Abstract
In spite of the usual combination form of methotrexate (MTX)/mitoxantrone (MIT) and various complex combination regimens of MTX/MIT with other anticancer drugs, the survival period, cure rate, and systemic toxicity still need to be improved. For this purpose, a nanostructured amino group-modified mesoporous silica nanoparticles (MSNN)−MTX/MIT was designed. In the preparation, the surface of mesoporous silica nanoparticles (MSNs) was modified with amino groups to form MSNN. The covalent modification of the amino groups on the surface of MSNN with MTX resulted in MSNN−MTX. The loading of MIT into the surface pores of MSNN−MTX produced nanostructured MSNN−MTX/MIT. Compared with the usual combination form (MTX/MIT), nanostructured MSNN−MTX/MIT increased the survival period greatly, heightened the cure rate to a great extent, and lowered the systemic toxicity of the treated S180 mice, significantly. These superior in vivo properties of nanostructured MSNN−MTX/MIT over the usual combination form (MTX/MIT) were correlated with the former selectively releasing MTX and MIT in tumor tissue and inside cancer cells in vitro. The chemical structure and the nanostructure of MSNN−MTX/MIT were characterized using infrared and differential scanning calorimeter spectra as well as transmission electron microscope images, respectively.
Introduction
Methotrexate (MTX) and mitoxantrone (MIT) exhibit antitumor actions in vitro and in vivo,Citation1–Citation4 as well as possess in vivo activity, which aids in treating multiple sclerosis,Citation5 but serious side effects of these drugs greatly limit the therapeutic efficacy. The side effects of MTX are stomatitis and alimentary canal bleeding,Citation6,Citation7 while MIT causes hematological and hepatic adverse effects.Citation8,Citation9 To prolong the survival period, heighten the cure rate, and reduce the systemic toxicity of MTX/MIT in treating cancer patients, various combination regimens, such as MIT/MTX/mitomycin followed by cisplatin/gemcitabine for treating unresectable malignant pleural mesothelioma,Citation10 MTX/MIT/mitomycin C for treating advanced breast cancer,Citation11–Citation14 MIT/cyclophosphamide/MTX/5-flurouracil/prednisone for treating advanced breast cancer,Citation15 MIT/MTX/folinic acid/methylprednisolone/vincristine/granulocyte for treating acute lymphoblastic leukemia,Citation16 carmustine/doxorubicin/etoposide/vincristine/cyclophosphamide/MIT/cytarabine/MTX/citrovorum factor for treating non-Hodgkin lymphoma,Citation17 and MIT/MTX/citrovorum for treating either metastatic or locally advanced transitional cell carcinoma of the bladder,Citation18 have been used. Besides, MTX- and MIT-related combinations were used to reduce the toxicity of other anticancer drugs, such as MTX/MIT/mitomycin C combination being used to reduce nausea, vomiting, alopecia, and cardiotoxicity of doxorubicin-based protocols.Citation19 Similar to the usual combination form (MTX/MIT), however, the survival period, the cure rate, and the systemic toxicity of the aforementioned combination regimensCitation20,Citation21 still need to be improved.
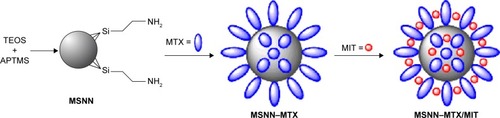
It is recognized commonly that loading a drug onto delivery vehicle leads to a decrease in toxicity.Citation22–Citation25 Among the various nanomaterials, mesoporous silica nanoparticles (MSNs) have an ordered structure, numerous holes, desirable hole size, acceptable biocompatibility, and biodegradability.Citation26 Functional modification of the surface of MSNs results in the establishment of a nanomedical platform either for various needs (such as imaging, diagnostics, and clinical therapy)Citation27 or for abolishing the side effects of the anticancer drugs in particular.Citation28 In this context, we modified the surface of MSNs with amino groups to prepare amino groups-modified mesoporous silica nanoparticles (MSNN), covalently linked MTX with the amino groups of MSNN to prepare MSNN−MTX, and loaded MIT into the surface pores to prepare MSNN−MTX/MIT (). Thus we used the nanomedical strategy of combining MTX and MIT rather than a usual combination form (MTX/MIT), for treating S180 mice, thereby showing the superiority of MSNN−MTX/MIT over MTX/MIT in the survival period, the cure rate, and the systemic toxicity.
Figure 1 Preparation of MSNN−MTX/MIT.
Abbreviations: MSN, mesoporous silica nanoparticles; MSNN, NH2-modified MSN; MTX, methotrexate; MIT, mitoxantrone; TEOS, tetraethyl orthosilicate; APTMS, 3-trimethoxysilyl-propane-1-amine on MSNN.
