Abstract
A systematic review was performed to evaluate how the maximum recommended starting dose (MRSD) was determined in first-in-human (FIH) studies with monoclonal antibodies (mAbs). Factors associated with the choice of each MRSD determination method were also identified. PubMed was searched for FIH studies with mAbs published in English between January 1, 1990 and December 31, 2013, and the following information was extracted: MRSD determination method, publication year, therapeutic area, antibody type, safety factor, safety assessment results after the first dose, and number of dose escalation steps. Seventy-nine FIH studies with mAbs were identified, 49 of which clearly reported the MRSD determination method. The no observed adverse effects level (NOAEL)-based approach was the most frequently used method, whereas the model-based approach was the least commonly used method (34.7% vs 16.3%). The minimal anticipated biological effect level (MABEL)- or minimum effective dose (MED)-based approach was used more frequently in 2011–2013 than in 1990–2007 (31.6% vs 6.3%, P=0.036), reflecting a slow, but steady acceptance of the European Medicines Agency’s guidance on mitigating risks for FIH clinical trials (2007). The median safety factor was much lower for the MABEL- or MED-based approach than for the other MRSD determination methods (10 vs 32.2–53). The number of dose escalation steps was not significantly different among the different MRSD determination methods. The MABEL-based approach appears to be safer and as efficient as the other MRSD determination methods for achieving the objectives of FIH studies with mAbs faster.
Introduction
Determining the safe starting dose for humans is one of the most important steps before any new biopharmaceutical product under development can enter clinical testing for the first time. Ideally, the starting dose should be low not to cause any harm in humans, while it is expected to be not too low for efficacy, thereby reducing the number of patients exposed to ineffective doses in the first-in-human (FIH) clinical trials.Citation1 The regulatory agencies such as the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) have published guidance documents to select the maximum recommended starting dose (MRSD) in the FIH study.Citation2,Citation3 The FDA guidance has been used in many FIH studies with new chemical entities of low-molecular weight, although it is also applicable to the FIH studies with biological agents. The emphasis in the FDA guidance is placed on the no observed adverse effects level (NOAEL) assessed in preclinical toxicology studies.Citation2 The NOAEL is then converted into the human equivalence dose by applying an appropriate scaling factors to adjust for body surface area among different species.Citation2 In contrast, the EMA guidance stresses the minimal anticipated biological effect level (MABEL) approach, in which all in vitro and in vivo information will be taken into consideration.Citation3 The NOAEL- or the MABEL-derived human equivalence dose can be reduced further by applying the safety factor, a number by which the calculated human equivalence dose is divided to increase the assurance that the first dose will not cause toxicity in humans.
Since the 1980s, monoclonal antibodies (mAbs) have been actively incorporated into clinical medicine as a beneficial therapeutic option, particularly in oncology and immunology.Citation4 However, protein-based drugs such as mAbs can have more uncertain safety profiles than those of chemistry-based drugs before an FIH study is conducted. For example, a severe life-threatening cytokine storm was developed in all the subjects who received the active drug in FIH study with TGN1412, a superagonist mAb against CD28, although a conservatively low starting dose was administered derived from the NOAEL (ie, a large safety factor of 160).Citation5 This tragic incident highlighted the importance of and difficulties in selecting the safest maximum starting dose in FIH studies with mAbs.Citation6 After the incident in the FIH study of TGN1412, several publications have proposed various ways to determine MRSD for FIH studies with biological agents. Many of these follow-up publications emphasized that MRSD for the FIH study with novel biological agents should be chosen after taking into account multiple points, for example, different endpoints, interspecies scaling, and safety factors.Citation7,Citation8 In support of this notion, a recent review found that the preclinical animal models and key toxicity parameters used to determine the starting dose for FIH studies with molecularly targeted agents in cancer patients were variable and heterogeneous.Citation9 To the best of our knowledge, however, no investigation has reported how MRSD was determined in FIH studies with mAbs and which factors were associated with the choice of MRSD determination methods. Furthermore, the consequences of various MRSD determination methods have not been assessed, particularly in terms of safety and efficiency in achieving the objectives of FIH clinical trials. On the basis of this understanding, the objectives of the present study were 1) to evaluate MRSD determination methods employed in FIH studies with mAbs, 2) to identify factors associated with choosing one method over the others, and 3) to compare the safety and efficiency of each MRSD determination method. To achieve these objectives, we performed a systematic review of the papers that reported the results of FIH studies with mAbs from 1990 to 2013.
Materials and methods
Literature search and selection of the FIH studies
To construct a database for the FIH studies with mAbs, we searched PubMed using the combination of the following terms: clinical trial, phase I or phase 1, first-in-human or first-in-man, first-time-in-human or first-time-in-man, starting dose or initial dose, and mAb. The literature search was complemented by an additional manual search of the references from the published papers and reviews focusing on mAbs. Eligible studies had to meet all of the following inclusion criteria: 1) the full text was available or there was at least a clear indication of how the MRSD was determined in the abstract or proceedings, 2) the text was written in English, and 3) the studies were published between January 1, 1990 and December 31, 2013.
