Abstract
The hepatitis B virus (HBV) has caused acute and chronic liver diseases in ~350 million infected people worldwide. Halofuginone (HF) is a plant alkaloid which has been demonstrated to play a crucial role in immune regulation. Our present study explored the function of HF in the immune response of HBV-infected Sprague Dawley (SD) rats. Plasmid containing pCDNA3.1-HBV1.3 was injected in SD rats for the construction of an acute HBV-infected animal model. Our data showed that HF reduced the high concentrations of serum hepatitis B e-antigen, hepatitis B surface antigen, and HBV DNA induced by HBV infection. HF also reduced the number of T helper (Th)17 cells and the expression of interleukin (IL)-17 compared with the pCDNA3.1-HBV1.3 group. Moreover, pro-inflammatory cytokine levels (IL-17, IL-23, interferon-γ, and IL-2) were downregulated and anti-inflammatory cytokine levels (IL-4 and IL-13) were upregulated by HF. Through further research we found that the expression of AMP-activated protein kinase (AMPK) and IKBA which suppressed NF-κB activation was increased while the expression of p-NF-κB P65 was decreased in pCDNA3.1-HBV1.3+HF group compared with pCDNA3.1-HBV1.3 group, indicating that HF may work through the activation of AMPK. Finally, our conjecture was further verified by using the AMPK inhibitor compound C, which counteracted the anti-inflammation effect of HF, resulting in the decreased expression of AMPK, IKBA and increased expression of p-NF-κB P65 and reduced number of Th17 cells. In our present study, HF was considered as an anti-inflammatory factor in acute HBV-infected SD rats and worked through AMPK-mediated NF-κB p65 inactivation. This study implicated HF as a potential therapeutic strategy for hepatitis B.
Introduction
The hepatitis B virus (HBV) is a kind of hepatotropic DNA virus which has caused acute and chronic liver diseases iñ350 million infected people worldwide.Citation1 HBV is transmitted by mother-to-child transmission, sex transmission, and blood transmission. It causes one of the most common infectious diseases (hepatitis B) in the world through a high level of viral replication. Researches have indicated that HBV plays a vital role in many liver diseases including cirrhosis and hepatocellular carcinoma.Citation2 The pathogenic mechanism of HBV is thought to be related to immune response instead of directly causing cytopathy of hepatocytes.Citation3,Citation4 HBV is able to trigger immune response in the presence of HBV-specific antigens which cause T-cell response.Citation5 However, the relationship between HBV infection and immune response still remains unclear. Hence, a better understanding of the pathogenic mechanisms of HBV infection and more effective therapeutic strategies for hepatitis B are required.
Halofuginone (HF), a natural low-molecular-weight plant alkaloid, isolated from the plant Dichroa febrifuga, has been reported to have antimalarial activity.Citation6 Researches revealed that HF worked as an anti-fibrotic agent.Citation7,Citation8 Studies also showed that HF had antitumor effect by inhibiting cancer cell proliferation, metastasis, and invasion in many kinds of tumors such as hepatocellular carcinoma, breast cancer, and melanoma.Citation9–Citation11 HF is also reported to be involved in several signal pathways such as PI3K/AKT, Stat3, NF-κB, and MAPK pathways.Citation12–Citation14 Evidence also supported the potential role of HF in immune regulation. A study by Park et al discovered that HF regulated the balance between T helper (Th)17 and Treg cells to ameliorate autoimmune arthritis in mice.Citation15 HF also acted as an attractive immunomodulator and anti-inflammatory agent to suppress activated peripheral blood T-cell functions through inhibiting NF-κB and p38 MAPK signaling pathway.Citation16 However, whether HF can ameliorate the inflammation induced by HBV infection still remains unknown and needs further research.
In this study, we aimed to elucidate the function of HF on inflammation in acute HBV-infected Sprague Dawley (SD) rats. We found that HF suppressed inflammation by decreasing the number of Th17 cells and pro-inflammatory cytokine levels via AMP-activated protein kinase (AMPK)-mediated NF-κB p65 inactivation. Taken together, our results suggest that HF provides a novel insight into the mechanism and treatment of hepatitis B.
