ scale) and coupling constant (J) values given in hertz (Hz). The splitting pattern is abbreviated as follows: s, singlet; d, doublet; and m, multiplet. Thin layer chromatography (TLC) analysis of reaction mixtures was performed on Merck (Merck Serono, Darmstadt, Germany) aluminum plates coated with silica gel (60 F254). Compounds were visualized by ultraviolet irradiation at 254 and 366 nm. Merck silica gel (60–120 mesh) was used for column chromatography.
Spectra of the compounds are available as Supplementary Materials.
General procedure for the synthesis of (1-(4-aryl)-1H-1,2,3-triazol-4-yl) methyl, substituted phenyl-6-methyl-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carboxylates (7a-l)
A 25 mL round bottom flask equipped with a condenser was charged with substituted aryl azides (1.0 mmol) (6) as well as DHPMs having terminal alkynyl group (1.0 mmol) (5). Compound 5 was synthesized by the four components Biginelli-like cyclocondensation reaction of tert-butyl β-ketoester (1.0 mmol) (1), propargyl alcohol (1.2 mmol) (2), substituted aryl aldehyde (1.0 mmol) (3), and urea (1.2 mmol) (4) by a reflux method.Citation39 The entire reaction mixture was allowed to stir for 3 hours at room temperature along with a catalytic amount of Cu(OAc)2 (0.1 mmol) and sodium ascorbate (0.2 mmol) in a 1:2 ratio of acetone and water (2 mL) as a solvent till the reaction was complete. The progress of the reaction was monitored on TLC (4:6 of hexane and ethyl acetate). After completion of the reaction as indicated on TLC, the contents were concentrated under reduced pressure to remove excess of the acetone and the crude reaction mixture was extracted with ethyl acetate and water. The combined organic extract, after drying over anhydrous sodium sulfate, was again concentrated under reduced pressure to obtain the crude product. For analytically pure products, the final solid mass was purified by column chromatography using the hexane/ethyl acetate (4:6) as the eluent to give the pure products 7a-l at 77%–92% yield. Physicochemical characteristics of the title compounds are tabulated in .
Table 1 Physicochemical constants of (1-(4-aryl)-1H-1,2,3-triazol-4-yl)methyl, substituted phenyl-6-methyl-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carboxylates 7a-l
(1-(4-(Trifluoromethyl)phenyl)-1H-1,2,3-triazol-4-yl)methyl 4-(4-fluorophenyl)-6-methyl-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate (7a)
IR (KBr) ν/cm−1 3,333, 2,946, 2,370, 1,670, 1,332, 844, 785, 753. 1H NMR (500 MHz, dimethyl sulfoxide [DMSO]) δ 9.29 (s, 1H), 8.72 (s, 1H), 8.12 (d, J=8.5 Hz, 2H), 7.99 (d, J=8.6 Hz, 2H), 7.77 (s, 1H), 7.24–7.21 (m, 2H), 7.06 (t, J=8.8 Hz, 2H), 5.21–5.17 (m, 3H), 2.27 (s, 3H). 13C NMR (126 MHz, DMSO) δ 165.3, 162.7, 160.7, 152.3, 150.04, 144.2, 141.3, 141.2, 139.6, 128.7, 128.6, 127.6, 123.2, 121.0, 115.6, 115.4, 99.0, 56.6, 53.7, 18.3. Liquid chromatography-mass spectrometry (LCMS): 475.2.
(1-(4-(Trifluoromethyl)phenyl)-1H-1,2,3-triazol-4-yl)methyl 4-(3-fluorophenyl)-6-methyl-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate (7b)
IR (KBr) ν/cm−1 3,367, 2,979, 2,345, 1,700, 1,636, 1,329, 873, 845, 757. 1H NMR (400 MHz, DMSO) δ 9.31 (s, 1H), 8.80 (s, 1H), 8.10 (d, J=7.9 Hz, 2H), 7.96 (d, J=8.0 Hz, 2H), 7.79 (s, 1H), 7.27 (d, J=6.6 Hz, 1H), 7.06–6.87 (m, 3H), 5.27–5.10 (m, 3H), 2.24 (s, 3H). 13C NMR (101 MHz, DMSO) δ 165.2, 163.6, 161.2, 152.3, 150.4, 147.9, 144.2, 139.7, 130.9, 130.8, 127.7, 127.6, 123.2, 122.5, 121.0, 114.6, 114.4, 113.5, 113.3, 98.5, 56.7, 53.8, 18.4. LCMS: 475.4.