Classification of MRSD determination methods and data extraction
If papers explicitly stated that the MRSD was determined based on a NOAEL, MABEL, minimum effective dose (MED), or pharmacologically active dose (PAD), they were classified as the respective dose- or level-based. Although a paper did not clearly indicate the MRSD determination method, it was also classified as NOAEL-, MABEL-, MED-, or PAD-based if the paper presented other information or supplemental data that enabled us to identify which method was used. For example, if a paper emphasized that no toxicity was found in the preclinical animal model up to a certain dose, which was used as the basis for determining the MRSD in humans, the method was NOAEL-based. Similarly, if the MRSD was determined from a dose identified in preclinical models that produced any or minimal pharmacological effect, the paper was classified as PAD- or MED-based, respectively. However, if animal pharmacokinetic (PK) data were the basis of MRSD determination or if a PK model was used to estimate the human PK parameters, which eventually resulted in the MRSD, the method was PK model-based. If the information about the receptor occupancy or other biomarkers was used to determine the MRSD, the method was pharmacodynamic (PD) model-based. If a PK–PD modeling approach was used to determine the MABEL, however, the paper was classified as MABEL-based. Because there were some similarities among MRSD determination methods, they were further grouped as follows: 1) MABEL- or MED-based (ie, MRSD was selected based on a dose associated with the minimal pharmacological effect) or 2) model-based (ie, PK, PD, or PK–PD, in which MRSD was determined using a model-based approach).
We also collected the information about the factors that could have been associated with the choice of MRSD determination method: publication year, therapeutic area (ie, oncology, immunology, infection, and others), and antibody type (ie, murine, chimeric, humanized, fully human, and others). Because the MABEL-based approach was officially first introduced in the EMA guidance in 2007, partly prompted by the TGN1412 incident,Citation3 we categorized the publication year into three periods: before 2007 (ie, 1990–2007) and two 3-year periods after 2007 (ie, 2008–2010 and 2011–2013) to investigate the impact of the EMA guidance.
Furthermore, we extracted or derived the safety factor using the information available in the paper. In addition, we collected the safety result after the first dose and the number of dose escalation steps to evaluate the consequence of each MRSD determination method.
Two authors (HYS and HL) independently reviewed the papers and performed data extraction. The extracted data were then cross-checked for concurrence, and any differences were discussed until an agreement was reached.
Statistical analysis
Safety factor and MRSD determination method were summarized using descriptive statistics. The Fisher’s exact test was performed to analyze whether MRSD determination method was significantly affected by the publication year, therapeutic area, and the type of mAbs. To test whether the median safety factor and the mean number of dose escalation steps were significantly different by MRSD determination method, the Kruskal–Wallis and the analysis of variance tests were performed, respectively. The SAS statistical software (version 9.4; SAS Institute, Inc., Cary, NC, USA) was used for the statistical analysis, and a two-tailed P-value ≤0.05 was considered statistically significant.
Results
Study identification
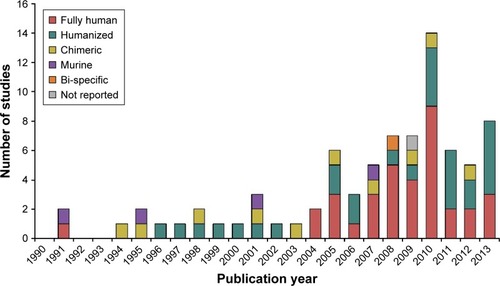
The literature search identified 140 candidate FIH studies with mAbs, 61 of which were excluded because they did not meet the selection criteria: full text unavailable (n=58) or not in English (n=1); published before January 1, 1990 or after December 31, 2013 (n=2). Hence, a total of 79 FIH studies were included in the final study database (). Overall, the majority of FIH studies with mAbs were performed in oncology (n=41, 51.9%), followed by immunology (n=14, 17.7%) and infection (n=10, 12.7%). The number of FIH studies with fully human antibodies and humanized antibodies has drastically increased since the early 2000s, whereas the number of FIH studies with murine or chimeric antibodies remained steadily low during the entire period ().
MRSD determination method
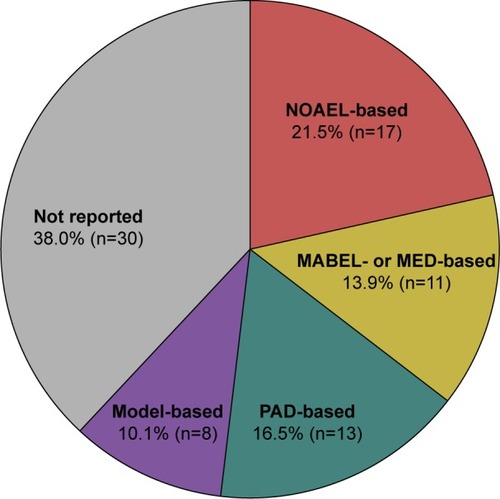
Of 79 FIH studies with mAbs included in the study database, 49 studies (62.0%) clearly indicated how the MRSD was determined, whereas the remaining 30 studies (38.0%) did not report the MRSD determination method (). Of the 49 studies that reported the MRSD determination method, more than one-third used the NOAEL-based approach (n=17, 34.7%), followed by the PAD-based approach (n=13, 26.5%) and the MABEL- or MED-based approach (n=11, 22.4%). The model-based approach was the least common method (n=8, 16.3%).