Materials and methods
Construction of animal model and grouping
Female, 6–9 weeks old SD rats were purchased from the Laboratory Animal Center of Henan Provincial People’s Hospital. All the animals were fed under specific pathogen-free conditions. Animal experiments and welfare were performed according to the guidelines of the National Institute of Health. The study was approved by the Medical Ethics Committee of Henan Provincial People’s Hospital. SD rats were transfected with pCDNA3.1-HBV1.3 plasmid which contained a 1.3-length transgene HBV or just pCDNA3.1 as control, respectively (constructed in the laboratory). Twenty micrograms of plasmids in phosphate-buffered saline (PBS; Double-Helix Biotech, London, UK) were injected into the tail vein of each rat for the acute HBV-infected animal model.Citation17
The animals were divided into 4 groups with 5 rats in each group as follows: pCDNA3.1 group, SD rats were transfected with pCDNA3.1 plasmid; pCDNA3.1-HBV1.3 group, SD rats were transfected with pCDNA3.1-HBV1.3 plasmid; pCDNA3.1-HBV1.3+HF group, pCDNA3.1-HBV1.3 plasmid transfected rats were treated with HF (1 mg/kg) by injection every other day; pCDNA3.1-HBV1.3+HF+compound C (CC) group, pCDNA3.1-HBV1.3 plasmid transfected rats were co-treated with CC (3 μM) and HF (1 mg/kg) by injection every other day. The peripheral blood of each rat in the different groups was collected and measured at 1, 4, 7, and 10 days post-transfection. After 10 days post-transfection, rats were sacrificed and livers were harvested for further analyses.
Quantification of hepatitis B e-antigen (HBeAg), hepatitis B surface antigen (HBsAg), and HBV DNA
Serum HBeAg, HBsAg, and HBV DNA levels were measured in the laboratory according to a method described previously.Citation18 Serum HBeAg was measured using the Abbott Diagnostics HBeAg kit (Abbott Diagnostics, Abbott Park, IL, USA), and HBsAg was measured on ARCHITECT platform (Abbott Laboratories, Abbott Park, IL, USA) by enzyme immunoassay according to the manufacturer’s instructions. The quantification of HBV DNA was tested using the Abbott RealTime HBV test (Abbott Molecular, Des Plaines, IL) according to the manufacturer’s instructions.
Flow cytometry
Peripheral blood samples from rats were lysed by NH4Cl lysis solution to remove red blood cells and washed twice with PBS. For the staining of intracellular cytokine, Caltag™, Fix & Perm® reagents (Thermo Fisher Scientific, Waltham, MA, USA) were used following the manufacturer’s instructions. Then, samples containing lymphomononuclear cells were incubated with fluorochrome-labeled antibodies at 5×105 cells for 30 minutes at 4°C and were analyzed on a flow cytometer (FACStar; BD Biosciences, San Jose, CA, USA). The following antibodies were used in this study: fluorescein isothiocyanate-conjugated anti-CD4 (BD Biosciences) and phycoerythrin-conjugated anti-interleukin (IL)-17 (BD Biosciences).
Western blot
Proteins were extracted from livers using RIPA lysis buffer (Beyotime Institute of Biotechnology, Shanghai, China) after grinding by liquid nitrogen. Lysates were centrifuged at 12,000 rpm for 15 minutes at 4°C, and the protein concentrations of supernatants were measured using the Bio-Rad protein assay kit (Beijing Solarbio Science & Technology Co, Ltd, Beijing, China) according to the manufacturer’s instructions. After incubation with 6× sample buffer (Sigma-Aldrich Co, St Louis, MO, USA) at 100°C for 5 minutes, the proteins were separated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis, and then transferred onto polyvinylidene difluoride membranes. After incubation with blocking buffer (5% nonfat milk in Tris-buffered saline) for 60 minutes at room temperature, the membranes were incubated with the following primary antibodies obtained from Abcam (Cambridge, UK), respectively: anti-IL-17 antibody, anti-AMPK antibody, anti-p-NF-κB P65 antibody, anti-IKBA antibody, and anti-GAPDH antibody at 4°C overnight. The membranes were then incubated with the corresponding secondary antibodies (1:2,000; Cell Signaling Technology, Danvers, MA, USA) for 1 hour at room temperature. Immunoreactive bands were visualized via enhanced chemiluminescence (Amersham Pharmacia Biotech, Little Chalfont, UK) reaction and measured by Quantity One analysis software (Bio-Rad Laboratories Inc, Hercules, CA, USA).
Quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA was extracted from livers using Trizol reagent (Thermo Fisher Scientific) following the manufacturer’s instructions and cDNA was synthesized using the PrimeScript RT Reagent Kit (TaKaRa, Dalian, China). A qRT-PCR study evaluating the expression of IL-17, IL-23, interferon (IFN)-γ, IL-2, IL-4, and IL-13 was carried out using the QuantiTect SYBR Green RT-PCR Kit on the StepOnePlus™ Real Time PCR System (Thermo Fisher Scientific). Gene expression was standardized to GAPDH expression as an endogenous control, and the relative expression level was calculated by relative quantification (2−ΔΔCt) method. The primers used were the following: IL-17 – forward: ACCAATCCCAAAAG GTCCTC, reverse: GGGGACAGAGTTCATGTGGT; IL-23 – forward: GGGAGATGAAGAGACTACAAATGATGT, reverse: ATCCTTTGCAAGCAGAACTGACT; IFN-γ – forward: GCATCCAAAAGAGTGTGGAG, reverse: GCAGGCAGGACAACCATTAC; IL-2 – forward: ATGTACAGGATGCAACTCCTG, reverse: TCAAGTCAGTG TTGAGATGATGCTTTGACAAAA; IL-4 – forward: GCACAAGCAGCTGATCCGAT, reverse: CAGGAATTCAAGCCCGCC; IL-13 – forward: TTCTACTCACG TGCTGACCT, reverse: CTGTCTCTGCAAATAATGAT.
Statistical analysis
SPSS 19.0 software (IBM Corporation, Armonk, NY, USA) was utilized for statistical analysis. The data are presented as mean ± standard deviation. Statistical analysis was performed with one-way analysis of variance or Student’s t-test. P<0.05 was considered statistically significant.
Results
HF alleviates pathologic features in HBV-infected SD rats
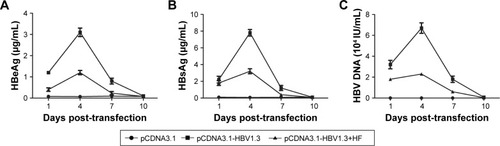
The pCDNA3.1 or pCDNA3.1-HBV1.3 plasmids were injected into SD rats to construct animal models of acute hepatitis B infection. In the pCDNA3.1-HBV1.3 group, the concentrations of HBeAg, HBsAg, and HBV DNA in the serum increased from day 1 to day 4 after transfection, and reached the peak of expression at day 4. Then, the levels of HBeAg, HBsAg, and HBV DNA decreased from day 4 to day 10, and were hardly detected at day 10. Compared with the pCDNA3.1-HBV1.3 group, the concentrations of HBeAg, HBsAg, and HBV DNA were obviously depressed by HF at every indicated time in the pCDNA3.1-HBV1.3+HF group (). Our results indicated that HF alleviated pathologic features in HBV-infected SD rats.
Figure 1 The expression of HBeAg, HBsAg, and HBV DNA in the serum of transfected rats. Rats were transfected with pCDNA3.1-HBV1.3 or pCDNA3.1, respectively. (A–C) The expression of HBeAg, HBsAg, and HBV DNA at different time points was detected through serological quantitation.
HF protects SD rats from acute hepatitis B by balancing Th17 cells responses
Th17 cells are one of the subtypes of CD4+ Th cells which secret IL-17 cytokine. In our present study, the ratios of Th17 cells in peripheral blood of rats in different groups were analyzed by flow cytometry. Our results indicated that the ratio of CD4+IL-17+ cells in rats of the pCDNA3.1-HBV1.3 group was significantly higher compared with the pCDNA3.1 control group. However, HF remarkably reduced the high ratio of CD4+IL-17+ cells caused by acute HBV infection in the pCDNA3.1-HBV1.3+HF group (***P<0.001, ##P<0.01; ). The protein level of IL-17 was higher in pCDNA3.1-HBV1.3 group than the pCDNA3.1 group at every indicated time by Western blot analysis. HF also reduced the expression level of IL-17 in the pCDNA3.1-HBV1.3+HF group compared with pCDNA3.1-HBV1.3 group. The expression of IL-17 increased from day 1 to day 4 and decreased from day 4 to day 10 postinjection in both pCDNA3.1-HBV1.3 and pCDNA3.1-HBV1.3+HF groups (*P<0.05, **P<0.01, ***P<0.001, #P<0.05, ##P<0.01; ).