(1-(4-(Trifluoromethyl)phenyl)-1H-1,2,3-triazol-4-yl)methyl 4-(3-chlorophenyl)-6-methyl-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate (7c)
IR (KBr) ν/cm−1 3,363, 2,969, 2,349, 1,695, 1,330, 843, 788, 757. 1H NMR (400 MHz, DMSO) δ 9.33 (s, 1H), 8.80 (s, 1H), 8.11 (d, J=8.4 Hz, 2H), 7.96 (d, J=8.5 Hz, 2H), 7.79 (s, 1H), 7.34–7.13 (m, 4H), 5.20–5.13 (m, 3H), 2.25 (s, 3H).13C NMR (101 MHz, DMSO) δ 165.2, 158.0, 152.2, 150.5, 147.5, 146.9, 144.2, 133.3, 130.8, 127.7, 127.6, 126.6, 125.2, 123.2, 121.0, 98.4, 56.7, 53.9, 18.4. LCMS: 491.2 (M+), 493.2 (M +2).
(1-(4-(Trifluoromethyl)phenyl)-1H-1,2,3-triazol-4-yl)methyl 6-methyl-2-oxo-4-(4-(trifluoromethyl)phenyl)-1,2,3,4-tetrahydropyrimidine-5-carboxylate (7d)
IR (KBr) ν/cm−1 3,412, 2,968, 2,345, 1,700, 1,641, 1,326, 844, 795, 718. 1H NMR (400 MHz, DMSO) δ 9.35 (s, 1H), 8.79 (s, 1H), 8.10 (d, J=8.1 Hz, 2H), 7.95 (d, J=8.2 Hz, 2H), 7.83 (s, 1H), 7.57 (d, J=7.7 Hz, 2H), 7.38 (d, J=7.6 Hz, 2H), 5.25–5.12 (m, 3H), 2.25 (s, 3H). 13C NMR (101 MHz, DMSO) δ 165.1, 152.2, 150.5, 144.2, 139.6, 127.6, 127.6, 127.6, 127.5, 125.8, 125.7, 123.2, 120.9, 98.3, 59.3, 56.7, 54.1, 18.4. LCMS: 525.1.
(1-(4-(Trifluoromethyl)phenyl)-1H-1,2,3-triazol-4-yl)methyl 4-(4-chlorophenyl)-6-methyl-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate (7e)
IR (KBr) ν/cm−1 3,365, 2,966, 2,309, 1,709, 1,638, 1,331, 843, 786, 746. 1H NMR (400 MHz, DMSO) δ 9.30 (s, 1H), 8.75 (s, 1H), 8.11 (d, J=8.5 Hz, 2H), 7.96 (d, J=8.6 Hz, 2H), 7.76 (s, 1H), 7.27 (d, J=8.4 Hz, 2H), 7.17 (d, J=8.4 Hz, 2H), 5.31–5.03 (m, 3H), 2.24 (s, 3H). 13C NMR (101 MHz, DMSO) δ 165.2, 152.2, 150.2, 144.3, 144.0, 139.7, 132.2, 129.4, 128.7, 128.6, 127.7, 127.6, 123.2, 121.0, 98.6, 56.7, 53.8, 18.4. LCMS: 491.2 (M+), 493.2 (M +2).
(1-(4-(Trifluoromethyl)phenyl)-1H-1,2,3-triazol-4-yl)methyl 4-(4-methoxyphenyl)-6-methyl-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate (7f)
IR (KBr) ν/cm−1 3,390, 2,964, 2,345, 1,695, 1,638, 1,336, 845, 790, 757. 1H NMR (400 MHz, DMSO) δ 9.21 (s, 1H), 8.68 (s, 1H), 8.10 (d, J=8.4 Hz, 2H), 7.96 (d, J=8.6 Hz, 2H), 7.66 (s, 1H), 7.08 (d, J=8.6 Hz, 2H), 6.76 (d, J=8.6 Hz, 2H), 5.18–5.08 (m, 3H), 3.59 (s, 3H), 2.23 (s, 3H). 13C NMR (101 MHz, DMSO) δ 165.3, 158.8, 152.4, 149.6, 144.4, 139.6, 137.3, 127.8, 127.6, 127.6, 123.1, 121.0, 114.0, 99.3, 56.6, 55.3, 53.7, 31.1, 18.3. LCMS: 487.4.
(1-(4-(Trifluoromethyl)phenyl)-1H-1,2,3-triazol-4-yl)methyl 4-(3-methoxyphenyl)-6-methyl-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate (7g)
IR (KBr) ν/cm−1 3,414, 2,964, 2,349, 1,718, 1,642, 1,333, 843, 788, 726. 1H NMR (400 MHz, DMSO) δ 9.25 (s, 1H), 8.74 (s, 1H), 8.10 (d, J=8.5 Hz, 2H), 7.97 (d, J=8.6 Hz, 2H), 7.72 (s, 1H), 7.15 (t, J=7.9 Hz, 1H), 6.82–6.65 (m, 3H), 5.19–5.11 (m, 3H), 3.59 (s, 3H), 2.24 (s, 3H). 13C NMR (101 MHz, DMSO) δ 165.3, 159.6, 152.5, 150.0, 146.6, 144.3, 130.0, 127.7, 127.6, 123.2, 121.0, 118.6, 112.8, 112.6, 98.9, 56.7, 55.3, 54.1, 18.3. LCMS: 487.3.
