Abstract
The purpose of this study was to induce experimental periodontitis in rats previously fed diets containing arginine silicate inositol (ASI) complex and examine the biochemical, immunological, and radiological effects. Fifty two 8-week-old female Sprague Dawley rats were equally divided into four groups. The control group included those fed a standard rat diet with no operation performed during the experiment. The periodontitis, ASI I, and ASI II groups were subjected to experimental periodontitis induction for 11 days after being fed a standard rat diet alone, a diet containing 1.81 g/kg ASI complex, or a diet containing 3.62 g/kg ASI complex, respectively, for 8 weeks. Throughout the 11-day duration of periodontitis induction, all rats were fed standard feed. The rats were euthanized on the eleventh day, and their tissue and blood samples were collected. In the periodontitis group, elevated tissue destruction parameters and reduced tissue formation parameters were found, as compared to the ASI groups. Levels of enzymes, cytokines, and mediators associated with periodontal tissue destruction were lower in rats fed a diet containing ASI complex after experimental periodontitis. These results indicate that ASI complex could be an alternative agent for host modulation.
Introduction
Periodontitis is a destructive, inflammatory, pathological condition that affects the connective tissue attachment between the alveolar bones of the teeth.Citation1 The main etiologic factor of periodontitis is an interaction between periodontal pathogenic bacteria and host tissue response.Citation2–Citation4 Since discovering the importance of host immune response in the pathogenesis of periodontal diseases, scientists have investigated host modulation therapy, which alters host tissue response to reduce destructive factors and increase preventive mediators.Citation5,Citation6 Various host modulatory agents have been investigated, such as growth factors, tetracycline, antiosteoclastic drugs, antioxidants, enamel matrix proteins, anti-inflammatory drugs, and bone morphogenetic proteins.Citation5,Citation7–Citation10 Despite continued research on host modulation therapy, only two agents for host modulation have been approved by the United States Food and Drug Administration (FDA): subantimicrobial-dose tetracycline and enamel matrix protein.
Arginine silicate inositol (ASI) complex is a novel, bioavailable source of arginine and silicon that could serve as an alternative agent for host modulation.Citation11–Citation14 The effects of arginine and silicon on bone and connective tissue metabolism in mammals are well documented, and the ASI complex has been shown to benefit bone and vascular health.Citation15 Several clinical studies have demonstrated the safety of ASI complex components. No adverse effects were observed following administration of arginine.Citation16,Citation17 Similarly, no adverse effects were seen following daily administration of 12 g of inositol for 4 weeks or 45 mg of silicon for 31 days.Citation18,Citation19
Recent studies suggest that dietary arginine and silicon play an important role in the development, growth, and remodeling of long bones.Citation20 Arginine is involved in collagen synthesis and the production of growth hormones, nitric oxide (NO), and insulin-like growth factor 1 (IGF-1).Citation21–Citation23 Arginine administration is clinically suggested for patients recovering from some illnesses and injuries and for conditions related to metabolic disturbance of calcium absorption, such as growth deficiency, dentition and ossification defects, rachitis, osteomalacia, and decalcification.Citation24,Citation25 Several roles for silicon have been defined, largely based on animal studies, and its most important function is in the growth and development of bone, cartilage, and connective tissue.Citation26,Citation27 There is a direct relationship between silicon and calcium,Citation28 and silicon is associated with calcium at an early stage of bone formation.Citation29 Additionally, silicon appears to be required for the synthesis of collagen, which is the protein matrix found in connective tissue and is the most abundant protein in the human body.Citation28
Although the roles of arginine and silicon in osteoporosis, bone metabolism, connective tissue formation, and collagen metabolism are well documented, information about the effects of ASI complex on host modulation in periodontal diseases is limited. This study aimed to investigate the effects of ASI complex supplementation on periodontal tissues during the periodontitis formation process.