Figure 2 HF protects SD rats from acute hepatitis B by regulating CD4+IL-17+ T-cells and expression levels of pro-inflammatory/anti-inflammatory cytokines. Rats were transfected with pCDNA3.1-HBV1.3 or pCDNA3.1, respectively. Rats in the pCDNA3.1-HBV1.3+HF group were transfected with pCDNA3.1-HBV1.3 and supplied with HF. (A) The frequency of CD4+IL-17+ T-cells in peripheral blood of transfected rats was detected through flow cytometry. (B) The ratio of IL-17 expression from the results of flow cytometry. (C) Relative protein level of IL-17 in livers of transfected rats at different time points was assayed through Western blot. (D) Quantitation of (C). (E–J) Relative mRNA levels of IL-17, IL-23, IFN-γ, IL-2, IL-4, and IL-13 at different time points were assayed through qRT-PCR, respectively. The bars show mean ± standard deviation of 3 independent experiments. *P<0.05, **P<0.01, ***P<0.001 versus pCDNA3.1 group; #P<0.05, ##P<0.01, ###P<0.001 versus pCDNA3.1-HBV1.3 group.
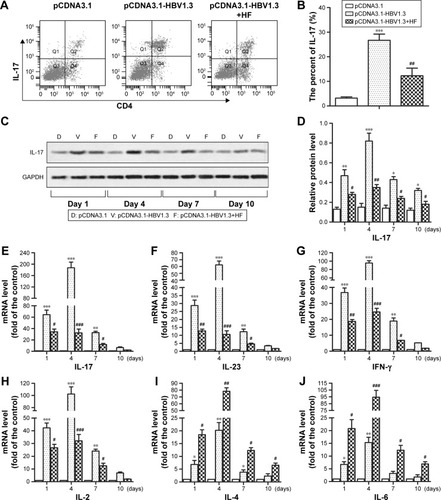
Relative mRNA levels of many cytokines (IL-17, IL-23, IFN-γ, IL-2, IL-4, and IL-13) were also compared among these 3 groups mentioned above. In the pCDNA3.1 group, relative mRNA levels of these cytokines were extremely low at different time points without change, indicating that injection of pCDNA3.1 did not influence the concentrations of these cytokines. Acute HBV infection remarkably increased the relative expression of pro-inflammatory cytokines (IL-17, IL-23, IFN-γ, and IL-2) and decreased the relative expression of anti-inflammatory cytokines (IL-4 and IL-13) in SD rats compared with the control group. However, the treatment of HF effectively abrogated the effect of HBV by downregulating the expression of pro-inflammatory cytokines and upregulating the expression of anti-inflammatory cytokines in pCDNA3.1-HBV1.3+HF group compared with the pCDNA3.1-HBV1.3 group (*P<0.05, **P<0.01, ***P<0.001, #P<0.05, ##P<0.01 ###P<0.001; ). Our results elucidated that HF suppressed the progression of acute hepatitis B by decreasing the high ratio of Th17 cells and pro-inflammatory cytokines and increasing the expression of anti-inflammatory cytokines in HBV-infected SD rats.