(1-(4-(Trifluoromethyl)phenyl)-1H-1,2,3-triazol-4-yl)methyl 6-methyl-2-oxo-4-(p-tolyl)-1,2,3,4-tetrahydropyrimidine-5-carboxylate (7h)
IR (KBr) ν/cm−1 3,367, 2,967, 2,349, 1,710, 1,646, 1,324, 841, 792, 703. 1H NMR (400 MHz, DMSO) δ 9.21 (s, 1H), 8.68 (s, 1H), 8.10 (d, J=8.5 Hz, 2H), 7.97 (d, J=8.6 Hz, 2H), 7.68 (s, 1H), 7.05–6.99 (m, 4H), 5.21–5.09 (m, 3H), 2.23 (s, 3H), 2.12 (s, 3H). 13C NMR (101 MHz, DMSO) δ 165.3, 152.4, 149.7, 144.4, 142.2, 139.7, 136.8, 129.2, 127.7, 127.6, 126.6, 125.6, 123.1, 121.0, 99.2, 56.6, 54.0, 20.9, 18.3. LCMS: 471.2.
(1-(4-(Trifluoromethyl)phenyl)-1H-1,2,3-triazol-4-yl)methyl 4-(4-ethoxyphenyl)-6-methyl-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate (7i)
IR (KBr) ν/cm−1 3,323, 2,982, 2,350, 1,696, 1,644, 1,331, 839, 769, 696. 1H NMR (400 MHz, DMSO) δ 9.20 (s, 1H), 8.65 (s, 1H), 8.09 (d, J=8.5 Hz, 2H), 7.96 (d, J=8.6 Hz, 2H), 7.65 (s, 1H), 7.05 (d, J=8.6 Hz, 2H), 6.72 (d, J=8.6 Hz, 2H), 5.26–5.05 (m, 3H), 3.88–3.72 (m, 2H), 2.23 (s, 3H), 1.19 (t, J=7.0 Hz, 3H). 13C NMR (101 MHz, DMSO) δ 165.3, 158.1, 152.4, 149.5, 144.4, 139.6, 137.2, 127.8, 127.6, 127.6, 123.0, 121.0, 114.5, 99.3, 63.3, 56.5, 53.7, 18.3, 14.9. LCMS: 501.6.
(1-(4-Fluorophenyl)-1H-1,2,3-triazol-4-yl) methyl 4-(4-chlorophenyl)-6-methyl-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate (7j)
IR (KBr) ν/cm−1 3,414, 2,960, 2,349, 1,712, 1,640, 1,323, 838, 792, 710. 1H NMR (400 MHz, DMSO) δ 9.36 (s, 1H), 8.63 (s, 1H), 7.93–7.79 (m, 3H), 7.59 (d, J=7.9 Hz, 2H), 7.45–7.38 (m, 4H), 5.27–5.09 (m, 3H), 2.25 (s, 3H). 13C NMR (101 MHz, DMSO) δ 165.2, 152.2, 150.5, 149.5, 143.8, 127.5, 125.8, 125.8, 123.2, 122.8, 122.8, 117.3, 117.0, 98.4, 56.8, 54.1, 31.1, 23.1, 18.4. LCMS: 441.2 (M+), 443.2 (M +2).
(1-(4-Fluorophenyl)-1H-1,2,3-triazol-4-yl)methyl 6-methyl-2-oxo-4-(4-(trifluoromethyl)phenyl)-1,2,3,4-tetrahydropyrimidine-5-carboxylate (7k)
IR (KBr) ν/cm−1 3,367, 2,960, 2,349, 1,711, 1,638, 1,313, 835, 787, 691. 1H NMR (400 MHz, DMSO) δ 9.30 (s, 1H), 8.58 (s, 1H), 7.90–7.87 (m, 2H), 7.76 (s, 1H), 7.43 (t, J=8.4 Hz, 2H), 7.28 (d, J=8.0 Hz, 2H), 7.18 (d, J=8.0 Hz, 2H), 5.20–5.09 (m, 3H), 2.24 (s, 3H). 13C NMR (101 MHz, DMSO) δ 165.2, 152.2, 150.2, 144.0, 143.9, 133.5, 132.2, 128.7, 128.6, 123.1, 123.0, 122.9, 98.6, 56.8, 53.8, 31.1, 18.4. LCMS: 475.3.