Figure 2 Overall proportion of the MRSD determination method in the first-in-human studies with monoclonal antibodies.
Abbreviations: MABEL, minimal anticipated biological effect level; MED, minimum effective dose; MRSD, maximum recommended starting dose; NOAEL, no observed adverse effects level; PAD, pharmacologically active dose; PD, pharmacodynamics; PK, pharmacokinetic.
Factors associated with the choice of MRSD determination method
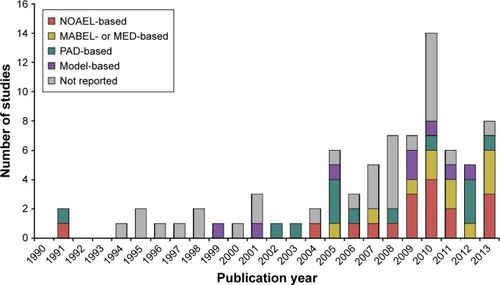
The more recent the publications were the more frequently they reported which method was used to determine the MRSD. Almost 90% of the studies published from 2011 to 2013 clearly indicated which method was used to determine the MRSD, whereas only half of the studies published before 2007 did (). The MABEL- or MED-based approach was used more frequently in 2011–2013 than in 1990–2007 (31.6% vs 6.3%, ). Notably, the MABEL-based approach was not used until 2005 (; ). In contrast, the proportions of the other MRSD determination methods, particularly the model-based approach, did not appear to change much over the entire period of 1990–2013. Collectively, MRSD determination method varied significantly by publication year (P=0.036, ), whereas therapeutic area or antibody type was not significantly associated with the choice of MRSD determination method (P=0.995 and 0.982, respectively, ).
Table 1 Publication year, therapeutic area, and antibody type by MRSD determination method
Figure 3 Yearly trend of the MRSD determination methods in the first-in-human studies with monoclonal antibodies (1990–2013).
Abbreviations: MABEL, minimal anticipated biological effect level; MED, minimum effective dose; MRSD, maximum recommended starting dose; NOAEL, no observed adverse effects level; PAD, pharmacologically active dose; PD, pharmacodynamics; PK, pharmacokinetic.
Safety factor and consequence of MRSD determination method
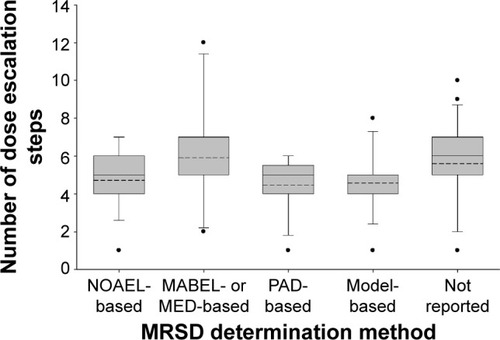
The median safety factor was numerically much lower for the MABEL- or MED-based approach than for the other approaches, although this difference failed to reach statistical significance (10 vs 32.2–53, P=0.416, ). Fourteen studies (17.7%) indicated that the first dose was safe, in which the MRSD was determined by the NOAEL-based (n=6) and the MABEL- or MED-based approaches (n=6). Only one study reported the first dose was not safe, in which the NOAEL was the basis for MRSD determination. The mean number of dose escalation steps was comparable among the different MRSD determination methods (P=0.177, ).
Table 2 Safety factors by MRSD determination method
Figure 4 Number of dose escalation steps by the MRSD determination method in the first-in-human studies with monoclonal antibodies.
Abbreviations: MABEL, minimal anticipated biological effect level; MED, minimum effective dose; MRSD, maximum recommended starting dose; NOAEL, no observed adverse effects level; PAD, pharmacologically active dose; PD, pharmacodynamics; PK, pharmacokinetic.