Experimental section
Preparing MSNN and MSNN−MTX
Based on previous literature, MSNN was prepared.Citation7 For the preparation of MSNN−MTX (MTX covalently modified MSNN), a solution of 285 mg (1.49 mmol) of N-ethyl-N′-(3-dimethyl-aminopropyl)-carbodiimide hydrochloride in 10 mL of anhydrous dimethyl sulfoxide was mixed with 95.7 mg (0.21 mmol) of MTX. Into this mixture, 275 mg of MSNN was added and the suspension stirred at room temperature for 24 hours to form MSNN−MTX. After centrifugation, the precipitates were successively washed with dimethyl sulfoxide, water, and ethanol to completely remove free MTX, ie, no MTX should be detected in the supernatant by absorption spectroscopy (Shimadzu UV-2550 spectrophotometer, Kyoto, Japan, 306 nm), and dried in vacuum for 24 hours. The content of covalently conjugated MTX was also identified using absorption spectroscopy.
Preparing MSNN−MTX/MIT
To a solution of 100 mg of MIT in 2 mL of ultrapure water, 1 g of MSNN−MTX was added. The suspension was ultrasonically oscillated for 20 minutes at room temperature and centrifuged at 120 rpm for 15 minutes; then, the collected precipitates were dried for loading. This loading procedure was repeated for five times by ultrasonically oscillating the dried precipitates in a solution of 100 mg MIT in 2 mL of ultrapure water to provide the MSNN−MTX/MIT. Gravimetric analysis showed that 1 g of MSNN−MTX/MIT contained 20 mg of MIT.
Measuring the release of MTX from MSNN−MTX
The release of MTX from 6.0 mg of MSNN−MTX was tested in 50 mL of phosphate-buffered solution (PBS; pH: 5.5, 37°C) with α-chymotrypsin (0.1 mg/mL). To alleviate the limitation of the release rate by external diffusion constraints, a constant rotation speed (120 rpm/min) was maintained during the test. The PBS was sampled and measured at 0.5, 1, 2, 3, 4, 6, 8, 10, 12, 24, 36, 51, 60, 72, 84, and 96 hours.
Measuring the release of MIT from MSNN−MTX/MIT
The release of MIT from 20.0 mg of MSNN−MTX/MIT was tested in 50 mL PBS (37°C, pH: 5.5 and 7.45). To alleviate the limitation of the release rate by external diffusion constraints, a constant rotation speed (120 rpm/min) was maintained during the test. The PBS was sampled and measured at 1, 3, 6, 9, 12, 24, 36, 48, 60, 72, 96, 120, 144, 180, 204, and 240 hours.
Measuring TEM images of MSNN−MTX/MIT
The nanostructures of MSN, MSNN−MTX, and MSNN−MTX/MIT were visualized on a transmission electron microscope (TEM; JSM-6360 LV, JEOL). Approximately 1 mg solution of MSNN or MSNN−MTX or MSNN−MTX/MIT in 1.5 mL of alcohol was added dropwise onto a formvar-coated copper grid, and then a drop of anhydrous ethanol was added to enhance the removal of water. Then, the grid was allowed to thoroughly dry in air and was then incubated at 37°C for 24 hours. The copper grids were viewed under a TEM and the nanostructures determined by counting over 100 species in randomly selected regions on the copper grids. All determinations were carried out in triplicate. The TEM was operated with an 80 kV electron beam. Images were recorded on an imaging plate (Gatan Bioscan camera model 1792, Gatan, Pleasanton, CA, USA) with a 20 eV energy window at 6,000–400,000× and were digitally enlarged.
Measuring the DSC spectra of MSNN−MTX/MIT
DSC (differential scanning calorimeter) spectra of MSNN, MIT, MTX, MSNN+MTX, MSNN−MTX, MSNN−MIT, and MSNN−MTX/MIT were recorded on a DSC (NETZSCH, 204F1). Approximately 1 mg sample of MSNN or MIT or MTX or MSNN+MTX or MSNN−MTX or MSNN−MIT or MSNN−MTX/MIT was added into aluminum crucible and analyzed at 20°C–300°C.
Measuring the IR spectra of MSNN−MTX/MIT
The infrared (IR) spectra of MSNN, MIT, MTX, MSNN+MTX, MSNN−MTX, MSNN−MIT, and MSNN−MTX/MIT were recorded using a FT-IR spectrometer (Thermo Nicolet IS5, Thermo Fisher Scientific, Waltham, MA, USA). Approximately 1 mg sample of MSNN or MIT or MTX or MSNN+MTX or MSNN−MTX or MSNN−MIT or MSNN−MTX/MIT was measured using the KBr pellet method at room temperature.
Particle size and zeta potential of MSNN−MTX/MIT
The particle size and zeta potential (ζ) potential of water (blank), MSNN, MSNN−MTX, and MSNN−MTX/MIT were measured using an instrument, Zeta PlusPotential Analyzer from Brookhaven Instruments Corporation (Holtsville, NY, USA) at room temperature. All samples were prepared by dissolving 1 mg of sample in 1.5 mL of water, and the experiments were repeated individually for three times.
In vivo tumor growth assays
Male Institute of Cancer Research (ICR) mice (6 weeks old, 18–22 g) were purchased from the Animal Center of Capital Medical University and housed in an air-conditioned room.