(1-(4-Fluorophenyl)-1H-1,2,3-triazol-4-yl) methyl 4-(3-fluorophenyl)-6-methyl-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate (7l)
IR (KBr) ν/cm−1 3,324, 3,079, 2,349, 1,663, 1,638, 1,239, 845, 761, 703. 1H NMR (400 MHz, DMSO) δ 9.31 (s, 1H), 8.62 (s, 1H), 7.90–7.87 (m, 2H), 7.79 (s, 1H), 7.43 (t, J=8.7 Hz, 2H), 7.28 (m, 1H), 7.06–6.86 (m, 3H), 5.23–5.10 (m, 3H), 2.25 (s, 3H). 13C NMR (101 MHz, DMSO) δ 165.2, 163.7, 161.2, 160.9, 152.3, 150.4, 147.9, 147.9, 143.8, 133.5, 130.9, 130.8, 123.2, 123.0, 122.9, 122.5, 117.3, 117.0, 114.6, 114.4, 113.5, 113.3, 98.5, 56.8, 53.8, 18.4. LCMS: 425.4.
Crystal growth and single crystal X-ray study
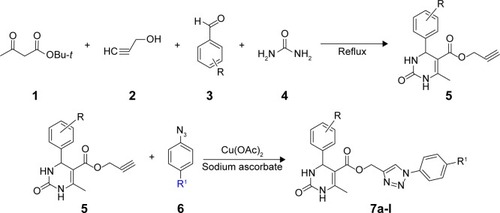
Title compound 7g was used to grow single crystals at room temperature (25°C) using benzene as solvent for crystallographic studies. Single crystal data were collected on the Bruker D8 VENTURE diffractometer equipped with CMOS type PHOTON 100 detector using monochromated Mo Kα radiation (λ=0.71073 Å). Unit cell measurement, data collection, integration, scaling, and absorption corrections for the crystal were done using Bruker Apex II software.Citation40 Data reduction was done by Bruker SAINT suite.Citation41 The crystal structure was solved by direct methods using SIR 2014Citation42 and refined by the full matrix least squares method using SHELXL 2014Citation43 present in the program suite WinGX (version 2014.1, Louis J. Farrugia, Glasgow, Scotland).Citation44 Absorption correction was applied using SADABS.Citation45 All non-hydrogen atoms were refined anisotropically and all hydrogen atoms (except H-atoms bonded to N4 and N5) were positioned geometrically and refined using a riding model with Uiso(H) =1.2Ueq. The H-atoms bonded to N4 and N5 were taken directly from difference Fourier maxima. ORTEP (Oak Ridge Thermal Ellipsoid Plot) was generated using Mercury 3.5.1 Cambridge Crystallographic Data Center (CCDC) program.Citation46 Geometrical calculations were done using PARSTCitation47 and PLATON.Citation48 Crystallographic and refinement data of the title compound 7g are tabulated in .
Table 2 Single crystal data collection and refinement for compound 7g
Safety studies
The safety of the test compounds 7a-l was evaluated by an MTT assay. The MTT cytotoxicity assay was used to evaluate the cytotoxic effect of the most promising compounds against peripheral blood mononuclear cells according to the protocol described.Citation49 Cells were pipetted (90 μL of cell culture, 1×105 cells/mL) into each well of 96-well microtiter plates, and the outer wells were filled with phosphate-buffered saline in order to prevent the medium from evaporation during incubation. Thereafter, plates were incubated at 37°C for 24 hours. Each well of the plate was then treated with 10 μL of the compounds (1,000–5 μg/mL). In the control wells, the negative control DMSO and media were added. Thereafter, the plates were incubated for 2 days at 37°C in a humidified incubator that contained a 5% CO2 atmosphere. After the incubation time, 20 μL of MTT reagent (5 mg/mL) was further added to individual well. The plate was then incubated for a further 4 hours at 37°C (5% CO2 incubator). The media were then removed after incubation, and an aliquot of 100 μL DMSO was added to each well in order to dissolve the formazan crystals that were formed in metabolically active cells. Thereafter, the plates were incubated for an extra hour. The absorbance of the formazan was evaluated at 590 nm using an ELISA plate reader.
Antitubercular activity
Resazurin microplate assay plate method
The susceptibility of clinical isolates comprising of both fully sensitive and MDR TB isolates were evaluated against test compounds 7a-l by the colorimetric resazurin microplate assay plate method.Citation50 An amount of 100 μL of Middlebrook 7H9 (Becton, Dickinson and Company, New Jersey, USA) broth was aseptically prepared and dispensed in each of the wells of a 96-well flat-bottomed microtiter plate with lids (Lasec, Ndabeni, South Africa). Each of the test compounds 7a-l was weighed out accordingly, dissolved in the appropriate solvent, and filter sterilized using a 0.2 micron polycarbonate filter. Stock solutions of the test samples were aliquoted into cryovials and stored at −20°C. An amount of 100 μL of the test samples was added to each of the well containing Middlebrook 7H9 broth supplemented with 0.1% casitone, 0.5% glycerol, and 10% oleic acid, albumin, dextrose, and catalase. The test samples were then further serially diluted two-fold directly in the broth of the microtiter plate to a desired concentration ranging from 40 to 0.625 μg/mL.