Discussion
We have found that the NOAEL-based approach was still the most commonly used MRSD determination method for FIH studies with mAbs, while the model-based approach was used far less frequently. Our results showed that more than one-third of the FIH studies employed the NOAEL-based approach, which was double the number of studies using the model-based approach (34.7% vs 16.3%, ). This trend was rather disappointing, given that the usefulness of the model-based approach has been repeatedly emphasized in determining the MRSD.Citation10–Citation13 For example, a PK–PD model derived from cynomolgus monkeys enabled choosing 0.01 mg/kg as the MRSD for the FIH study with TRC105, an antibody with antiangiogenic effect to solid tumors. On the basis of the PK–PD model, the MRSD would successfully result in concentrations above the dissociation constant for the antibody, leading to a pharmacologic effect in humans.Citation14 However, the infrequent use of the model-based approach to determine the MRSD can be attributed to the fact that animal data may not be available in sufficient detail to construct a model at the time of the FIH studies with mAbs.Citation2,Citation11,Citation15 Furthermore, concerns about interspecies differences in bioavailability and metabolism could be another factor that has prevented the model-based approach from being applied more frequently in FIH studies with mAbs.Citation16
Our results also showed that publication year was significantly associated with the choice of MRSD determination method, which was demonstrated in two ways. First, the proportion of FIH studies not reporting the MRSD determination method fell sharply to 10.5% in 2011–2013 from 42.9% in 2008–2010 and 50.0% in 1990–2007 (; ). It is encouraging that more FIH studies started reporting the MRSD determination method because this not only indicates increased transparency, but also it may allow for evaluating whether a certain type of MRSD determination method was useful or not in a particular study setting. Second, the MABEL- or MED-based approaches were more frequently used in 2011–2013 (31.6%) than in 1990–2007 (6.2%) and 2008–2010 (10.7%, ). In particular, the first MABEL-based FIH study with mAbs was published in 2005, followed by another in 2007 and six during 2010–2013 (). This sharp increase during the latest period certainly reflects the impact of the tragic TGN1412 incident and the EMA guidance that followed the incident, which strongly recommended the use of the MABEL-based approach to determine MRSD.Citation8,Citation17 This trend is expected to continue in the future given the heightened concern about the potential safety issues of biological agents including mAbs. However, the MABEL-based approach requires extensive knowledge regarding the pharmacological mechanisms and their integration, preferably via PK–PD modeling.Citation10,Citation18
The present study indicates that the safety factor varied widely by MRSD determination method. Namely, the MABEL- or MED-based approaches had much smaller median values of safety factor than the other MRSD determination methods (). The safety factor accounts for uncertainties such as potential interspecies differences and thereby serves as an additional means of assuring that toxicity dose not develop in humans at the first dose in FIH studies.Citation19 Therefore, smaller safety factors indicated greater confidence for human safety at the time of FIH studies.Citation2 The MABEL-based approach always results in a smaller human equivalence dose than the other MRSD determination methods, particularly the NOAEL-based approach.Citation20,Citation21 Therefore, the safety factor tends to be smaller with the MABEL-based approach than with the other methods, as shown in our results.
Although the MABEL-based approach came up with an MRSD lower than those derived by the other approaches, the average number of dose escalation steps was similar (). Fewer dose escalation steps indicated more efficient FIH studies. Therefore, the MABEL-based approach did not appear to be inferior to the other MRSD determination methods. Furthermore, more than half (6/11=54.5%) of the papers that employed the MABEL-based approach explicitly indicated that the first dose was safe, which was almost 20% points higher than that with the NOAEL-based approach (6/17=35.3%). Of course, this interpretation needs caution because >80% of the papers did not explicitly mention about the safety results after the first dose.
The major limitation of the present study was the possibility of misclassifying MRSD determination method, particularly between the model- and MABEL-based approaches. Because the EMA guidance suggests that
all information available from PK/PD data … wherever possible … should be integrated in a PK/PD modeling approach for the determination of the MABEL (emphasis added)
some FIH studies classified as using the model-based approach had, in fact, used the MABEL-based approach. However, this possible misclassification was very unlikely to influence our final conclusion because only a small number of FIH studies (n=8, 10.1%, ) were classified as model-based. Another limitation was that the MRSD determination method was not identifiable in 30 (=38%) FIH studies with mAbs because the authors did not report which method was used. Although our study database was constructed by a thorough literature search, further studies are warranted to circumvent this type of publication bias.Citation22
Conclusion
We anticipate that the MABEL-based approach will be more frequently used in FIH studies with mAbs in the future, while the NOAEL-based approach is still likely to be the most commonly used method. The MABEL-based approach appears to be safer and as efficient as the other MRSD determination methods for achieving the objectives of FIH clinical trials faster. To the best of our knowledge, this is the first report showing the rapid acceptance of the MABEL-based approach in FIH studies with mAbs, reinforcing the impact of the EMA guidance. Our study can also illuminate the trends of the choice of MRSD determination methods, which may contribute to a safer design and conduct of FIH studies with mAbs in humans.
Acknowledgments
The authors thank the members of the Department of Clinical Pharmacology and Therapeutics, College of Medicine, Seoul National University Hospital. Particular thanks goes to Dr In-Jin Jang for providing helpful comments and suggestions. This work was partly supported by the Education and Research Encouragement Fund of Seoul National University Hospital.