The care and handling of the animals were performed with the approval of Institutional Authority for Laboratory Animal Care of Capital Medical University and adhered to these guidelines. S180 cells were purchased from Vital River Laboratory Animal Technology Co., Ltd. Beijing, People’s Republic of China and xenografted subcutaneously in the mice. The mice were housed in sterile isolated cages at constant temperatures (22°C–25°C) and given free access to food and water. Tumor growth was monitored by measuring the perpendicular diameter of the tumors using a caliper. Tumor volume was calculated using the formula, Volume = Length × Width2/2 cm3. When the average volume of tumors xenograft reached 0.5 cm3, the mice were randomly divided into sodium carboxyl methyl cellulose (CMCNa; 0.5%, 10 mL/kg/d) group, MTX (6 mg/kg/d) + MIT (1 mg/kg/d) group, and MSNN−MTX/MIT (50 mg/kg/d) group, each with 12 mice. The mice were then intraperitoneally injected with CMCNa, MTX+MIT, and MSNN−MTX/MIT for 7 consecutive days. The mice were bred for another 6 days and then sacrificed for further investigations, such as survival analysis, tumor volume determination, and tumor weight test.
Liver/kidney toxicity assays
Plasma ALT and AST assays
Approximately 0.9 mL of blood of the mice receiving antitumor assay was collected into a syringe containing 0.1 mL of 3.8% sodium citrate and immediately centrifuged at 3,000 rpm for 10 minutes to get the plasma samples. The separated plasma was used to measure plasma levels of alanine transaminase (ALT) and aspartate transaminase (AST) according to the manufacturer’s instructions (AST/GOT testing kit, ALT/GPT testing kit; JCBIO Co., Nanjing, People’s Republic of China). In brief, 5 mL of plasma was added into the test wells of 96 microtiter plates, then 20 mL of Matrix liquid was added and incubated at 37°C for 30 minutes; then, 5 mL of plasma was added into the control wells of the 96 microtiter plates followed by addition of 20 mL of 2,4-dinitrophenylhydrazine to all wells. The plates were incubated at 37°C for 20 minutes. Then, 200 mL of 0.4 mol L−1 NaOH was added and incubated at 37°C for 15 minutes, and the absorbance of the yellow solutions was recorded at 510 nm to calculate vitality using the formula: (ODtest – ODcontrol)/Kcurve, where ODtest refers to the absorbance of the test well, ODcontrol refers to the absorbance of the control well, and Kcurve refers to the standard curve, which is calculated using the absorbance of the standard well following the manufacturer’s protocols.
Plasma Cr assay
Approximately 0.9 mL of blood of the mice receiving antitumor assay was collected into a syringe containing 0.1 mL of 3.8% sodium citrate and immediately centrifuged at 3,000 rpm for 10 minutes to get the plasma samples. The separated plasma was used to measure plasma levels of creatinine (Cr) according to the guidelines provided in the kit (Cr testing kit; JCBIO Co., Nanjing, People’s Republic of China). In brief, 6 mL of plasma or standard solution was added into the wells of 96 microtiter plates; then, 180 mL of the enzyme solution A was added and incubated at 37°C for 5 minutes followed by addition of 60 mL of the enzyme solution B. The samples were again incubated at 37°C for 5 minutes, and the absorbance of the purple solutions was recorded at 546 nm to calculate vitality using the formula: (ODtest – ODcontrol)/Kcurve, where ODtest refers to the absorbance of the test well, ODcontrol refers to the absorbance of the control well, and Kcurve refers to the standard curve.
Results and Discussion
As depicted in , the reaction of 3-trimethoxysilyl-propane-1-amine resulted in the formation of MSNN. The reaction between MSNN and MTX formed MSNN−MTX, ie, MTX covalently modified MSNN. UV analysis showed that 1 g of MSNN−MTX contained 120 mg of MTX. By repeated absorptions, more MIT was loaded into the holes of MSNN−MTX to form MSNN−MTX/MIT (20 mg MIT/g MSNN−MTX/MIT). Gravimetric analysis showed that 1 g of MSNN−MTX/MIT contained 20 mg of MIT.
In vivo activities of MSNN−MTX/MIT and MTX/MIT
In vivo antitumor evaluations were performed on male ICR mice. In brief, S180 cells were xenografted subcutaneously in mice housed in sterile isolated cages at 22°C–25°C. Tumor growth was monitored by measuring the perpendicular diameter of the tumors using a caliper. Tumor volume was calculated using the formula, Volume = Length × Width2/2 cm3. Seven days after the xenograft of S180 cells, the volume of the tumor reached ~0.5 cm3 and the mice were randomly divided into three groups of 12 mice each. The mice were intraperitoneally injected with CMCNa (0.5%, 10 mL/kg/d), MTX (6 mg/kg/d)/MIT (1 mg/kg/d), and MSNN−MTX/MIT (50 mg/kg/d) for 7 consecutive days. The mice were bred for an additional 6 days and then sacrificed for further investigations.