HF-induced AMPK activation is negatively associated with NF-κB activation with p65 phosphorylation in livers infected with HBV
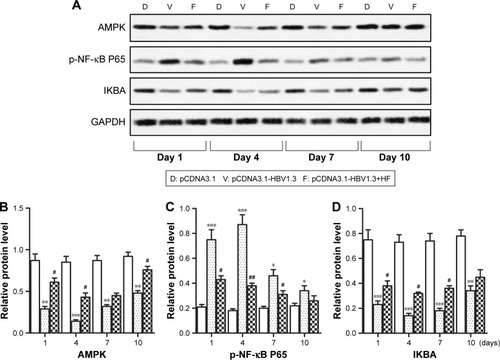
The AMPK pathway was reported to play an important role in immune response. Therefore, we further investigated whether HF regulated HBV-induced inflammation through the AMPK pathway. Relative expression levels of AMPK and IKBA in the pCDNA3.1-HBV1.3 group were much lower than pCDNA3.1 group and pCDNA3.1-HBV1.3+HF group at every indicated time. In contrast, the expression of p-NF-κB P65 was largely increased in the pCDNA3.1-HBV1.3 group compared with pCDNA3.1 group, but the elevated level of p-NF-κB P65 was remarkably decreased by HF in the pCDNA3.1-HBV1.3+HF group compared with pCDNA3.1-HBV1.3 group (*P<0.05, **P<0.01, ***P<0.001, #P<0.05, ##P<0.01, ###P<0.001; ). These results suggested that suppression of AMPK by HBV infection was activated by HF and the activation of AMPK was negatively associated with NF-κB activation with p65 phosphorylation in HBV-infected SD rats.
Figure 3 HF-induced AMPK activation is negatively associated with NF-κB activation with p65 phosphorylation in livers infected with HBV. (A) Relative protein levels of AMPK, p-NF-κB P65, and IKBA in livers of transfected rats at different time points were assayed through Western blot. (B–D) Quantitation of the expression of AMPK, p-NF-κB P65, and IKBA in (A), respectively. The bars show mean ± standard deviation of 3 independent experiments. *P<0.05, **P<0.01, ***P<0.001 compared with pCDNA3.1 group; #P<0.05, ##P<0.01 compared with pCDNA3.1-HBV1.3 group.
HF inhibits HBV infection via AMPK-mediated NF-κB p65 inactivation
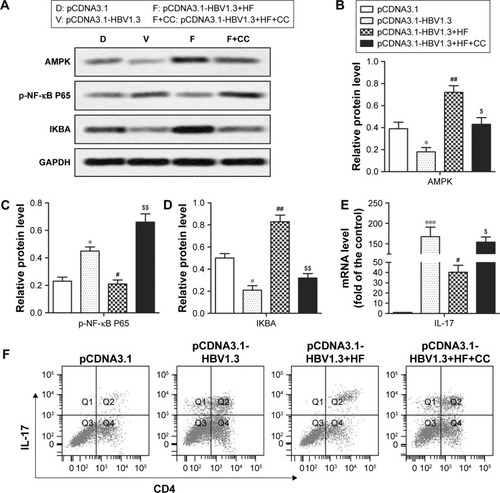
In order to further elucidate the potential molecular mechanism of how HF inhibited HBV infection, AMPK inhibitor CC was used in the animal model. After CC treatment, relative expression levels of AMPK and IKBA were decreased while the expression level of p-NF-κB P65 was increased significantly in pCDNA3.1-HBV1.3+HF+CC group compared with pCDNA3.1-HBV1.3+HF group (*P<0.05, ***P<0.001, #P<0.05, ##P<0.01, $P<0.05, $$P<0.01; ). CC also upregulated the relative expression of IL-17 compared with pCDNA3.1-HBV1.3+HF group (#P<0.05, $P<0.05, ***P<0.001; ). Results from flow cytometry showed an increased ratio of Th17 cells after CC treatment compared with the pCDNA3.1-HBV1.3+HF group (). These data indicated that HF inhibited HBV infection via AMPK-mediated NF-κB p65 inactivation.
Figure 4 HF inhibits HBV infection via AMPK-mediated NF-κB p65 inactivation. Rats in pCDNA3.1-HBV1.3+HF+CC group were transfected with pCDNA3.1-HBV1.3 and supplied with HF and CC. (A) Relative protein levels of AMPK, p-NF-κB P65, and IKBA in transfected rats were assayed through Western blot. (B–D) Quantitation of the expression of AMPK, p-NF-κB P65, and IKBA in (A), respectively. (E) Relative mRNA level of IL-17 in transfected rats was assayed by qRT-PCR. (F) The frequency of CD4+IL-17+ T-cells in the peripheral blood of transfected rats was detected through flow cytometry. The bars show mean ± standard deviation of 3 independent experiments. *P<0.05, ***P<0.001 compared with pCDNA3.1 group; #P<0.05, ##P<0.01 compared with pCDNA3.1-HBV1.3 group; $P<0.05, $$P<0.01 compared with pCDNA3.1-HBV1.3+HF group.