Supplementary material
Table S1 Study characteristics
References
- DrobyskiWRGottliebMCarriganDPhase I study of safety and pharmacokinetics of a human anticytomegalovirus monoclonal antibody in allogeneic bone marrow transplant recipientsTransplantation1991516119011961646504
- KleinBWijdenesJZhangXGMurine anti-interleukin-6 monoclonal antibody therapy for a patient with plasma cell leukemiaBlood1991785119812041715218
- MaloneyDGLilesTMCzerwinskiDKPhase I clinical trial using escalating single-dose infusion of chimeric anti-CD20 monoclonal antibody (IDEC-C2B8) in patients with recurrent B-cell lymphomaBlood1994848245724667522629
- HandgretingerRAndersonKLangPA phase I study of human/mouse chimeric antiganglioside GD2 antibody ch14. 18 in patients with neuroblastomaEur J Cancer1995312261267
- BrooksDTaylorCDos SantosBPhase Ia trial of murine immunoglobulin A antitransferrin receptor antibody 42/6Clin Cancer Res1995111125912659815920
- EverittDEDavisCBThompsonKThe pharmacokinetics, antigenicity, and fusion-inhibition activity of RSHZ19, a humanized monoclonal antibody to respiratory syncytial virus, in healthy volunteersJ Infect Dis199617434634698769601
- VincentiFLantzMBirnbaumJA phase I trial of humanized anti-interleukin 2 receptor antibody in renal transplantation 1Transplantation199763133389000657
- ZaanenVDer LelieVOersVChimaeric anti-interleukin 6 monoclonal antibodies in the treatment of advanced multiple myeloma: a phase I dose-escalating studyBr J Haematol199810237837909722307
- BowenJDPetersdorfSHRichardsTLPhase I study of a humanized anti-CD11/CD18 monoclonal antibody in multiple sclerosisClin Pharmacol Ther19986433393469757158
- HarderSKirchmaierCMKrzywanekHJWestrupDBaeJWBreddinHKPharmacokinetics and pharmacodynamic effects of a new antibody glycoprotein IIb/IIIa inhibitor (YM337) in healthy subjectsCirculation1999100111175118110484537
- GottliebAKruegerJGBrightREffects of administration of a single dose of a humanized monoclonal antibody to CD11a on the immunobiology and clinical activity of psoriasisJ Am Acad Dermatol200042342843510688712
- CrombetTTorresORodriguezVPhase I clinical evaluation of a neutralizing monoclonal antibody against epidermal growth factor receptor in advanced brain tumor patients: preliminary studyHybridoma200120213113611394532
- GordonMSMargolinKTalpazMPhase I safety and pharmacokinetic study of recombinant human anti-vascular endothelial growth factor in patients with advanced cancerJ Clin Oncol200119384385011157038
- VerbonADekkersPEten HoveTIC14, an anti-CD14 antibody, inhibits endotoxin-mediated symptoms and inflammatory responses in humansJ Immunol200116653599360511207321
- ChowFSBenincosaLJShethSBPharmacokinetic and pharmacodynamic modeling of humanized anti–factor IX antibody (SB 249417) in humansClin Pharmacol Ther200271423524511956506
- PoseyJANgTCYangBA phase I study of anti-kinase insert domain-containing receptor antibody, IMC-1C11, in patients with liver metastases from colorectal carcinomaClin Cancer Res2003941323133212684400
- KauffmanCLAriaNToichiEA phase I study evaluating the safety, pharmacokinetics, and clinical response of a human IL-12 p40 antibody in subjects with plaque psoriasisJ Invest Dermatol200412361037104415610511
- BekkerPJHollowayDLRasmussenASA single-dose placebo-controlled study of AMG 162, a fully human monoclonal antibody to RANKL, in postmenopausal womenJ Bone Miner Res20041971059106615176987
- AgusDBGordonMSTaylorCPhase I clinical study of pertuzumab, a novel HER dimerization inhibitor, in patients with advanced cancerJ Clin Oncol200523112534254315699478
- DowlingTCChavaillazPAYoungDGPhase 1 safety and pharmacokinetic study of chimeric murine-human monoclonal antibody cαStx2 administered intravenously to healthy adult volunteersAntimicrob Agents Chemother20054951808181215855500
- PaceySPlummerRAttardGPhase I and pharmacokinetic study of HGS-ETR2, a human monoclonal antibody to TRAIL R2, in patients with advanced solid malignanciesJ Clin Oncol2005233055
- SubramanianGMCroninPWPoleyGA phase 1 study of PAmAb, a fully human monoclonal antibody against Bacillus anthracis protective antigen, in healthy volunteersClin Infect Dis2005411122015937757
- RibasACamachoLHLopez-BeresteinGAntitumor activity in melanoma and anti-self responses in a phase I trial with the anti-cytotoxic T lymphocyte-associated antigen 4 monoclonal antibody CP-675,206J Clin Oncol200523358968897716204013
- ReilleySWenzelEReynoldsLBennettBPattiJMHetheringtonSOpen-label, dose escalation study of the safety and pharmacokinetic profile of tefibazumab in healthy volunteersAntimicrob Agents Chemother200549395996215728889
- SuntharalingamGPerryMRWardSCytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412N Engl J Med2006355101018102816908486
- NgCMStefanichEAnandBSFielderPJVaickusLPharmacokinetics/pharmacodynamics of nondepleting anti-CD4 monoclonal antibody (TRX1) in healthy human volunteersPharm Res20062319510316308668
- LacyMAlsinaMMelvinCLPhase 1 first-in-human dose escalation study of cp-751,871, a specific monoclonal antibody against the insulin like growth factor 1 receptorJ Clin Oncol2006247609
- TabriziMARoskosLKPreclinical and clinical safety of monoclonal antibodiesDrug Discov Today20071213–1454054717631248
