Abstract
Morbidity and mortality rates in patients with active acromegaly are higher than the general population. Adequate biochemical control restores mortality to normal rates. Now, medical therapy has an increasingly important role in the treatment of patients with acromegaly. Somatostatin receptor ligands (SRLs) are considered the standard medical therapy, either after surgery or as a first-line therapy when surgery is deemed ineffective or is contraindicated. Overall, octreotide and lanreotide are first-generation SRLs and are effective in ~20%–70% of patients. Pegvisomant, a growth hormone receptor antagonist, controls insulin-like growth factor 1 in 65%–90% of cases. Consequently, a subset of patients (nonresponders) requires other treatment options. Drug combination therapy offers the potential for more efficacious disease control. However, the development of new medical therapies remains essential. Here, emphasis is placed on new medical therapies to control acromegaly. There is a focus on pasireotide long-acting release (LAR) (Signifor LAR®), which was approved in 2014 by the US Food and Drug Administration and the European Medicine Agency for the treatment of acromegaly. Pasireotide LAR is a long-acting somatostatin multireceptor ligand. In a Phase III clinical trial in patients with acromegaly (naïve to medical therapy or uncontrolled on a maximum dose of first-generation SRLs), 40 and 60 mg of intramuscular pasireotide LAR achieved better biochemical disease control than octreotide LAR, and tumor shrinkage was noted in both pasireotide groups. Pasireotide LAR tolerability was similar to other SRLs, except for a greater frequency and degree of hyperglycemia and diabetes mellitus. Baseline glucose may predict hyperglycemia occurrence after treatment, and careful monitoring of glycemic status and appropriate treatment is required. A precise definition of patients with acromegaly who will derive the greatest therapeutic benefit from pasireotide LAR remains to be established. Lastly, novel therapies and new potential delivery modalities (oral octreotide) are summarized.
An introduction to disease management
Acromegaly is most commonly caused by a somatotroph pituitary adenoma with autonomous overproduction of growth hormone (GH) ().Citation1–Citation7 Its worldwide prevalence is ~40–70 cases per million and incidence is approximately three to ten cases per million.Citation8–Citation10
Table 1 Etiology of acromegaly
Somatotrophs comprise ~45% of anterior pituitary cells, and GH comprises a single-chain polypeptide of 191 amino acids.Citation11 The excess GH causes increased transcription, synthesis, and release of insulin-like growth factor 1 (IGF-1) from the liver.Citation11–Citation13 Clinical manifestations include acral enlargement, diaphoresis, and facial malformations.
If untreated, serious consequences such as diabetes mellitus, hypertension, sleep apnea, pulmonary hypertension, and heart failure can increase patients’ mortality by up to 2.5–3.5 times in comparison with the general population.Citation14,Citation15 Disease control by reducing GH to <2.5 µg/L (as determined by radioimmunoassay) or <1 µg/dL (as determined by sensitive immunometric assays) and normalizing IGF-1 (adjusted for age and sex) may lessen the increased mortality risk.Citation14,Citation16,Citation17 In addition to normalizing GH and IGF-1, treatment aims to prevent tumor growth or, ideally, induce tumor shrinkage. Currently, therapeutic modalities for acromegaly include neurosurgical intervention, medical therapies, and radiotherapy.Citation11,Citation18–Citation23
Medical therapy, such as somatostatin receptor ligands (SRLs) and dopamine agonists or the GH-receptor antagonist pegvisomant target pituitary adenoma GH secretion or block peripheral GH action, respectively, and are mostly used to treat persistent or recurrent acromegaly after noncurative neurosurgery.Citation8,Citation9,Citation24–Citation26 These medical therapies can be also used as a primary therapy for patients in whom surgery is contraindicated or as a short-term therapy before the intervention.Citation27–Citation29 However, even with these therapies, a substantial proportion of patients (30%–60%) require further treatment for ongoing disease.Citation21,Citation25 The multireceptor-targeted SRL pasireotide (Signifor®) was initially approved in 2012 by the US Food and Drug Administration (FDA) for use in patients who were considered uncontrolled after surgical treatment of a corticotroph pituitary adenoma (Cushing’s disease).Citation30 Somatotroph pituitary adenomas predominantly express somatostatin receptor (SSTR) types 2 and 5. The efficacy of pasireotide in reducing GH and IGF-1 levels and in shrinking tumor in patients with acromegaly has been evaluated in Phase III clinical trials. Injectable pasireotide long-acting release (LAR) (Signifor LAR) was approved in 2014 by the US FDACitation31 for the treatment of acromegaly in patients who have experienced an inadequate response to surgery or for those in whom surgery is not an option. This review outlines the role of pasireotide LAR for the medical management of patients with acromegaly.