Inoculums from clinical isolates were prepared fresh from Middlebrook 7H11 agar plates by scraping and resuspending loopful of colonies into Middlebrook 7H9 broth containing glass beads. The inoculum turbidity was adjusted to a McFarland number 1 standard and further diluted 1:10 in M7H9 broth prior to addition (100 μL) to each of the test samples and drug-free wells. A growth control and a sterile control were also included for each isolate. Sterile M7H9 broth was added to all perimeter wells to avoid evaporation during the incubation. The plate was covered, sealed in a plastic bag, and incubated at 37°C. After 8 days of incubation, 30 μL of 0.02% working solution of resazurin salt was inoculated into each microtiter well. The plates were then incubated overnight and read the following day. A positive reaction resulted in a color change from blue to pink owing to the reduction of resazurin to rezarufin, which confirmed MTB cell viability/growth and hence drug resistance. The minimum inhibitory concentrations were defined as the minimum drug concentration to inhibit the growth of the organism with no color changes present in the well. The anti-TB results of title compounds 7a-l are tabulated in .
Table 3 In vitro antitubercular activity of title compounds 7a-l against Mycobacterium tuberculosis
Results and discussion
Chemistry
As a continuing aspect of our earlier workCitation6,Citation37,Citation51 and after much efforts over the years to develop efficient synthetic procedures for multicomponent reactions under greener conditions, there was a requirement to synthesize a huge library of diversified 1,2,3-triazole hybrids with DHPMs analogs with reduced time, outstanding yields, and excellent biological activities. The DHPMs with terminal alkynyl group were synthesized following a previously reported procedure.Citation52 Aromatic azides were synthesized in high yields from arenediazonium tosylates and sodium azide in water at room temperature. An in situ diazotization followed by azidation in the presence of p-toluenesulfonic acid allows the direct transformation of aromatic amines.Citation53
For our initial studies, 4-trifluoromethyl aryl azide and prop-2-yn-1-yl 4-(4-fluorophenyl)-6-methyl-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate were chosen as model substrates (entry 1; compound 7a, ) with a catalytic amount of Cu(OAc)2 and sodium ascorbate in a 1:2 ratio of acetone and water (2 mL) as a solvent. A whole reaction mixture of 4-trifluoromethyl aryl azide and terminal alkynyl DHPMs was stirred at room temperature (25°C). The starting material was consumed within 3 hours as indicated by TLC analysis. It was observed that when aryl azide, terminal alkynyl DHPMs, Cu(OAc)2, and sodium ascorbate were used in the ratio of 1:1:0.1:0.2 in 2 mL of a mixture of 1:2 ratio of acetone and water as a solvent, they gave the best result. After workup and purification by silica gel column chromatography, the final desired product 1,2,3-triazole hybrid with DHPMs was isolated in 85% yield.
The practicality of optimized reaction conditions was further extended to the synthesis of more functionalized 1,2,3-triazole hybrid DHPM derivatives 7b-l and experiments were performed by making use of a wide range of aryl azides. It was found that in all the cases the reaction occurred smoothly. We have also reacted a variety of DHPMs analogs having both electron-releasing and -withdrawing substituent to synthesize the diversified DHPMs derivatives with 1,2,3-triazole linkage and all the results are appended in . The partition coefficient of the title compounds was calculated by ChemBioDraw Ultra 13.0v (PerkinElmer Inc., Waltham, MA, USA) and the results were in the range of 3.7767–5.3856. The purity of the compounds was confirmed by high performance liquid chromatography and it was over 99%.
Crystallographic studies
Test compound 7g emerged as one of the promising compounds for anti-TB activity from the series subjected to single crystal X-ray studies. The compound crystallizes in the centrosymmetric monoclinic space group P21/c with one molecule in the asymmetric unit (Z=4). ORTEP is shown in . Structural investigation shows that the DHPM ring of the molecule exists in a boat-like conformation due to minimization of the steric repulsion between ester moiety containing a triazole ring with meta methoxy phenyl ring. The para trifluoro substituted benzene ring and triazole unit remain almost in the same molecular plane whereas the methoxy phenyl substituted DHPM unit is almost perpendicular to the plane. Crystal packing is mainly controlled by strong intermolecular N-H…O dimeric motif along the crystallographic axis a and weak C-H…O hydrogen bonding dimers along the axis c (shaded regions in ). In addition, molecules form centrosymmetric dimers via N-H…N, C-H…N, C-H…π (Cg1) hydrogen bonds and weak π…π stacking interactions along the crystallographic b-axis wherein these dimers are linked with weak C-H…F (involving H20, F2) hydrogen bond and F…F (involving F1, F3) interactions (). Hence, it is noteworthy to mention that interactions involving fluorine atom are one of the important contributors to the overall packing.Citation54–Citation56 The list of all the intermolecular interactions is given in .