Systemic toxicity and survival period of S180 mice treated with MSNN−MTX/MIT and MTX/MIT
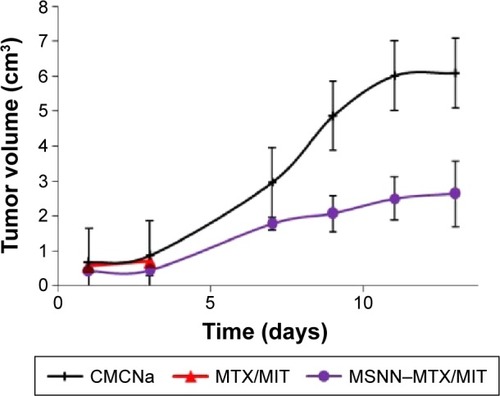
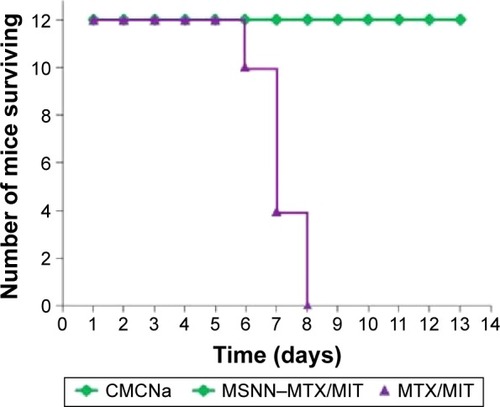
Lethality and the ratio of organ/bodyweight are used to indicate systemic toxicity and survival period after therapy. shows the lethality during a 13-day period in which S180 mice received CMCNa, MSNN−MTX/MIT, and MTX/MIT at the same dose. The lethality of CMCNa- or MSNN−MTX/MIT-treated S180 mice on day 13 was 0%, while the lethality of MTX/MIT-treated S180 mice on days 6, 7, and 8 was 17%, 67%, and 100%, respectively. The significant lethality suggests that for prolonging survival period, nanostructured MSNN−MTX/MIT is superior over the usual combination form (MTX/MIT).
Figure 2 Survival of S180 mice treated with CMCNa, MTX/MIT, and MSNN−MTX/MIT during the 7-day administration and the 13-day observation period.
shows the body weight and the ratios of organ weight/body weight of S180 mice receiving nanostructured MSNN−MTX/MIT and the usual combination form (MTX/MIT) at the same dose. The body weight and the ratios of liver, spleen, brain, and kidney weights to body weight of the mice that received MTX/MIT were significantly different from those of the mice that received CMCNa or MSNN−MTX/MIT, while the body weight and the ratios of liver, spleen, brain, and kidney weights to body weight of the mice that received CMCNa were at the same level as those of the mice that received MSNN−MTX/MIT. This suggests that treatment for even 6, 7, and 8 days with the usual combination form (MTX/MIT) still significantly slowed the growth and damaged the liver, spleen, brain, and kidney of the mice. In contrast, 13-day treatment with the nanostructured MSNN−MTX/MIT did not slow the growth and damage the liver, spleen, brain, and kidney of the mice. Therefore, for limiting systemic toxicity, the nanostructured MSNN−MTX/MIT was superior over the usual combination form (MTX/MIT).
Table 1 Body weight and ratios of organ weight/body weight of MSNN−MTX/MIT- and MTX/MIT-treated S180 mice
Tumor volume of S180 mice treated with MSNN−MTX/MIT and MTX/MIT
The superiority of nanostructured MSNN−MTX/MIT over the usual combination form (MTX/MIT) was demonstrated by comparing the tumor volume of S180 mice treated with the two different regimens. The treatment with MTX/MIT for 8 days induced all mice to die, and so the tumor volume of the mice treated for 13 days was unavailable (). On day 13, however, the tumor volume of CMCNa-treated mice was greater than twofold that of the mice treated with MSNN−MTX/MIT. Therefore, at the same dose as the usual combination form (MTX/MIT), nanostructured MSNN−MTX/MIT effectively slowed tumor growth and was found to be superior over the usual combination form (MTX/MIT) in tumor therapy.
Tumor weight of S180 mice treated with MSNN−MTX/MIT and MTX/MIT
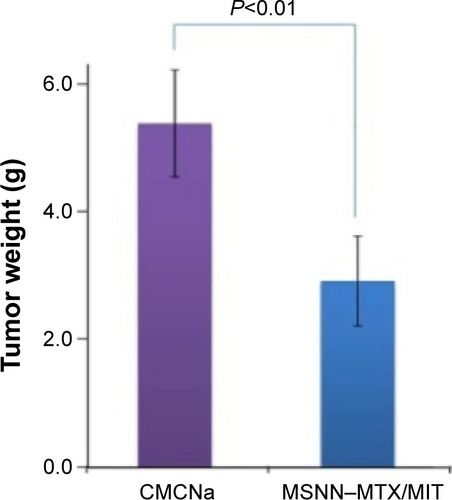
The superiority of nanostructured MSNN−MTX/MIT over the usual combination form (MTX/MIT) was mirrored with the tumor weight of the treated S180 mice. The treatment of MTX/MIT for 8 days induced all mice to die, and so the tumor weight of the mice treated for 13 days was unavailable. On day 13, the tumor weight of the mice treated with CMCNa was significantly higher than that of the mice treated with MSNN−MTX/MIT at the same dose as the usual combination form (MTX/MIT; ). Again, at the given dose, nanostructured MSNN−MTX/MIT effectively slowed tumor growth and was found to be superior over the usual combination form (MTX/MIT) in tumor therapy.