- TolcherAWMitaMMeropolNJPhase I pharmacokinetic and biologic correlative study of mapatumumab, a fully human monoclonal antibody with agonist activity to tumor necrosis factor-related apoptosis-inducing ligand receptor-1J Clin Oncol200725111390139517416859
- VonderheideRHFlahertyKTKhalilMClinical activity and immune modulation in cancer patients treated with CP-870,893, a novel CD40 agonist monoclonal antibodyJ Clin Oncol200725787688317327609
- ScottAMLeeFTTebbuttNA phase I clinical trial with monoclonal antibody ch806 targeting transitional state and mutant epidermal growth factor receptorsProc Natl Acad Sci U S A2007104104071407617360479
- MullamithaSATonNCParkerGJPhase I evaluation of a fully human anti-αv integrin monoclonal antibody (CNTO 95) in patients with advanced solid tumorsClin Cancer Res20071372128213517404096
- FurieRStohlWGinzlerEMBiologic activity and safety of belimumab, a neutralizing anti-B-lymphocyte stimulator (BLyS) monoclonal antibody: a phase I trial in patients with systemic lupus erythematosusArthritis Res Ther2008105R10918786258
- HagenbeekAGadebergOJohnsonPFirst clinical use of ofatumumab, a novel fully human anti-CD20 monoclonal antibody in relapsed or refractory follicular lymphoma: results of a phase 1/2 trialBlood2008111125486549518390837
- Bouman-ThioEFransonKMillerBA phase I, single and fractionated, ascending-dose study evaluating the safety, pharmacokinetics, pharmacodynamics, and immunogenicity of an erythropoietin mimetic antibody fusion protein (CNTO 528) in healthy male subjectsJ Clin Pharmacol200848101197120718812609
- BargouRLeoEZugmaierGTumor regression in cancer patients by very low doses of a T cell-engaging antibodyScience2008321589197497718703743
- SznolMHodiFMargolinKPhase I study of BMS-663513, a fully human anti-CD137 agonist monoclonal antibody, in patients (pts) with advanced cancer (CA)J Clin Oncol2008263007
- MendelsonDDinolfoMCohenRFirst-in-human dose escalation safety and pharmacokinetic (PK) trial of a novel intravenous (IV) thrombospondin-1 (TSP-1) mimetic humanized monoclonal CovX Body (CVX-045) in patients (pts) with advanced solid tumorsJ Clin Oncol200826Suppl 153524
- TaylorCPTummalaSMolrineDOpen-label, dose escalation phase I study in healthy volunteers to evaluate the safety and pharmacokinetics of a human monoclonal antibody to Clostridium difficile toxin AVaccine200826273404340918502001
- WeismanLEFischerGWThackrayHMSafety and pharmacokinetics of a chimerized anti-lipoteichoic acid monoclonal antibody in healthy adultsInt Immunopharmacol20099563964419268719
- LazarHHornMPZuercherAWPharmacokinetics and safety profile of the human anti-Pseudomonas aeruginosa serotype O11 immunoglobulin M monoclonal antibody KBPA-101 in healthy volunteersAntimicrob Agents Chemother20095383442344619451304
- LachmannHJKone-PautIKuemmerle-DeschnerJBUse of canakinumab in the cryopyrin-associated periodic syndromeN Engl J Med2009360232416242519494217
- HerbstRSHongDChapLSafety, pharmacokinetics, and antitumor activity of AMG 386, a selective angiopoietin inhibitor, in adult patients with advanced solid tumorsJ Clin Oncol200927213557356519546406
- TolcherAWSarantopoulosJPatnaikAPhase I, pharmacokinetic, and pharmacodynamic study of AMG 479, a fully human monoclonal antibody to insulin-like growth factor receptor 1J Clin Oncol200927345800580719786654
- LumPRuixoJJPOgbagabrielSAbstract B167: identifying first in human (FIH) doses and schedule of U3-1287 (AMG 888), a fully human anti-HER3 mAb, based on preclinical pharmacokinetic (PK), pharmacodynamic (PD) and efficacy dataMol Cancer Ther20098Suppl 12B167
- WhiteBLeonFWhiteWRobbieGTwo first-in-human, open-label, phase I dose-escalation safety trials of MEDI-528, a monoclonal antibody against interleukin-9, in healthy adult volunteersClin Ther200931472874019446146
- GordonMSSweeneyCJMendelsonDSSafety, pharmacokinetics, and pharmacodynamics of AMG 102, a fully human hepatocyte growth factor-neutralizing monoclonal antibody, in a first-in-human study of patients with advanced solid tumorsClin Cancer Res201016269971020068101
- HerbstRSKurzrockRHongDSA first-in-human study of conatumumab in adult patients with advanced solid tumorsClin Cancer Res201016235883589120947515
- CamidgeDRHerbstRSGordonMSA phase I safety and pharmacokinetic study of the death receptor 5 agonistic antibody PRO95780 in patients with advanced malignanciesClin Cancer Res20101641256126320145186
- SpratlinJLCohenRBEadensMPhase I pharmacologic and biologic study of ramucirumab (IMC-1121B), a fully human immunoglobulin G1 monoclonal antibody targeting the vascular endothelial growth factor receptor-2J Clin Oncol201028578078720048182
- BeigelJHNordstromJLPillemerSRSafety and pharmacokinetics of single intravenous dose of MGAWN1, a novel monoclonal antibody to West Nile virusAntimicrob Agents Chemother20105462431243620350945
- BurrisHARosenLSRocha-LimaCMPhase 1 experience with an anti-glycotope monoclonal antibody, RAV12, in recurrent adenocarcinomaClin Cancer Res20101651673168120179219
- VerhammePPakolaSJensenTJTolerability and pharmacokinetics of TB-402 in healthy male volunteersClin Ther20103261205122020637972
- KropIEBeeramMModiSPhase I study of trastuzumab-DM1, an HER2 antibody-drug conjugate, given every 3 weeks to patients with HER2-positive metastatic breast cancerJ Clin Oncol201028162698270420421541