Materials and methods
Animals and study design
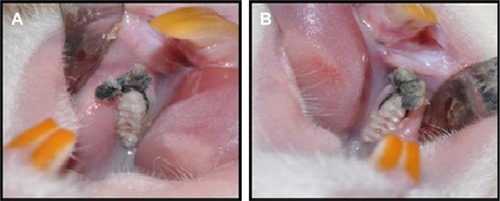
Fifty-two female Sprague Dawley rats were used in the experiment. Their average body weight was 135±3 g at the beginning of the experiment. The animals were kept in temperature-controlled cages (~25°C), exposed to 24-hour light–dark cycles of equal time, and had free access to food and water. The study protocol and experimental design were approved by the animal ethics committee of Inonu University. All rats received humane care according to the standards defined in the Guide for the Care and Use of Laboratory Animals organized by the Ministry of Agriculture and permitted by the Ethics Committee of the University of Inonu, Malatya, Turkey. Procedures for animal selection, feeding duration, and feeding methods were taken from Proctor et al.Citation12 The rats were randomly divided into the following four groups: 1) control group (n=13), fed a basal diet () for 8 weeks with no ligature placement; 2) periodontitis group (n=13), fed a basal diet for 8 weeks prior to 11-day ligature placement; 3) ASI I group (n=13), fed a supplementation of 1.81 g/kg of ASI complex for 8 weeks prior to 11-day ligature placement; and 4) ASI II group (n=13), fed a supplementation of 3.62 g/kg of ASI complex for 8 weeks prior to 11-day ligature placement. General anesthesia was administered using ketamine (40 mg/kg; Pfizer, Istanbul, Turkey). To induce experimental periodontitis, 4-0 silk sutures were placed and knotted submarginally by the same operator around the gingival margin of the right and left mandibular molars of the rats. The sutures were checked after application, and lost or loose sutures were replaced (). All rats were fed a standard diet for the 11 days during experimental periodontitis and euthanized by anesthetic overdose (two to three times the anesthetic dosage – of ketamine, administered intramuscularly) on the eleventh day. The mandibles were then separated from muscle and soft tissues.
Table 1 Composition of basal diet
Western blot analysis
Gingival tissue samples were collected around the right mandibular first molar tooth and were stored at −80°C. Gingival tissues from each group of rats were pooled together for Western blot analysis. Frozen tissue samples were homogenized in phosphate-buffered saline (PBS) with protease inhibitor cocktail, and the protein concentration was quantitated. The gingival tissue samples (20 mg of protein per lane) were mixed with sample buffer, boiled for 5 minutes, separated by sodium dodecyl sulfate–polyacrylamide (12%) gel electrophoresis under denaturing conditions, and electroblotted onto nitrocellulose membranes. The nitrocellulose blots were washed in PBS and blocked with 1% bovine serum albumin in PBS for 1 hour prior to application of the primary antibody. The primary antibody was diluted 1:1,000 in the same buffer containing 0.05% Tween 20. The nitrocellulose membrane was incubated overnight at 4°C with protein antibodies. Antibodies against interleukin 1β (IL-1β), matrix metalloproteinase 8 (MMP-8), receptor activator of nuclear factor-κB (RANK) and RANK ligand (RANKL), osteoprotegerin (OPG), myeloperoxidase (MPO), and β-actin (purchased from Santa Cruz Biotechnology Inc, Dallas, TX, USA) were used to probe the separate membranes. The next day, the immune reaction was continued with secondary goat anti-rabbit horseradish peroxidase-conjugated antibody after washing for 2 hours at room temperature. Specific binding was detected using diaminobenzidine and H2O2 as substrates. Blotting was performed at least four times to confirm data reproducibility. Protein levels were analyzed densitometrically using an image analysis system.