Liver and kidney toxicity of S180 mice treated with MSNN−MTX/MIT and MTX/MIT
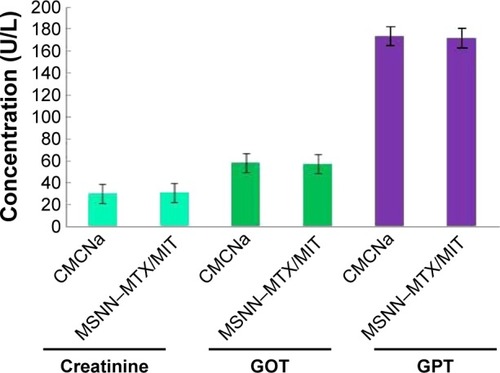
The superiority of nanostructured MSNN−MTX/MIT over the usual combination form (MTX/MIT) was also mirrored by the serum ALT, AST, and Cr levels of the treated S180 mice. Treatment with the usual combination form (MTX/MIT) for 8 days induced all S180 mice to die, and so the levels of serum ALT, AST, and Cr on day 13 were unavailable. The levels of serum ALT, AST, and Cr on day 13 in the S180 mice treated with CMCNa were equal to those of the S180 mice treated with MSNN−MTX/MIT at the same dose as the usual combination form (MTX/MIT) (). The serum levels of ALT and AST indicate liver toxicity, and the serum level of Cr represents kidney toxicity. The fact that S180 mice treated with CMCNa and MSNN−MTX/MIT, showed equal serum levels of ALT, AST, and Cr, implies that nanostructured MSNN−MTX/MIT caused no liver and kidney toxicity and is superior to the usual combination form (MTX/MIT) in limiting liver and kidney toxicity.
Figure 5 Serum ALT, AST, and Cr levels of the mice treated with CMCNa, MTX/MIT, and MSNN−MTX/MIT.
Release property of MSNN−MTX and MSNN−MTX/MIT
To understand the advantage of nanostructured MSNN−MTX/MIT in prolonging the survival period, heightening the cure rate, and limiting the toxicity of the treated S180 mice, the in vitro release of MSNN−MTX and MSNN−MTX/MIT was examined.
MSNN−MTX and MSNN−MTX/MIT releasing MTX
The release of MTX from 6 mg of MSNN−MTX was carried out in 50.0 mL of PBS (37°C, pH: 7.45 and 5.5) with or without protease. The cumulative release was monitored by sampling the PBS at 0.5, 1, 2, 3, 4, 6, 8, 10, 12, 24, 36, 51, 60, 72, 84, and 96 hours, and the curves are shown in . In pH 7.45 and 5.5 PBS, MSNN−MTX released less than 10% of MTX at all time points; however, in pH 5.5 PBS with 0.5 mM of α-chymotrypsin, the release of MTX was rapidly increased at 35 hours and reached the maximum at 70 hours, exhibiting a prolonged release manner.
Figure 6 α-Chymotrypsin induces the cumulative release profile of MTX from (A) MSNN−MTX and (B) MSNN−MTX/MIT in pH 5.4 PBS.
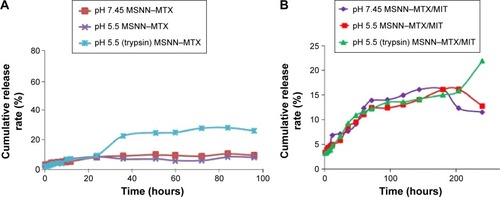
The release of MTX from 15.0 mg of MSNN−MTX/MIT was carried out in 50.0 mL PBS (37°C, pH: 7.45 and 5.5) with or without α-chymotrypsin (0.1 mg/mL), and these curves are shown in . Within 200 hours, in pH 5.5 and 7.45 PBS, MSNN−MTX/MIT slowly released MTX, and at 200 hours the release rate rapidly decreased. Although within 200 hours in pH 5.5 PBS with 0.5 mM of α-chymotrypsin, MSNN−MTX/MIT slowly released MTX, at 200 hours the release rate of MTX rapidly increased, and this was in a controlled manner.
It is well documented that the tumor tissue pH value is 5.5 and the level of α-chymotrypsin inside cancer cells is higher than that of α-chymotrypsin outside cancer cells.Citation29,Citation30 Therefore, the observations suggest that inside tumor tissue, but not inside normal tissue, and inside cytosol of cancer cells, but not outside cytosol of cancer cells, MTX will be optionally released from MSNN−MTX and MSNN−MTX/MIT. The loading of MIT onto the surface holes of MSNN−MTX changes the release manner, but does not influence the release of MTX.