Figure 2 ORTEP (Oak Ridge Thermal Ellipsoid Plot) of the compound 7g drawn with 50% ellipsoidal probability.
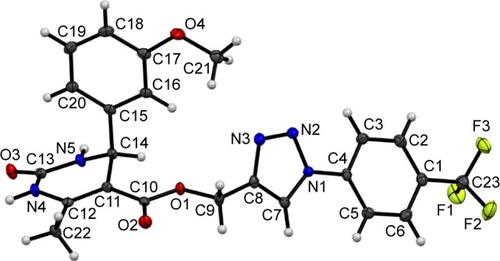
Figure 3 Packing network of compound 7g (A) down the ac plane associated with N-H…O and CH…O dimers (noninteracting H-atoms have been removed for clarity); (B) molecules forming centrosymmetric dimers via N-H…N, C-H…N, C-H…π (Cg1) hydrogen bonds and π…π stacking connected down the ab plane through weak C-H…F hydrogen bond and F…F interactions.
Table 4 Intermolecular interactions in 7g
Safely studies
Test compounds 7a-l were evaluated for safely studies by an MTT assay and it was found that up to 500 μg/mL no toxicity on PBM cell lines was observed.
Antitubercular activity
Anti-TB activity of the test compounds 7a-l was evaluated against H37Rv and MDR-MTB by resazurin microplate assay plate method and the results are tabulated in . Compound 7d with trifluoro methyl group at fourth position of two-phenyl ring appeared as a promising agent against H37Rv and MDR-MTB at 10 and 15 μg/mL, respectively. However, compound 7f with methoxy group on phenyl ring of pyrimidine nucleus and trifluoromethyl group on phenyl ring of triazole ring exhibited activity at 10 μg/mL against H37Rv and no activity against MDR-MTB. Test compounds 7b, 7g, and 7l exhibited similar activity against H37Rv and MDR-MTB in spite of varying functional groups on phenyl rings that are on pyrimidine and triazole nucleus.
Conclusion
We have established an operationally simple and straightforward one-pot synthesis for the synthesis of 1,2,3-triazole hybrid with DHPMs analogs via click chemistry. The purity of the compound was over 99% and yield of the compounds was excellent. Compound 7d emerged as a promising compound from the series for anti-TB activity. Crystallographic studies for the compound 7g revealed that the interplay of strong (such as N-H…O, N-H…N, C-H…O) and weak interactions (eg, C-H…F, C-H…π, F…F) stabilizes the overall crystal packing in the solid state.
Acknowledgments
The authors are grateful to Deanship of Scientific Research (150199), King Faisal University, Kingdom of Saudi Arabia, and IISER Bhopal for providing access to their facility and for their encouragement. GBDR thanks IISER Bhopal for postdoctoral fellowship. DC thanks IISER Bhopal for research facilities and infrastructure.
Disclosure
The authors report no conflicts of interest in this work.
References
- MayerTUKapoorTMHaggartySJKingRWSchreiberSLMitchisonTJSmall molecule inhibitor of mitotic spindle bipolarity identified in a phenotype-based screenScience1999286544197197410542155
- HurstEWHullRTwo new synthetic substances active against viruses of the psittacosis-lymphogranuloma-trachoma groupJ Med Pharm Chem19613221522914450164
- ZhuXZhaoGZhouX2,4-Diaryl-4,6,7,8-tetrahydroquinazolin-5(1H)-one derivatives as anti-HBV agents targeting at capsid assemblyBioorg Med Chem Lett201020129930119897363
- KarnailSABrianNSStevenEUDihydropyrimidine calcium channel blockers. 3, 3-Carbamoyl-4-aryl-1,2,3,4-tetrahydro-6-methyl-5-pyrimidinecarboxylic acid esters as orally effective antihypertensive agentsJ Med Chem19913428068111995904
- JaukBPernatTKappeCODesign and synthesis of a conformationally rigid mimic of the dihydropyrimidine calcium channel modulator SQ 32,926Molecules20005227239
- VenugopalaKNNayakSKPillayMPrasannaRCoovadiaYMOdhavBSynthesis and antitubercular activity of 2-(substituted phenyl/benzyl-amino)-6-(4-chlorophenyl)-5-(methoxycarbonyl)-4-methyl-3,6-dihydropyrimidin-1-ium chloridesChem Biol Drug Des201381221922723150983
- WaelAESIbrahimFNAdelAHAbdelRC-furyl glycosides, II: synthesis and antimicrobial evaluation of C-furyl glycosides bearing pyrazolines, isoxazolines, and 5,6-dihydropyrimidine-2(1H)-thionesMonatsh Chem2009140365370
- ShahTBGupteAPatelMRChaudhariVSPatelHPatelVCSynthesis and in vitro study of biological activity of heterocyclic N-mannich bases of 3,4-dihydropyrimidine-2(1H)-thionesInd J Chem201049B05578586