Radiological analysis
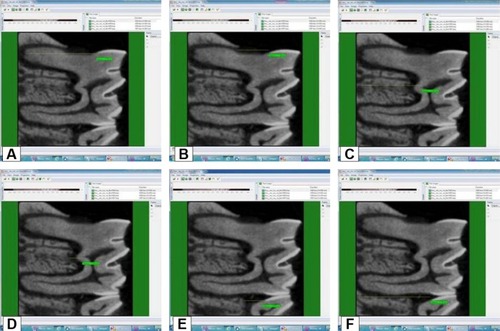
Micro-computed tomography (micro-CT) analysis was performed to quantify interradicular and interproximal alveolar bone of the right first lower molars. The X-ray source was operated at 100 kV with a 0.5 mm aluminum filter, and the image size was set to 11 megapixels. A total of 400–450 reslices with a thickness of 13–14 µm were selected from the top of the cusp down to the alveolar bone in the sagittal plane for each specimen. Samples were scanned with 360° rotation with a rotation step of 0.40°. Cross-sectional images (compatible with the Digital Imaging and Communication in Medicine standard) were converted to bitmap file format. The crude radiological images were obtained after micro-CT analysis using NRecon software (version 1.6.9.4; SkyScan, Kontich, Belgium). Pollution and image artifacts were eliminated prior to mathematical analysis. Processed radiological images were repositioned in each of the three planes of space using DataViewer (version 1.5.0, 64-bit; SkyScan), and areas outside the field of radiological images were removed. Sizes were reduced to make mathematical analysis faster. The resulting new data series was loaded onto CT analyzer (version 1.13.5.1; SkyScan), and the first and last sections in the sagittal plane sections were established. Considering the number of sections in the selected cross section range, the linear function of distance was measured between the following locations, using the program in ten sections: mesial cementoenamel junction to mesial root apex, mesial cementoenamel junction to mesial crestal bone, furcation roof to furcation root apex, furcation roof to furcation crestal bone, distal cementoenamel junction to distal root apex, and distal cementoenamel junction to distal crestal bone (). An average of ten measurements was taken from each specimen by a blinded and calibrated examiner.
Figure 2 Measurement of the distances between (A) MMCJ and MRA, (B) MMCJ and MRA, (C) FR and FRCB, (D) FR and FRRA, (E) DMCJ and DCB, (F) DMCJ and DRA.
Statistical analysis
Statistical analysis was performed using SPSS Statistics (version 22.0; IBM Corporation, Armonk, NY, USA). The appropriateness of the normal distribution of the parameters was assessed by the Kolmogorov–Smirnov test. The differences between groups were tested. A one-way analysis of variance was performed for the parameters showing a normal distribution. Tukey’s honestly significant difference test was used to detect which group caused the difference. Between-group differences were tested using the Kruskal–Wallis H test for parameters showing a non-normal distribution, and the Mann–Whitney U-test was used to detect which group caused the difference. Results yielding P-values <0.05 were considered statistically significant.
Results
Western blot results
The RANK level in the control group was significantly lower than that in the periodontitis, ASI I, and ASI II groups (P<0.05). The RANK level in the ASI I group was significantly higher than that in the control and ASI II groups, and the RANK level in the periodontitis group was significantly higher than that in the ASI II group (P<0.05). No statistically significant differences were detected for RANK levels between the ASI I and ASI II groups and between the periodontitis and ASI I groups (P>0.05) () (; ). The RANKL level in the periodontitis group was significantly higher than that in the control, ASI I, and ASI II groups (P<0.05). The RANKL level in the control group was significantly lower than that in the ASI I and ASI II groups (P<0.05). No statistically significant differences were found between the ASI I and ASI II groups for RANKL levels (P>0.05) (; ).
Table 2 Western blot findings of the groups
Figure 3 (A) Control, (B) Periodontitis, (C) ASI I and (D) ASI II.
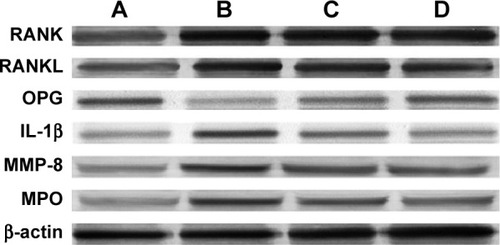
The OPG level in the periodontitis group was significantly lower than that in the control, ASI I, and ASI II groups (P<0.05). The OPG level in the ASI I group was significantly lower than that in the control and ASI II groups (P<0.05). The OPG level in the ASI II group was significantly lower than that in the control group (P<0.05) (; ).
Significantly higher tissue level of IL-1β was found in the ligature-induced periodontitis group in comparison to control animals, independently of experimental periods (P<0.05). However, significantly lower concentrations of IL-1β were found in the ASI-treated groups (ligature-induced periodontitis and ASI) in comparison to positive control animals, independently of experimental periods (P<0.05; , ).
A significant increase in MMP-8 and MPO levels, an index of tissue granulocytic infiltration, was found in the periodontitis group when compared with the control group. A significant decrease in MMP-8 and MPO levels was found in the ASI groups when compared with the positive and control groups (P<0.05; , ).