Discussion
HBV is a common human hepadnavirus which results in various clinical outcomes including acute or chronic hepatitis, liver fibrosis, cirrhosis, or even hepatocellular carcinoma.Citation19 It has been reported that HBV is a stealth virus which establishes itself efficiently without causing apoptosis or gene expression exchange of hepatocytes.Citation20,Citation21 On the contrary, the injury of liver cells is reported to be induced by immune response which may promote hepatic fibrosis during HBV infection.Citation22 Therefore, it is essential to elucidate the related immune reaction after HBV infection.
HF is a plant alkaloid which is extracted from the Chinese herb D. febrifuga Lour.Citation6 Previous researches have demonstrated the antimalarial, anti-fibrotic, and antitumor functions of HF. Moreover, HF has gained attention for its function as an immune regulator in arthritis.Citation15,Citation23 However, the therapeutic efficacy of HF in HBV-infected hepatitis is still elusive; so, further studies are needed.
In our present study, acute HBV-infected animal models were constructed according to previous studies.Citation17,Citation24 The concentrations of HBeAg, HBsAg, and HBV DNA were reduced remarkably by HF compared with the control group, indicating that HF suppressed the pathologic progression of HBV infection in SD rats. Moreover, the possible reason for decreasing HBeAg, HBsAg, and HBV DNA concentrations from day 4 to day 10 was probably caused by the appearance of the corresponding antibodies heralding the onset of an immune response to the virus in immunocompetent animals.Citation17 Thus, we further explored the immune response after HBV infection. Previous studies described IL-17 producing CD4+ Th17 cells as a subtype of helper CD4+ T-cells.Citation25,Citation26 Th17 cells which secrete several kinds of cytokines including IL-17 have been reported to play an important role in chronic HBV infection.Citation27,Citation28 It has also been reported that Th17 cells are highly enriched in the liver of chronic HBV-infected patients and these were likely to exacerbate liver damage during chronic HBV infection.Citation28 In addition, previous studies revealed that HF decreased the differentiation or the number of Th17 cells in liver fibrosis, autoimmune arthritis, and viral myocarditis animal models.Citation15,Citation29,Citation30 In accordance with these previous studies, our data indicated that HF treatment decreased the high expression of Th17 induced by HBV infection, indicating that HF ameliorated the severe Th17-mediated immune response caused by HBV infection. However, no single cytokine could explain the immune response for there existed many complex cytokine networks in immune reaction. Moreover, our results also showed that HF treatment downregulated the expression of IL-17, IL-23, IFN-γ, and IL-2 and upregulated the expression of IL-4 and IL-13 in acute HBV-infected rats. Cytokines in our study were classified into 2 types: Th1 (pro-inflammatory) cytokines (IL-17, IL-23, IFN-γ, and IL-2) that caused stimulation of virus-specific CD8+ cytolytic T lymphocytes, leading to viral clearance and Th2 (anti-inflammatory) cytokines (IL-4 and IL-13) that stimulated activation or differentiation of B cells.Citation31,Citation32 However, reports suggest that Th1 lymphocytes contributed not only to virus clearance but also to liver damage and the imbalance of Th1/Th2 played a crucial role in the pathogenesis of HBV infection.Citation33,Citation34 Moreover, Sun’s research indicated that HF downregulated pro-inflammatory cytokine levels and increased anti-inflammatory cytokine levels in acute viral myocarditis.Citation30 In agreement with Sun’s study, our present data showed that HF reduced the pro-inflammatory cytokines levels and increased anti-inflammatory cytokines in acute HBV-infected SD rats, indicating the anti-inflammatory role of HF in HBV infection.