MSNN−MTX/MIT releasing MIT in pH 5.5 and 7.45 PBS
The release of MIT from 15.0 mg of MSNN−MTX/MIT was carried out in 50.0 mL PBS (pH: 7.45 and 5.5) at 37°C by sampling the PBS at 1, 3, 6, 9, 12, 24, 36, 48, 60, 72, 96, 120, 144, and 180 hours, and the curves are shown in . In pH 7.45 PBS, no MIT was released, suggesting that in the pH environment of blood and healthy tissues, MSNN−MTX/MIT does not release MIT.Citation7 In pH 5.5 PBS, however, with prolonged duration, MIT is gradually released, suggesting that in the pH environment of tumor tissues MSNN−MTX/MIT releases MIT in a time-dependent manner.Citation31
Figure 7 MSNN−MTX/MIT releases MIT in a time- and pH-dependent manner.
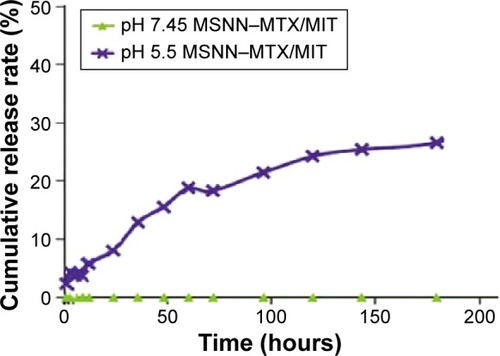
Nanostructured MSNN−MTX/MIT selectively releasing both MTX and MIT in tumor tissue, but not in normal organ tissue, would be responsible for prolonged survival period, heightened cure rate, and lowered systemic toxicity. Therefore, the release profiles of MSNN−MTX/MIT in tumor evidence the advantage of the nanostructured regimen.
Nanoproperty of MSNN−MTX/MIT
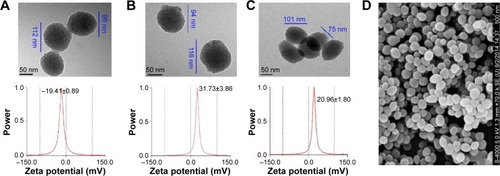
The size, shape, and surface feature of the nanoparticles of MSNN−MTX/MIT were studied. The images were recorded on a TEM (JEOL-200CX, Tokyo, Japan) and are shown in . The TEM images indicate that the diameter of the nanoparticles of MSN ranged from 96 to 112 nm and the diameter of the surface holes was ~2.5 nm (). The TEM images indicate that the diameter of the nanoparticles of MSNN−MTX was 94–116 nm and the diameter of the surface holes was ~2.0 nm (). The TEM images indicate that the diameter of the nanoparticles of MSNN−MTX/MIT was 75–101 nm and the diameter of the surface holes was ~1.5 nm (). It is likely that the covalent modification of MTX resulted in a slight increase in the size of the nanoparticle and that the load of MIT resulted in an obvious decrease in the diameter of their surface holes. It is generally accepted that nanoparticles of 77–116 nm in diameter are suitable for cell uptake and that holes of 1.3–2.5 in diameter benefit drug loading and release.Citation31,Citation32 The structure of MSNN−MTX/MIT in solid state was assessed by scanning electron microscopy (HITACHI S-4800, Tokyo, Japan), and these images are also provided (). Scanning electron microscopy images indicate that, in the solid state, MSNN−MTX/MIT exists as well-distributed nanoparticles of ~100 nm in diameter.
Figure 8 TEM images and ζ potentials.
Abbreviations: TEM, transmission electron microscopy; MTX, methotrexate; MIT, mitoxantrone; MSN, mesoporous silica nanoparticle; MSNN, amino group-modified MSN; SEM, scanning electron microscopy.
Characterization of MSNN−MTX/MIT
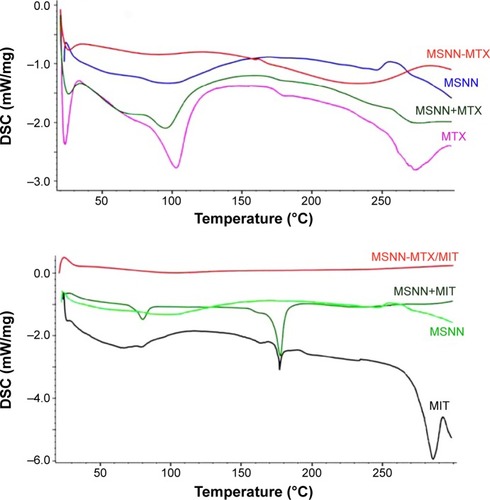
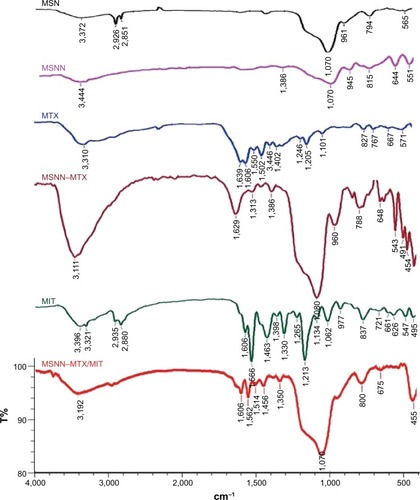
The chemical structure was explained with IR and DSC (Netzsch, Selb, Germany) spectra. As can be seen in , after the conversion of MSN to MSNN, a peak appeared at 3,444 cm−1, suggesting that the surface of MSN was modified by NH groups; after the conversion of MSNN to MSNN−MTX, a broad peak appeared at ~2,500 to ~3,400 cm−1 and another peak appeared at 1,629 cm−1, suggesting that the NH2 groups on the surface of MSNN were amidated by one carboxyl group of MTX. After the loading of MIT onto the surface holes of MSNN−MTX, the significant peaks of both MIT and MSNN−MTX are involved in the IR spectrum of MSNN−MTX/MIT. Therefore, IR spectra support the reliability of the preparation.