Radiological results
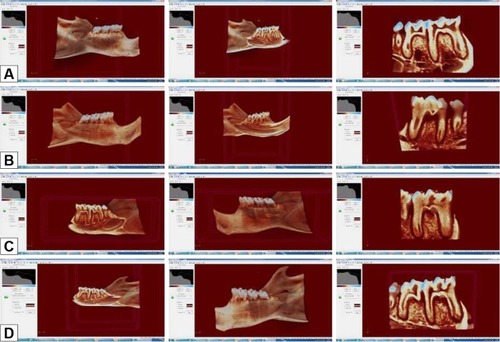
The mesial bone loss ratio in the control group was significantly lower than that in the periodontitis, ASI I, and ASI II groups (P<0.05). The mesial bone loss ratio in the periodontitis group was significantly higher than that in the ASI I and ASI II groups (P<0.05). No statistically significant difference was detected between the ASI I and ASI II groups for the ratio of mesial bone loss (P>0.05) (; ). The furcation bone loss ratio in the control group was significantly lower than that in the periodontitis, ASI I, and ASI II groups (P<0.05). The furcation bone loss ratio of the periodontitis group was significantly higher than that in the ASI I and ASI II groups (P<0.05). No statistically significant difference was detected between the ASI I and ASI II groups for the ratio of furcation bone loss (P>0.05) (; ). No statistically significant difference was detected between the ASI I and ASI II groups for the ratio of distal bone loss (P>0.05) (; ).
Table 3 MCT findings of the groups
Figure 4 Radiologic images of the groups.
Abbreviation: ASI, arginine silicate inositol.
Discussion
Although research on new agents for host modulation therapy to treat periodontal diseases is ongoing, the FDA has only approved subantimicrobial-dose tetracycline and enamel matrix protein for host modulatory therapy.Citation5,Citation7–Citation10 Research has demonstrated that arginine and silicon increase immune function and osteoblastic activity, as well as wound healing in bone and connective tissues.Citation11–Citation15 Based on these data, this study aimed to investigate the effects of ASI complex supplementation on periodontal tissues during periodontitis formation.
The ligature-induced periodontitis model was used in accordance with the literature.Citation30–Citation32 The tissue destruction parameters in the periodontitis group were higher than those in the control group, which is in line with previously reported studies that suggest ligature-induced periodontitis.Citation32–Citation35 Toker et al,Citation31,Citation32 in their study inducing periodontitis by binding silk sutures around rat molar teeth, reported that plaque and bacteria accumulation increased in the region where silk sutures contacted the tissue.
Two components of the product used in this study, arginine and silicon, have been shown to increase osteogenesis.Citation24,Citation28,Citation29 Arginine deficiency may be involved in osteoporosis, fractures, and bone defects.Citation21 Arginine is involved in the synthesis of growth hormone, IGF, as well as polyamines and L-proline, which act as substrates for collagen synthesis.Citation21–Citation23 As the precursor of NO, which has been shown to prevent corticosteroid-induced bone loss in mature rats,Citation36 arginine is an essential amino acid and is necessary for the antimicrobial activity of polymorphonuclear leukocytes (PMNLs), macrophages, and mononuclear cells.Citation37 Moffat et alCitation38 found in an in vitro study that arginine had an effect on NO synthase (NOS) and also caused an increase in phagocytic effects of PMNLs against Staphylococcus aureus. When leukocytes are stimulated with lipopolysaccharides, they produce inducible NOS from NO. This mechanism is an important part of the innate and adaptive immune systems. The presence of inducible NOS in macrophages and neutrophils plays an essential role in the antimicrobial function of these cells against fungi, viruses, parasites, bacteria, malignant cells, and intracellular protozoa.Citation39 Torricelli et alCitation40 reported that arginine in human osteoblasts markedly increased certain parameters, called bone formation markers, such as alkaline phosphatase, NO, type I collagen, and IGF. These markers increased the synthesis of bone matrix, acting at the cellular level in bone tissue. Torricelli et alCitation40 also reported that the effects of arginine on bone tissue were directly related to the effect on the cell proliferation mechanism. In the same study, IL-6 production was determined to be lower in osteoblasts exposed to arginine.Citation40