- HusseinMBerensonJRNiesvizkyRA phase I multidose study of dacetuzumab (SGN-40; humanized anti-CD40 monoclonal antibody) in patients with multiple myelomaHaematologica201095584584820133895
- KuenenBWitteveenPORuijterRA phase I pharmacologic study of necitumumab (IMC-11F8), a fully human IgG1 monoclonal antibody directed against EGFR in patients with advanced solid malignanciesClin Cancer Res20101661915192320197484
- BrahmerJRDrakeCGWollnerIPhase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlatesJ Clin Oncol201028193167317520516446
- GenoveseMCVan den BoschFRobersonSLY2439821, a humanized anti-interleukin-17 monoclonal antibody, in the treatment of patients with rheumatoid arthritis: a phase I randomized, double-blind, placebo-controlled, proof-of-concept studyArthritis Rheum201062492993920131262
- AdlerSGSchwartzSWilliamsMEPhase 1 study of anti-CTGF monoclonal antibody in patients with diabetes and microalbuminuriaClin J Am Soc Nephrol2010581420142820522536
- BusseWWKatialRGossageDSafety profile, pharmacokinetics, and biologic activity of MEDI-563, an anti-IL-5 receptor α antibody, in a phase I study of subjects with mild asthmaJ Allergy Clin Immunol201012561237124420513521
- RiddleVLeesePBlansetDAdamcioMMeldorfMLowyIPhase I study evaluating the safety and pharmacokinetics of MDX-1303, a fully human monoclonal antibody against Bacillus anthracis protective antigen, in healthy volunteersClin Vaccine Immunol201118122136214221976227
- XuZBouman-ThioEComisarCPharmacokinetics, pharmacodynamics and safety of a human anti-IL-6 monoclonal antibody (sirukumab) in healthy subjects in a first-in-human studyBr J Clin Pharmacol201172227028121392075
- Martinsson-NiskanenTRiisbroRLarssonLMonoclonal antibody TB-403: a first-in-human, phase I, double-blind, dose escalation study directed against placental growth factor in healthy male subjectsClin Ther20113391142114921906811
- Paz-AresLGGomez-RocaCDelordJPPhase I pharmacokinetic and pharmacodynamic dose-escalation study of RG7160 (GA201), the first glycoengineered monoclonal antibody against the epidermal growth factor receptor, in patients with advanced solid tumorsJ Clin Oncol201129283783379021900113
- PadhiDJangGStouchBFangLPosvarESingle-dose, placebo-controlled, randomized study of AMG 785, a sclerostin monoclonal antibodyJ Bone Miner Res2011261192620593411
- BurmesterGRFeistESleemanMAWangBWhiteBMagriniFMavrilimumab, a human monoclonal antibody targeting GM-CSF receptor-alpha, in subjects with rheumatoid arthritis: a randomised, double-blind, placebo-controlled, phase I, first-in-human studyAnn Rheum Dis20117091542154921613310
- RosenLSHurwitzHIWongMKA phase I first-in-human study of TRC105 (anti-endoglin antibody) in patients with advanced cancerClin Cancer Res201218174820482922767667
- MorrisMJEisenbergerMAPiliRA phase I/IIA study of AGS-PSCA for castration-resistant prostate cancerAnn Oncol201223102714271922553195
- CurtinFLangABPerronHGNbAC1, a humanized monoclonal antibody against the envelope protein of multiple sclerosis-associated endogenous retrovirus: a first-in-humans randomized clinical studyClin Ther201234122268227823200102
- SteinEAMellisSYancopoulosGDEffect of a monoclonal antibody to PCSK9 on LDL cholesterolN Engl J Med2012366121108111822435370
- ZonderJAMohrbacherAFSinghalSA phase 1, multicenter, open-label, dose escalation study of elotuzumab in patients with advanced multiple myelomaBlood2012120355255922184404
- AbilaBCunninghamESimeoniMFirst-time-in-human study with GSK249320, a myelin-associated glycoprotein inhibitor, in healthy volunteersClin Pharmacol Ther201393216316923267856
- GoldwaterRKeirnsJBlahunkaPA phase 1, randomized ascending single-dose study of antagonist anti-human CD40 ASKP1240 in healthy subjectsAm J Transplant20131341040104623356210
- HodsmanPAshmanCCahnAA phase 1, randomized, placebo-controlled, dose-escalation study of an anti-IL-13 monoclonal antibody in healthy subjects and mild asthmaticsBr J Clin Pharmacol201375111812822616628
- SandhuSKPapadopoulosKFongPCA first-in-human, first-in-class, phase I study of carlumab (CNTO 888), a human monoclonal antibody against CC-chemokine ligand 2 in patients with solid tumorsCancer Chemother Pharmacol20137141041105023385782
- InfanteJRBendellJCGoffLWSafety, pharmacokinetics and pharmacodynamics of the anti-A33 fully-human monoclonal antibody, KRN330, in patients with advanced colorectal cancerEur J Cancer20134961169117523294608
- VugmeysterYRohdeCPerreaultMGimenoRESinghPAgonistic TAM-163 antibody targeting tyrosine kinase receptor-B: applying mechanistic modeling to enable preclinical to clinical translation and guide clinical trial designMAbs20135337338323529133
- ReillyMMillerRMThomsonMHRandomized, double-blind, placebo-controlled, dose-escalating phase I, healthy subjects study of intravenous OPN-305, a humanized anti-TLR2 antibodyClin Pharmacol Ther201394559360023880971
- ZhuAXGoldPJEl-KhoueiryABFirst-in-man phase I study of GC33, a novel recombinant humanized antibody against glypican-3, in patients with advanced hepatocellular carcinomaClin Cancer Res201319492092823362325
Disclosure
The authors report no conflicts of interest in this work.