- SushilkumarSBDevanandBSSynthesis and anti-inflammatory activity of some 2-amino-6-(4-substituted aryl)-4-(4-substituted phenyl)-1,6-dihydropyrimidine-5-yl-acetic acid derivativesActa Pharm20035322322914769245
- NofalZMFahmyHHZareaESEl-ErakyWSynthesis of new pyrimidine derivatives with evaluation of their anti-inflammatory and analgesic activitiesActa Pol Pharm201168450751721796933
- RajanarendarEReddyMNMurthyKRSynthesis, antimicrobial, and mosquito larvicidal activity of 1-aryl-4-methyl-3,6-bis-(5-methylisoxazol-3-yl)-2-thioxo-2,3,6,10b-tetrahydro-1H-pyrimido[5,4-c] quinolin-5-onesBioorg Med Chem Lett201020206052605520813527
- VenugopalaKNGleiserRMChalannavarRKOdhavBAntimosquito properties of 2-substituted phenyl/benzylamino-6-(4-chlorophenyl)-5-methoxycarbonyl-4-methyl-3,6-dihydropyrimidinium chlorides against Anopheles arabiensisMed Chem201410221121924506684
- BuckleDROutredDJRockellCJSmithHSpicerBAStudies on v-triazoles. 7. Antiallergic 9-oxo-1H,9H-benzopyrano[2,3-d]-v-triazolesJ Med Chem19832622512546131133
- GłowackaIEBalzariniJWróblewskiAEThe synthesis, antiviral, cytostatic and cytotoxic evaluation of a new series of acyclonucleotide analogues with a 1,2,3-triazole linkerEur J Med Chem20137070372224219992
- DabiriMSalehiPBahramnejadMKoohshariMAliahmadiAOne-pot synthesis of (1,2,3-triazolyl)methyl 3,4-dihydro-2-oxo-1H-pyrimidine-5-carboxylates as potentially active antimicrobial agentsHelv Chim Acta2014973375383
- PatpiSRPulipatiLYogeeswariPDesign, snthesis, and structure–activity correlations of novel Dibenzo[b,d]furan, Dibenzo[b,d]thiophene, and N-Methylcarbazole clubbed 1,2,3-triazoles as potent inhibitors of mycobacterium tuberculosisJ Med Chem20125583911392222449006
- GiffinMJHeasletHBrikAA copper(I)-catalyzed 1,2,3-triazole azide-alkyne click compound is a potent inhibitor of a multidrug-resistant HIV-1 protease variantJ Med Chem200851206263627018823110
- ZouYZhaoQLiaoJNew triazole derivatives as antifungal agents: synthesis via click reaction, in vitro evaluation and molecular docking studiesBioorg Med Chem Lett20122282959296222437114
- BillingJFNilssonUJC2-symmetric macrocyclic carbohydrate/amino acid hybrids through copper(I)-catalyzed formation of 1,2,3-triazolesJ Org Chem200570124847485015932327
- SpeersAEAdamGCCravattBFActivity-based protein profiling in vivo using a copper(I)-catalyzed azide-alkyne [3+2] cycloadditionJ Am Chem Soc2003125164686468712696868
- CollmanJPDevarajNKChidseyCED“Clicking” functionality onto electrode surfacesLangmuir20042041051105315803676
- LöberSRodriguez-LoaizaPGmeinerPClick linker: efficient and high-yielding synthesis of a new family of SPOS resins by 1,3-dipolar cycloadditionOrg Lett20035101753175512735769
- GeninMJAllwineDAAndersonDJSubstituent effects on the antibacterial activity of nitrogen–carbon-linked (azolylphenyl)oxazolidinones with expanded activity against the fastidious gram-negative organisms haemophilus influenzae and moraxella catarrhalisJ Med Chem200043595397010715160
- ThibaultRJTakizawaKLowenheilmPA versatile new monomer family: functionalized 4-vinyl-1,2,3-triazoles via click chemistryJ Am Chem Soc200612837120841208516967955
- KolbHCFinnMGSharplessKBClick chemistry: diverse chemical function from a few good reactionsAngew Chem Int Ed Engl200140112004202111433435
- KolbHCSharplessKBThe growing impact of click chemistry on drug discoveryDrug Discov Today20038241128113714678739
- ClaesPCappoenDUythethofkenC2,4-Dialkyl-8,9,10,11-tetrahydrobenzo[g]pyrimido[4,5-c]isoquinoline-1,3,7,12(2H,4H)-tetraones as new leads against Mycobacterium tuberculosisEur J Med Chem20147740942124681334
- CappoenDClaesPJacobsJ1,2,3,4,8,9,10,11-Octahydrobenzo[j] phenanthridine-7,12-diones as new leads against Mycobacterium tuberculosisJ Med Chem20145772895290724611928
- ClaesPCappoenDMbalaBMSynthesis and antimycobacterial activity of analogues of the bioactive natural products sampangine and cleistopholineE J Med Chem20136798110