Silicon has been implicated as an important component in bone formation, and its deficiency negatively influences skeletal development.Citation41 The primary effect of silicon on bone and cartilage is thought to be through matrix synthesis, although it also has a significant influence on calcification.Citation42,Citation43 Silicon supplementation inhibits bone resorption and increases trabecular bone volume and bone mineral density in postmenopausal women with osteoporosis.Citation42,Citation43 Although limited in vivo research has been done, silicon increases osteoblastic activity and decreases osteoclastic activity in vitro.Citation13,Citation14,Citation44 Schroder et alCitation45 and Wiens et alCitation46 reported that the OPG produced increased osteoblast-like cells exposed to silicate, but no changes occurred in the production of the receptor activator of RANKL. These researchers stated that the increased OPG emission suppressed RANKL activity and consequently increased osteoclastogenesis. Additionally, Sahin et alCitation13 and Onderci et alCitation14 examined the effects of ASI complex on bone tissue in a study on poultry fed a diet supplemented with either 500 mg/kg or 1,000 mg/kg ASI. In these studies, increased levels of bone mineral density, calcium, and phosphorus were seen in the tibia, and increased levels of osteocalcin and alkaline phosphatase were found in the serum, depending on dose in both of the experimental groups compared to the controls. The results of this study, which indicate that the ASI complex could be effective in reducing tissue destruction parameters, confirm these previous results.
Periodontitis can initiate tissue and alveolar bone destruction through the activation of several proinflammatory cytokines, including IL-1β.Citation47 Cytokines might have a direct influence on bone remodeling, as IL-1 seems to stimulate osteoblasts to produce a set of cytokines (IL-6 and tumor necrosis factor [TNF]-α) that are capable of directly influencing bone resorption processes.Citation48 These cytokines play key roles in the breakdown of periodontal tissue through collagenolytic enzymes such as MMPs (a family of related zinc-containing proteinases), which can degrade most of the extracellular matrix.Citation49 Additionally, the balance between bone resorption and bone formation is regulated by the production of RANKL and its decoy receptor, OPG, both essential molecules for osteoclast differentiation supported by osteoblasts.Citation49,Citation50 This study demonstrated that the administration of ASI significantly reduced the levels of bone-related molecules (RANK and RANKL), neutrophil infiltration, and cytokine production in gingival tissue (IL-1β), as well as collagenolytic enzymes (MMP8), in the experimental periodontitis-induced rats. However, ASI supplementation increased OPG levels in the experimental periodontitis-induced rats. These results are compatible with results from other studies reporting that the compounds of ASI were effective on important mechanisms of the immune system, such as lymphocyte growth and the excessive release of proinflammatory cytokines.Citation13,Citation16 Similar to our results, Sahin et alCitation13 reported that dietary ASI supplementation decreased serum concentrations of TNF-α and C-reactive protein in stressed animals.
Although this study showed that dietary ASI complex was associated with decreased levels of indicators of periodontal tissue destruction, the effects of arginine and silicon were not evaluated separately. This limitation must be considered when evaluating the findings. It is difficult to examine the difference between the synergistic interaction formed by the distinctive impact levels of the individual materials and the combined use of the same materials.
Conclusion
Although this study has limitations that must be considered, it appears that ASI complex supplementation could have decreased periodontal tissue destruction in the rat experimental ligature-induced periodontitis model. Further studies on the effects of ASI complex supplementation on periodontal tissue destruction are needed, as well as research on the effects of new agents on the mechanisms of tissue destruction.
Acknowledgments
We thank Nurhan Sahin and Cemal Orhan for their collaboration in our experiments. This work was supported by the Scientific Research Project Fund of Inonu University under project number 2013/88 and the Turkish Academy of Sciences (to KS).
Disclosure
The authors report no conflicts of interest in this work.