AMPK has gained much attention as it acts as an attractive target in inflammation control. It was reported that the activation of AMPK inhibited inflammation, serving as a potential target for inflammation disorder diseases treatment.Citation35,Citation36 Moreover, the NF-κB pathway was reported to be associated with the connections between AMPK and inflammation in some researches. A study by Mo et al revealed that activation of AMPK suppressed NF-κB signaling and inflammation induced by fatty acid in macrophages.Citation35 Moreover, HF was reported to suppress peripheral blood T-cell functions and pro-inflammatory cytokine production through inactivating NF-κB.Citation16 Coincident with previous reports, the treatment of HF abolished the effect of HBV infection by increasing the expression of AMPK and IKBA and decreasing the expression of p-NF-κB P65. In contrast, the AMPK inhibitor CC weakened the anti-inflammation effect of HF. Our results indicated that HF played an anti-inflammatory role through AMPK-mediated NF-κB p65 inactivation in acute HBV-infected hepatitis.
Conclusion
This study explored the function of HF in regulating immune reaction in severe acute HBV-infected SD rats. HF-treated rats exhibited lower concentration of serum antigens and HBV DNA than the control group. HF also reduced the ratio of Th17 cells and pro-inflammatory cytokine levels and increased the anti-inflammatory cytokine levels, demonstrating an anti-inflammatory role via AMPK-mediated NF-κB p65 inactivation. This study implicated that HF is a potential therapeutic strategy for HBV-infected hepatitis.
Abbreviations
| HBV | = | hepatitis virus B |
| HF | = | halofuginone |
| CC | = | compound C |
| qRT-PCR | = | quantitative real-time polymerase chain reaction |
| Th | = | T helper |
Disclosure
The authors report no conflicts of interest in this work.
Notes
All authors contributed equally toward the conception and design of the study, acquisition and interpretation of data, drafting the article, and gave final approval of the version to be published.
References
- LiangTJHepatitis B: the virus and diseaseHepatology2009495 Suppl S13 S2119399811
- SzpakowskiJLTuckerLYCauses of death in patients with hepatitis B: a natural history cohort study in the United StatesHepatology2013581 21 3023080403
- ChisariFVRous-Whipple Award Lecture. Viruses, immunity, and cancer: lessons from hepatitis BAm J Pathol20001564 1117 113210751335
- GuidottiLGChisariFVImmunobiology and pathogenesis of viral hepatitisAnnu Rev Pathol20061 23 6118039107
- RehermannBFowlerPSidneyJThe cytotoxic T lymphocyte response to multiple hepatitis B virus polymerase epitopes during and after acute viral hepatitisJ Exp Med19951813 1047 10587532675
- JiangSZengQGettayacaminMAntimalarial activities and therapeutic properties of febrifugine analogsAntimicrob Agents Chemother2005493 1169 117615728920
- GnainskyYKushnirskyZBiluGGene expression during chemically induced liver fibrosis: effect of halofuginone on TGF-beta signalingCell Tissue Res20073281 153 16617180598
- PinesMSnyderDYarkoniSNaglerAHalofuginone to treat fibrosis in chronic graft-versus-host disease and sclerodermaBiol Blood Marrow Transplant200397 417 42512869955
- HuoSYuHLiCZhangJLiuTEffect of halofuginone on the inhibition of proliferation and invasion of hepatocellular carcinoma HepG2 cell lineInt J Clin Exp Pathol2015812 15863 1587026884857
- JinMLParkSYKimYHParkGLeeSJHalofuginone induces the apoptosis of breast cancer cells and inhibits migration via downregulation of matrix metalloproteinase-9Int J Oncol2014441 309 31824173318
- JuarezPMohammadKSYinJJHalofuginone inhibits the establishment and progression of melanoma bone metastasesCancer Res20127223 6247 625623002206
- RoffeSHagaiYPinesMHalevyOHalofuginone inhibits Smad3 phosphorylation via the PI3K/Akt and MAPK/ERK pathways in muscle cells: effect on myotube fusionExp Cell Res20103166 1061 106920060825