Comorbidities
Acromegaly is characterized by a high incidence of cardiovascular disease and represents the main cause of morbidity and mortality. Hypertension is present in about one-third of all patients; however, some authors have reported a prevalence of 60%.Citation32 Cardiomyopathy is a consequence of chronic GH exposure, causing biventricular concentric hypertrophy with thickened ventricle walls but normal sized chambers.Citation33 Functional alterations are common such as cardiac insufficiency with decreased ejection fraction, valve abnormalities, and high incidence of arrhythmias. By the time of diagnosis, arrhythmias, hypertension, and valvular heart disease are present in up to 60% of patients.Citation11,Citation34 Patients with acromegaly have swelling of the nasopharyngeal tissue, sleep apnea, and lethargy.
Excess of GH induces insulin resistance by counteracting the ability of insulin to suppress gluconeogenesis, decreasing peripheral glucose utilization, and reducing insulin receptor numbers and binding affinity.Citation33 Excess of GH also counteracts insulin action on lipid metabolism. Altered glucose metabolism is the most frequent metabolic complication in patients with acromegaly. The prevalence of impaired glucose tolerance can be up to 46%, and diabetes mellitus is present in 19%–56% of cases.Citation33
Despite initial reports, cancer incidence per se is not increased in patients with acromegaly.Citation35,Citation36 Moreover, recent epidemiological studies in the cohorts of patients with acromegaly found that cancer death rates were similar to those in the general population.Citation16,Citation37 Patients with acromegaly have an increased risk of colonic polyps that may transform to colorectal cancer, and colonoscopy screening, while controversial, is recommended.Citation38,Citation39 In addition to increased GH and IGF-1 levels, hyperinsulinemia may also play a significant role in the development of hyperplasic polyps, adenomatous polyps, and adenocarcinoma.Citation40
Acromegalic arthropathy affects up to 70% of patients and is the most significant cause of functional disability. Symptomatic carpal tunnel syndrome is also common in patients with acromegaly.Citation11–Citation13
Overview of current and emerging therapeutic strategies
Somatostatin physiology
Somatostatin (SST) is a cyclical peptide of 14 (SST-14) or 28 (SST-28) amino acids with a short half-life (<3 minutes) (). SST-producing cells can be found throughout the central nervous system (CNS) and peripheral nervous systems and in the endocrine pancreas and gut.Citation41 In the CNS, after SST is released by the hypothalamus, GH secretion is inhibited via action on SSTRs.
Figure 1 Somatostatin and pasireotide structure.
Abbreviations: SST, somatostatin; SSTR, somatostatin receptor; SRL, somatostatin receptor ligand.
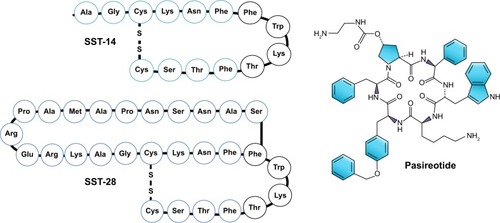
SSTRs are five 7-domain G-protein-coupled receptors named SSTR1 to SSTR5. The adult human pituitary gland mainly expresses SSTR1, SSTR2, SSTR3, and SSTR5. In normal human pituitary, SSTR5 is highly expressed, followed by SSTR2, SSTR1, and SSTR3. SSTR4 is expressed in extremely low levels.Citation42 SSTR2 and SSTR5 are expressed in 95% and 85% of GH-secreting pituitary adenomas, respectively, whereas SSTR1 and SSTR3 are expressed in ~40%.Citation43,Citation44
At the level of the pituitary, the main effect of SST is the inhibition of both hormone secretion and cell growth;Citation42,Citation45,Citation46 a potent antiproliferative mechanism and antisecretory action are also evident.Citation47 SSTRs are an important pharmacological treatment target. SRLs were developed to increase SST half-life and action, decrease multiple and simultaneous actions in different organs, and also reduce post-SST infusion rebound in GH, insulin, and glucagon. SRLs are classified as first- generation (predominantly acting on SSTR; octreotide and lanreotide) and second-generation or multiligand acting on SSTR5 and SSTR2 (pasireotide).