References
- GumusPNizamNNalbantsoyAOzcakaOBuduneliNSaliva, serum levels of pentraxin-3 and interleukin-1beta in generalised aggressive or chronic periodontitisJ Periodontol20148534046
- SalviGELangNPHost response modulation in the management of periodontal diseasesJ Clin Periodontol200532suppl 610812916128833
- HasturkHKantarciAVan DykeTEOral inflammatory diseases and systemic inflammation: role of the macrophageFront Immunol2012311811722679445
- Yucel-LindbergTBageTInflammatory mediators in the pathogenesis of periodontitisExpert Rev Mol Med20131517
- ElavarasuSSekarSMuruganTHost modulation by therapeutic agentsJ Pharm Bioallied Sci20124suppl 2256259
- UstunKErciyasKKisacikBHost modulation in rheumatoid arthritis patients with TNF blockers significantly decreases biochemical parameters in periodontitisInflammation20133651171117723649513
- TariqMIqbalZAliJTreatment modalities and evaluation models for periodontitisInt J Pharm Investig201223106122
- KaraAAkmanSOzkanlarSImmune modulatory and antioxidant effects of melatonin in experimental periodontitis in ratsFree Radic Biol Med201355212623146767
- AkmanSCanakciVKaraATozogluUArabaciTDagsuyuIMTherapeutic effects of alpha lipoic acid and vitamin C on alveolar bone resorption after experimental periodontitis in rats: a biochemical, histochemical, and stereologic studyJ Periodontol201384566667422702517
- HakkiSSFosterBLNagatomoKJBone morphogenetic protein-7 enhances cementoblast function in vitroJ Periodontol201081111663167420681807
- ProctorSDKellySERussellJCA novel complex of arginine-silicate improves micro- and macrovascular function and inhibits glomerular sclerosis in insulin-resistant JCR:LA-cp ratsDiabetologia20054891925193215991019
- ProctorSDKellySEVineDFRussellJCMetabolic effects of a novel silicate inositol complex of the nitric oxide precursor arginine in the obese insulin-resistant JCR:LA-cp ratMetabolism200756101318132517884439
- SahinKOnderciMSahinNDietary arginine silicate inositol complex improves bone mineralization in quailPoult Sci200685348649216553280
- OnderciMSahinNSahinKDietary arginine silicate inositol complex during the late laying period of quail at different environmental temperaturesBr Poult Sci200647220921516641032
- RussellJCA novel complex of arginine – silicate improves micro- and macrovascular function and inhibits glomerular sclerosis in insulin-resistant JCR: LA-Cp ratsDiabetologia20054891925193215991019
- AdamsMRForsythCJJessupWRobinsonJCelermajerDSOral L-arginine inhibits platelet aggregation but does not enhance endothelium dependent dilation in healthy young menJ Am Coll Cardiol1995264105410617560599
- LermanABurnettJCJrHiganoSTMckinleyLJHolmesDRJrLong term L-arginine supplementation improves small-vessel coronary endothelial function in humansCirculation19989721212321289626172
- LevineJControlled trials of inositol in psychiatryEur Neuropsychopharmacol1997721471559169302
- Van DyckKVan CauwenberghRRobberechtHDeelstraHBioavailability of silicon from food and food supplementsFresenius J Anal Chem1999363541544
- SeabornCDNielsenFHSilicon deprivation decreases collagen formation in wounds and bone, and ornithine transaminase enzyme activity in liverBiol Trace Elem Res200289325126112462748
- ChevalleyTRizzoliRManenDCaverzasioJBonjourJPArginine increases insulin-like growth factor-I production and collagen synthesis in osteoblast-like cellsBone19982321031099701468
- TrippelSBPotential role of insulin-like growth factors in fracture healingClin Orthop1998355301313
- ColaoADi SommaCPivonelloRBone loss is correlated to the severity of growth hormone deficiency in adult patients with hypopituitarismJ Clin Endocrinol Metab19998461919192410372687
- FioreCEPennisiPCutuliVMPratoAMessinaRClementiGL-arginine prevents bone loss and bone collagen breakdown in cyclosporin A-treated ratsEur J Pharmacol2000408332332611090650
- ClementiGFioreCEMarganoNGRole of soy diet and L-arginine in cyclosporine A-induced osteopenia in ratsPharmacol Toxicol2001881161911169156