- CarlsonTJPellerinADjureticIMHalofuginone-induced amino acid starvation regulates Stat3-dependent Th17 effector function and reduces established autoimmune inflammationJ Immunol20141925 2167 217624489094
- PopovYPatsenkerEBauerMNiedobitekESchulze-KrebsASchuppanDHalofuginone induces matrix metalloproteinases in rat hepatic stellate cells via activation of p38 and NFkappaBJ Biol Chem200628122 15090 1509816489207
- ParkMKParkJSParkEMHalofuginone ameliorates autoimmune arthritis in mice by regulating the balance between Th17 and Treg cells and inhibiting osteoclastogenesisArthritis Rheumatol2014665 1195 120724782183
- LeibaMCahalonLShimoniAHalofuginone inhibits NF-kappaB and p38 MAPK in activated T cellsJ Leukoc Biol2006802 399 40616769768
- YangPLAlthageAChungJChisariFVHydrodynamic injection of viral DNA: a mouse model of acute hepatitis B virus infectionProc Natl Acad Sci U S A20029921 13825 1383012374864
- MatthewsGVAliRJAvihingsanonAQuantitative HBsAg and HBeAg predict hepatitis B seroconversion after initiation of HAART in HIV-HBV coinfected individualsPloS One201384 e6129723593455
- McMahonBJThe natural history of chronic hepatitis B virus infectionHepatology2009495 Suppl S45 S5519399792
- RehermannBNascimbeniMImmunology of hepatitis B virus and hepatitis C virus infectionNat Rev Immunol200553 215 22915738952
- WielandSFChisariFVStealth and cunning: hepatitis B and hepatitis C virusesJ Virol20057915 9369 938016014900
- ChisariFVFerrariCHepatitis B virus immunopathogenesisAnnu Rev Immunol199513 29 607612225
- ZengSWangKHuangMHalofuginone inhibits TNF-alpha-induced the migration and proliferation of fibroblast-like synoviocytes from rheumatoid arthritis patientsInt Immunopharmacol201743 187 19428013187
- SongXFHuTTLeiYActivation of intrahepatic CD4+CXCR5+ T and CD19+ B cells is associated with viral clearance in a mouse model of acute hepatitis B virus infectionOncotarget2016732 50952 5096227447555
- SakaguchiSNaturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responsesAnnu Rev Immunol200422 531 56215032588
- WeaverCTHattonRDManganPRHarringtonLEIL-17 family cytokines and the expanding diversity of effector T cell lineagesAnnu Rev Immunol200725 821 85217201677
- GeJWangKMengQHQiZXMengFLFanYCImplication of Th17 and Th1 cells in patients with chronic active hepatitis BJ Clin Immunol2010301 60 6719756987
- CallJAWilsonRJLakerRCZhangMKunduMYanZUlk1-mediated autophagy plays an essential role in mitochondrial remodeling and functional regeneration of skeletal muscleAm J Physiol Cell Physiol20173126 C724 C73228356270
- LiangJZhangBShenRWThe effect of antifibrotic drug halofugine on Th17 cells in concanavalin A-induced liver fibrosisScand J Immunol2014793 163 17224383550
- SunXHFuJSunDQHalofuginone alleviates acute viral myocarditis in suckling BALB/c mice by inhibiting TGF-beta1Biochem Biophys Res Commun20164732 558 56427021682
- BudhuAWangXWThe role of cytokines in hepatocellular carcinomaJ Leukoc Biol2006806 1197 121316946019
- SaxenaRKaurJTh1/Th2 cytokines and their genotypes as predictors of hepatitis B virus related hepatocellular carcinomaWorld J Hepatol2015711 1572 158026085916
- PennaADel PreteGCavalliAPredominant T-helper 1 cytokine profile of hepatitis B virus nucleocapsid-specific T cells in acute self-limited hepatitis BHepatology1997254 1022 10279096614
- YeYXieXYuJInvolvement of Th17 and Th1 effector responses in patients with hepatitis BJ Clin Immunol2010304 546 55520393789
- MoCWangLZhangJThe crosstalk between Nrf2 and AMPK signal pathways is important for the anti-inflammatory effect of berberine in LPS-stimulated macrophages and endotoxin-shocked miceAntioxid Redox Signal2014204 574 58823875776
- ZongYSunLLiuBResveratrol inhibits LPS-induced MAPKs activation via activation of the phosphatidylinositol 3-kinase pathway in murine RAW 264.7 macrophage cellsPloS One201278 e4410722952890