Pharmacodynamic characteristics
First-generation SRLs
SST analogs, or SRLs, such as octreotide or lanreotide, are considered the mainstay of medical therapeutic options for the treatment of acromegaly. Octreotide and lanreotide display high-affinity binding to SSTR2, with half-lives of 2 hours and <1 hour, respectively. Both drugs show a small volume of distribution and a low clearance that results in a longer duration of exposure and long-lasting biological activity compared with SST. Moreover, rebound hormonal hypersecretion is not present; thus, these SRLs are optimal for clinical use.Citation48
Mixing octreotide with microspheres of carboxymethylcellulose sodium as biodegradable glucose polymers and mannitol and water as diluent resulted in an octreotide LAR formulation, which remains therapeutic for 24–42 days.Citation48
Lanreotide is available in two LAR/slow-release formulations. Slow-release lanreotide was created after mixing lanreotide with microspheres of lactide/glycolide copolymers, which allows for administration of every 7–28 days.Citation11 A second formulation is lanreotide autogel, which is a viscous aqueous formulation supplied in ready-to-use prefilled syringes and is administered every 28–56 days ().
Table 2 Medical therapy for acromegaly that is commercially available and in different stages of clinical research
Octreotide LAR (Sandostatin® LAR Depot) and lanreotide autogel (Somatuline® Depot) are US FDA approved as a long-term maintenance therapy in patients with acromegaly who have had an inadequate response to surgery and/or radiotherapy or for whom surgery and/or radiotherapy is not an option.Citation11,Citation20,Citation23,Citation49
Second-generation SRLs
Pasireotide (SOM230) is a novel multireceptor-targeted SST created by the incorporation of four synthetic and two essential amino acids of SST in a novel cyclohexapeptide structure (). Pasireotide has a high affinity for SSTR5 followed by SSTR2, SSTR3, and SSTR1 ( and ). When compared with octreotide, pasireotide displays a 40, 30, and five times higher binding affinity to SSTR5, SSTR1, and SSTR3, respectively, and a 2.5 times lower binding affinity to SSTR2.Citation50 Pasireotide has 106-fold higher affinity for SSTR5 in comparison with lanreotide.Citation50 Pasireotide LAR was developed using biodegradable polymers using a method similar to that of octreotide LAR.Citation20,Citation23
Figure 2 Pasireotide effects.
Abbreviations: SRL, somatostatin receptor ligand; SSTR, somatostatin receptor; GH, growth hormone; GLP-1, glucagon-like peptide 1; CYP450, cytochrome P450.
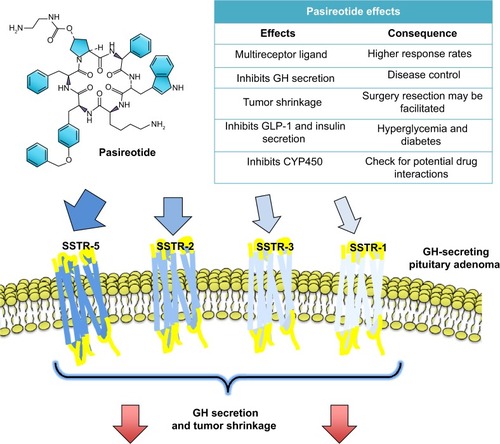
Since somatotroph tumors express higher levels of SSTR2 and SSTR5, the SSTR-targeting profile of pasireotide adds a potential benefit compared with more selective SSTR2 ligands such as lanreotide or octreotide.