References
- IvySPSiuLLGarrett-MayerERubinsteinLApproaches to phase 1 clinical trial design focused on safety, efficiency, and selected patient populations: a report from the clinical trial design task force of the national cancer institute investigational drug steering committeeClin Cancer Res20101661726173620215542
- US Food Drug AdministrationGuidance for Industry: Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy VolunteersRockville, MDFood and Drug Administration2005 Available from: http://www.fda.gov/downloads/drugs/guidances/ucm078932.pdfAccessed July 6, 2005
- Committee for Medicinal Products for Human UseGuideline on Strategies to Identify and Mitigate Risks for First-in-Human Clinical Trials with Investigational Medical ProductsLondon, UKEuropean Medicines Agency2007 Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500002988.pdfAccessed July 19, 2007
- ReichertJMRosensweigCJFadenLBDewitzMCMonoclonal antibody successes in the clinicNat Biotech200523910731078
- StebbingsRPooleSThorpeRSafety of biologics, lessons learnt from TGN1412Curr Opin Biotechnol200920667367719892543
- SuntharalingamGPerryMRWardSCytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412N Engl J Med2006355101018102816908486
- TibbittsJCavagnaroJAHallerCAMarafinoBAndrewsPASullivanJTPractical approaches to dose selection for first-in-human clinical trials with novel biopharmaceuticalsRegul Toxicol Pharmacol201058224325120558225
- SharmaVMcNeillJHTo scale or not to scale: the principles of dose extrapolationB J Pharmacol20091576907921
- Le TourneauCStathisAVidalLMooreMJSiuLLChoice of starting dose for molecularly targeted agents evaluated in first-in-human phase I cancer clinical trialsJ Clin Oncol20102881401140720124167
- ZouPYuYZhengNApplications of human pharmacokinetic prediction in first-in-human dose estimationAAPS J201214226228122407287
- AgoramBMUse of pharmacokinetic/pharmacodynamic modelling for starting dose selection in first-in-human trials of high-risk biologicsBr J Clin Pharmacol200967215316019076987
- HansenARCookNRicciMSChoice of starting dose for biopharmaceuticals in first-in-human phase I cancer clinical trialsOncologist201520665365925964306
- TosiDLaghzaliYVinchesMClinical development strategies and outcomes in first-in-human trials of monoclonal antibodiesJ Clin Oncol201533192158216526014300
- RosenLSHurwitzHIWongMKA phase I first-in-human study of TRC105 (anti-endoglin antibody) in patients with advanced cancerClin Cancer Res201218174820482922767667
- AgoramBMMartinSWvan der GraafPHThe role of mechanism-based pharmacokinetic–pharmacodynamic (PK–PD) modelling in translational research of biologicsDrug Discov Today200712231018102418061880
- WangWWangEBalthasarJMonoclonal antibody pharmacokinetics and pharmacodynamicsClin Pharmacol Ther200884554855818784655
- MiltonMNHorvathCJThe EMEA guideline on first-in-human clinical trials and its impact on pharmaceutical developmentToxicol Pathol200937336337119246571
- LowePJHijaziYLuttringerOYinHSarangapaniRHowardDOn the anticipation of the human dose in first-in-man trials from preclinical and prior clinical information in early drug developmentXenobiotica20073710–111331135417968748
- BokkersBGSlobWDeriving a data-based interspecies assessment factor using the NOAEL and the benchmark dose approachCrit Rev Toxicol200737535537317612951
- ZhaoLRenT-hWangDDClinical pharmacology considerations in biologics developmentActa Pharmacol Sin201233111339134723001474
- MullerPYMiltonMLloydPSimsJBrennanFRThe minimum anticipated biological effect level (MABEL) for selection of first human dose in clinical trials with monoclonal antibodiesCurr Opin Biotechnol200920672272919896825
- EasterbrookPJGopalanRBerlinJMatthewsDRPublication bias in clinical researchLancet199133787468678721672966