- CarlisleEMSilicon as an essential element for the chickScience197217840616196215086395
- SchwarzKMilneDBGrowth promoting effects of silicon in ratsNature1972239537133333412635226
- CarlisleEMIn vivo requirement for silicon in articular cartilage and connective tissue formation in the chickJ Nutr197610644784841255267
- CarlisleEMSilicon: a possible factor in bone calcificationScience197016739162792805410261
- SakalliogluEEAyasBLutfiogluMKelesGCAcikgozGFiratliEGingival levels of monocyte chemoattractant protein-1 (MCP-1) in diabetes mellitus and periodontitis: an experimental study in ratsClin Oral Investig20081218389
- TokerHOzanFOzerHOzdemirHErenKYelerHA morphometric and histopathologic evaluation of the effects of propolis on alveolar bone loss in experimental periodontitis in ratsJ Periodontol20087961089109418533788
- TokerHOzdemirHErenKOzerHSahinGN-acetylcysteine, a thiol antioxidant, decreases alveolar bone loss in experimental periodontitis in ratsJ Periodontol200980467267819335088
- WeiPFHoKYHoYPWuYMYangYHTsaiCCThe investigation of glutathione peroxidase, lactoferrin, myeloperoxidase and interleukin-1beta in gingival crevicular fluid: implications for oxidative stress in human periodontal diseasesJ Periodontal Res200439528729315324348
- BostanciNIlgenliTEmingilGGingival crevicular fluid levels of RANKL and OPG in periodontal diseases: implications of their relative ratioJ Clin Periodontol200734537037617355365
- GomesDAPiresJRZuzaEPMyeloperoxidase as inflammatory marker of periodontal disease: experimental study in ratsImmunol Invest200938211712219330621
- WimalawansaSJChapaMTYallampalliCZhangRSimmonsDJPrevention of corticosteroid-induced bone loss with nitric oxide donor nitroglycerin in male ratsBone19972132752809276093
- PopovicPJZehHJ3rdOchoaJBArginine and immunityJ Nutr20071376 suppl 216811686
- MoffatFLJrHanTLiZMSupplemental L-arginine HCl augments bacterial phagocytosis in human polymorphonuclear leukocytesJ Cell Physiol1996168126338647919
- LiPYinYLiDKimSWWuGAmino acids and immune functionBr J Nutr200798223725217403271
- TorricelliPFiniMGiavaresiGL-arginine and L-lysine stimulation on cultured human osteoblastsBiomed Pharmacother2002561049249712504270
- KimMHKimEJJungJYChoiMKEffect of water-soluble silicon supplementation on bone status and balance of calcium and magnesium in male miceBiol Trace Elem Res2014158223824224664270
- SeabornCDNielsenFHEffects of germanium and silicon on bone mineralizationBiol Trace Elem Res19944221511647981005
- EisingerJClairetDEffects of silicon, fluoride, etidronate and magnesium on bone mineral density: a retrospective studyMagnes Res1993632472498292498
- SchutzeNOurslerMJNolanJRiggsBLSpelsbergTCZeolite A inhibits osteoclast-mediated bone resorption in vitroJ Cell Biochem199558139467642721
- SchroderHCWangXHWiensMSilicate modulates the crosstalk between osteoblasts (SaOS-2) and osteoclasts (RAW 264.7 cells): inhibition of osteoclast growth and differentiationJ Cell Biochem2012113103197320622615001
- WiensMWangXSchroderHCThe role of biosilica in the osteoprotegerin/RANKL ratio in human osteoblast-like cellsBiomaterials201031307716772520673584
- NapimogaMHBenattiBBLimaFOCannabidiol decreases bone resorption by inhibiting RANK/RANKL expression and pro-inflammatory cytokines during experimental periodontitis in ratsInt Immunopharmacol20099221622219070683
- SudaTTakahashiNUdagawaNJimiEGillespieMTMartinTJModulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand familiesEndocr Rev199920334535710368775
- AraújoAAVarelaHBritoGAAzilsartan increases levels of IL-10, down-regulates MMP-2, MMP-9, RANKL/RANK, Cathepsin K and up-regulates OPG in an experimental periodontitis modelPLoS One201495e9675024819928
- GuimarãesMVMeloIMAdriano AraújoVMDry extract of Matricaria recutita L. (chamomile) prevents ligature-induced alveolar bone resorption in rats via inhibition of tumor necrosis factor-α and interleukin-1βJ Periodontol201687670671526777767