Figure 9 IR spectra of MSN, MSNN, MTX, MIT, MSNN−MTX/MIT, and MSNN−MTX.
The DSC spectra of MTX and the mixture of MSNN+MTX are characterized by three peaks at ~25°C, ~100°C, and ~270°C, whereas the DSC spectrum of MSNN−MTX had no comparable peak (), suggesting that covalent modification, but not simple mixing, resulted in MSNN−MTX. The DSC spectra of MIT and the mixture of MSNN+MTX are characterized by two peaks at ~80°C and ~180°C, whereas the DSC spectrum of MSNN−MTX/MIT had no comparable peak, suggesting that the loading of MIT into the surface holes of MSNN−MTX resulted in the complete integration of MSNN−MTX and MIT. Therefore, DSC spectra also support the reliability of the preparation.
Conclusion
To prolong the survival period, heighten the cure rate, and lower the systemic toxicity of the usual combination form (MTX/MIT) in treating cancer patients, various combinations of MTX/MIT plus other anticancer drugs have been clinically used. Because all these regimens and the usual combination form (MTX/MIT) have similar limitations, the combination researches remain to be of clinical importance. The in vitro selective release of MTX and MIT in tumor tissue and inside cancer cells and the in vivo prolongation of the survival period, obvious heightening of the cure rate, and significant lowering of the systemic toxicity of the treated S180 mice emphasize that nanostructured MSNN−MTX/MIT is a promising regimen to alter the usual combination form (MTX/MIT) and various combinations of MTX/MIT plus other anticancer drugs.
Acknowledgments
This work was supported by the Beijing area major laboratory of peptide and small molecular drugs, by PHR (IHLB), by the NSFC (21171120, 81172930, 81270046, and 81373265), Beijing Natural Science Foundation (7132020 and 7132032), Beijing Nova Programme xx2013039, 863 project 2015AA020902, TJSHG201310025008, Project for Science and Technology Development, Beijing Commission of Education (SQKM201210025007), and the Project of Construction of Innovative Teams and Teacher Career Development for Universities and Colleges Under Beijing Municipality. We thank Engineering Research Center of Endogenous Prophylactic of Ministry of Education of China for technical support.
Disclosure
The authors report no conflicts of interest in this work.
References
- LünenbürgerHLanvers-KaminskyCLechtapeBFrühwaldMCSystematic analysis of the antiproliferative effects of novel and standard anticancer agents in rhabdoid tumor cell linesAnticancer Drugs20102151452220147838
- VolkELRohdeKRheeMMethotrexate cross-resistance in a mitoxantrone-selected multidrug-resistant MCF7 breast cancer cell line is attributable to enhanced energy-dependent drug effluxCancer Res200060133514352110910063
- SenguptaSEavaroneDCapilaITemporal targeting of tumour cells and neovasculature with a nanoscale delivery systemNature200543656857216049491
- ShinHCAlaniAWRaoDARockichNCKwonGSMulti-drug loaded polymeric micelles for simultaneous delivery of poorly soluble anticancer drugsJ Control Release2009140329430019409432
- DoughtyJCKaneECookeTGMcArdleCSMitoxantrone and methotrexate chemotherapy with and without mitomycin C in the regional treatment of locally advanced breast cancerBreast2002111979914965654
- KumarAEffectiveness of mitoxantrone and oral methotrexate combination therapy in relapsing-remitting multiple sclerosisJ Neurol Sci2009285S1S155S339
- KadiaTMKantarjianHMThomasDAPhase II study of methotrexate, vincristine, pegylated-asparaginase, and dexamethasone (MOpAD) in patients with relapsed/refractory acute lymphoblastic leukemiaAm J Hematol201590212012425368968
- UkmarTMaverUPlaninšekOKaučičVGaberščekMGodecAUnderstanding controlled drug release from mesoporous silicates: theory and experimentJ Control Release2011155340941721763374
- RossatoLGCostaVMDallegraveECumulative mitoxantrone-induced haematological and hepatic adverse effects in a subchronic in vivo studyBasic Clin Pharmacol Toxicol2014114325426224119282
- PintoCMarinoAManziniVDPSequential chemotherapy with cisplatin/gemcitabine (CG) followed by mitoxantrone/methotrexate/mitomycin (MMM) in patients with malignant pleural mesothelioma. A multicenter Italian Phase II Study (SITMP1)Lung Cancer200652219920616542747