- SmitsRCadicamoCDBurgerKKokschBSynthetic strategies to alpha-trifluoromethyl and alpha-difluoromethyl substituted alpha-amino acidsChem Soc Rev20083781727173918648692
- BoechatNFerreiraVFFerreiraSBNovel 1,2,3-triazole derivatives for use against Mycobacterium tuberculosis H37Rv (ATCC 27294) strainJ Med Chem201154175988599921776985
- LeesonPDrug discovery: chemical beauty contestNature2012481738245545622281594
- van MeerlooJKaspersGJCloosJCell sensitivity assays: the MTT assayMethods Mol Biol201173123724521516412
- VenugopalaKNKrishnappaMNayakSKSynthesis and antimosquito properties of 2,6-substituted benzo[d]thiazole and 2,4-substituted benzo[d]thiazole analogues against Anopheles arabiensisEur J Med Chem20136529530323727539
- VenugopalaKNNayakSKGleiserRMSanchez-BorzoneMEGarciaDAOdhavBSynthesis, polymorphism, and insecticidal activity of methyl 4-(4-chlorophenyl)-8-iodo-2-methyl-6-oxo-1,6-dihydro-4H- pyrimido[2,1-b]quinazoline-3-carboxylate against Anopheles arabiensis mosquitoChem Biol Drug Des Epub201623
- NayakSKVenugopalaKNChopraDRowTNGInsights into conformational and packing features in a series of aryl substituted ethyl- 6-methyl-4-phenyl-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carboxylatesCrystEngComm2011132591605
- PaniniPVenugopalaKNOdhavBChopraDPolymorphism in two biologically active dihydropyrimidinium hydrochloride derivatives: quantitative inputs towards the energetics associated with crystal packingActa Crystallogr Sect B2014704681696
- PaniniPVenugopalaKNOdhavBChopraDQuantitative analysis of intermolecular interactions in 7-hydroxy-4-methyl-2H-chromen-2-one and its hydrateProc Natl Acad Sci India Sect A Phy Sci2014842281295
- PuripatMRamozziRHatanakaMParasukWParasukVMorokumaKThe biginelli reaction is a urea-catalyzed organocatalytic multicomponent reactionJ Org Chem201580146959696726066623
- Apex2, Version 2 User Manual, M86-E01078, Bruker analytical X-ray systems Madison, WI; 2006.
- Siemens, SMART SystemSiemens Analytical X-ray Instruments IncMadison, MI1995
- BurlaMCCaliandroRCarrozziniBCrystal structure determination and refinement via SIR2014J Appl Cryst2015481306309
- SheldrickGA short history of SHELXActa Cryst Sect A200864111212218156677
- FarrugiaLWinGX suite for small-molecule single-crystal crystallographyJ Appl Cryst1999324837838
- SheldrickGMSADABSMadison, WIBruker AXS, Inc2007
- MacraeCFBrunoIJChisholmJAMercury CSD 2.0 – new features for the visualization and investigation of crystal structuresJ Appl Cryst2008412466470
- NardelliMPARST95 – an update to PARST: a system of Fortran routines for calculating molecular structure parameters from the results of crystal structure analysesJ Appl Cryst1995285659
- SpekASingle-crystal structure validation with the program PLATONJ Appl Cryst2003361713
- MossmanTRapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assaysJ Immunol Methods19836555636606682
- MartinAMorcilloNLemusDMulticenter study of MTT and resazurin assays for testing susceptibility to first-line anti-tuberculosis drugsInt J Tuberc Lung Dis20059890190616104638
- VenugopalaKNAlbericioFCoovadiaYMTotal synthesis of a depsidomycin analogue by convergent solid-phase peptide synthesis and macrolactonization strategy for antitubercular activityJ Pep Sci20111710683689
- RaoGBDAnjaneyuluBKaushikMPGreener and expeditious one-pot synthesis of dihydropyrimidinone derivatives using noncommercial [small beta]-ketoesters via the Biginelli reactionRSC Adv20144824332143325
- KutonovaKVTrusovaMEPostnikovPSFilimonovVDParelloJA simple and effective synthesis of aryl azides via arenediazonium tosylatesSynthesis2013451927062710
- ChopraDIs organic fluorine really “not” polarizable?Cryst Growth Des2012122541546
- PaniniPChopraDIn hydrogen bonded supramolecular structuresLiZWuLLecture Notes in ChemistryBerlin, HeidelbergSpringer-Verlag2015873767
- DeyDThomasSPSpackmanMAChopraD‘Quasi-isostructural polymorphism’ in molecular crystals: inputs from interaction hierarchy and energy frameworksChem Comm201652102141214426693707