- SteinRCBowerMLawMMitozantrone and methotrexate chemotherapy with and without mitomycin C in the treatment of advanced breast cancer: a randomised clinical trialEuropean Journal of Cancer199228A12196319651419290
- PintoCMarinoAGuaraldiMCombination chemotherapy with mitoxantrone, methotrexate, and mitomycin (MMM Regimen) in malignant pleural mesothelioma: a phase II studyAm J Clin Oncol200124214314711319288
- Harper-WynneCEnglishJMeyerLRandomized trial to compare the efficacy and toxicity of cyclophosphamide, methotrexate and 5-fluorouracil (CMF) with methotrexate mitoxantrone (MM) in advanced carcinoma of the breastBr J Cancer199981231632210496359
- KakolyrisSSamonisGKoukourakisMFirst-line treatment with mitoxantrone, methotrexate, vincristine, and carboplatine (MIMOC) plus cyclical hormonotherapy with tamoxifen and megestrol acetate in advanced breast cancerAm J Clin Oncol199922327327710362335
- LeeCKGebskiVJCoatesASTradeoffs in quality of life and survival with chemo-therapy for advanced breast cancer: mature results of a randomized trial comparing single-agent mitoxantrone with combination cyclophosphamide, methotrexate, 5-fluorouracil and prednisoneSpringerplus2013239140024024086
- Di BonaEPoglianiERossiGTransplant-finalized salvage of adult acute lymphoblastic leukemia: results of a mitoxantrone and methotrexate-based regimen in 36 patientsLeuk Lymphoma200546687988416019533
- RigacciLCarraiVNassiLCombined chemotherapy with carmustine, doxorubicin, etoposide, vincristine, and cyclophosphamide plus mitoxantrone, cytarabine and methotrexate with citrovorum factor for the treatment of aggressive non-Hodgkin lymphoma: a long-term follow-up studyCancer2005103597097715666323
- PectasidesDMylonakisAAntoniouFChemotherapy with methotrexate, carbo-platin, mitoxantrone (Novantrone) and vincristine (Oncovin) in transitional-cell urothelial cancerOncology19985521391449499188
- RainaVSharmaADeoSVMitoxantrone, mitomycin-C, methotrexate combination chemotherapy with radiotherapy and/or surgery in stage III (T4B, NO-2, M0) breast cancerJ Assoc Physicians India1998461192692911229215
- van OutryveSSchrijversDvan den BrandeJMethotrexate-associated liver toxicity in a patient with breast cancer: case report and literature reviewNeth J Med200260521622212365478
- ConwayDCohenJACombination therapy in multiple sclerosisLancet Neurol20109329930820170843
- LammersTImproving the efficacy of combined modality anticancer therapy using HPMA copolymer-based nanomedicine formulationsAdv Drug Deliv Rev201062220323019951732
- KrishnamachariYSalemAKInnovative strategies for codelivering antigens and CpG oligonucleotidesAdv Drug Deliv Rev200961320521719272328
- PasquierEKavallarisMAndréNMetronomic chemotherapy: new rationale for new directionsNature Reviews Clinical Oncology201078455465
- MasciGLosurdoAGandiniCLow-dose “metronomic chemotherapy” with oral cyclophosphamide and methotrexate in metastatic breast cancer: a case report of extraordinarily prolonged clinical benefitEcancermedicalscience20126127523130087
- MaTYLiuLYuanZYDirect synthesis of ordered mesoporous carbonsChem Soc Rev20134293977400323132523
- Giménez-BonaféPTortosaAPérez-TomásROvercoming drug resistance by enhancing apoptosis of tumor cellsCurr Cancer Drug Targets20099332034019442052
- KunjachanSBłaużAMöckelDOvercoming cellular multidrug resistance using classical nanomedicine formulationsEuropean Journal of Pharmaceutical Sciences Official Journal of the European Federation for Pharmaceutical Sciences201245442142821907796
- YadaKShibataKMatsumotoTOhtaMYokoyamaSKitanoSProtease-activated receptor-2 regulates cell proliferation and enhances cyclooxygenase-2 mRNA expression in human pancreatic cancer cellsJ Surg Oncol2005892798515660373
- PimentaDCChenVCChaoJAlpha1-antichymotrypsin and kallistatin hydrolysis by human cathepsin DJournal of Protein Chemistry200019541141811131147
- ChungTHWuSHYaoMThe effect of surface charge on the uptake and biological function of mesoporous silica nano-particles in 3T3-L1 cells and human mesenchymal stem cellsBiomaterials200728192959296617397919
- HuangXTengXChenDTangFHeJThe effect of the shape of mesoporous silica nanoparticles on cellular uptake and cell functionBiomaterials201031343844819800115