Pharmacology, mechanism of action, and pharmacokinetics of pasireotide LAR
Pharmacodynamics and mechanism of action in acromegaly
Many G-protein-coupled receptors, including SSTR1–SSTR5, regulate responsiveness to continued agonist effect with different degrees of receptor internalization and degradation. Therefore, continued exposure to the peptide causes progressive reduction of the inhibitory effects. However, the desensitization of SSTRs in patients with acromegaly who respond well to SRLs shows a persistent inhibitory effect of GH secretion without escape after prolonged treatment periods.Citation51 Pasireotide modulates SSTR trafficking differently than octreotide, resulting in quicker recycling of SSTRs, particularly SSTR2, to the plasma membrane after endocytosis.Citation52 Pasireotide stimulates phosphorylation of serine residues 341 and 343 of SSTR2. This mechanism results in accelerated recycling that may counteract the desensitization of SSTR2.Citation53
In contrast to receptor desensitization, the expression of SSTRs is highly variable in pituitary adenomas. SSTR2 mRNA expression correlates positively with in vivo GH suppression induced by SRLs.Citation54–Citation56 In patients treated with octreotide or lanreotide, which mainly acts on the SSTR2 receptor, the expression of SSTR2 is a key determinant for responsiveness and efficient inhibition of GH release. However, the loss of SSTR2 is not the only determinant. Some partially SRL-sensitive GH-secreting adenomas have a loss of SSTR2 expression. In such tumors, SSTR5 mRNA expression is higher than SSTR2, and this would seem to explain the incomplete sensitivity of the SRLs.Citation54,Citation57 Also, a SSTR2- and SSTR5-bispecific compound was shown to exhibit better GH suppression when compared with a selective agonist to each receptor.Citation58 Therefore, some GH-secreting adenomas show better response to SSTR2-specific ligands, while in others, SSTR5-specific ligands are more potent.
Pharmacokinetics
Pasireotide LAR administered via intramuscular injection is widely distributed, primarily through plasma,Citation31,Citation59 and exhibits 88% protein binding.
After three 28-day (3 months) intramuscular injections of pasireotide LAR (20, 40, or 60 mg/month) in patients with acromegaly, steady-state circulating levels were reached.Citation60 After the first injection, independent of the dose, pasireotide LAR quickly increased (within the first 2–10 hours), and pasireotide plasma levels then plateaued or decreased. A dose–response serum increment was also observed.Citation60 Furthermore, a higher response rate was noted with an increased pasireotide LAR dose.Citation61 Pasireotide exhibits high metabolic stability; however, it may inhibit cytochrome P450 enzyme;Citation61 therefore, some concomitant drugs need to be avoided if possible, as some are contraindicated, or a dose adjustment may be required (). Pasireotide is cleared through liver metabolism, and ~60% of the drug is eliminated in the feces or urine after 10 days following a single subcutaneous (SC) dose.Citation31,Citation59 Therefore, pasireotide-circulating levels may increase in patients with moderate-to-severe hepatic impairment compared with normal liver function.Citation62 In patients with kidney failure, an increment of circulating pasireotide is also expected.Citation59 The pharmacokinetic parameters of pasireotide were shown not to be influenced by age, body weight, sex, or race.Citation59
Table 3 Potential drug interactions with pasireotide LAR
Clinical efficacy, safety, and tolerability of pasireotide LAR
Clinical efficacy
Approval of pasireotide LAR for the treatment of adults with acromegaly was based on data from two large, international, multicenter, double-blinded randomized Phase III studies, C2305 and C2402. These studies confirmed higher rates of composite biochemical control (defined as mean GH level <2.5 µg/L and normal IGF-1 levels) than the first-generation SRLs.
A proof-of-concept trial compared a single dose of pasireotide (100 or 250 µg) with 100 µg octreotide. GH circulation levels were significantly reduced with all three interventions, with a marked dose-dependent reduction in the two pasireotide groups (100 or 250 µg).Citation63 Interestingly, those cases with presumably SSTR2 predominant expression responded to both octreotide and pasireotide treatments. In others, pasireotide was more effective in reducing GH circulating levels that are likely due to the higher abundance of SSTR5. However, in some cases, there was a better response to octreotide, presumably due to predominant SSTR2 and low SSTR5 expression. A subsequent Phase II, multicenter, open-label, randomized, cross-over trial of pasireotide was conducted.Citation64 A total of 60 patients with active acromegaly were initially given 100 µg octreotide over the course of 1 month to evaluate the response to standard treatment. Patients were then randomized to 200, 400, or 600 µg/day of pasireotide. After 1 month, patients taking pasireotide showed a full response rate with normalization of both GH and IGF-1 levels in 14%, 12%, and 30% with 200, 400, and 600 µg, respectively. After 3 months, 38% of patients achieved normal IGF-1 levels, and in 49%, GH levels were <2.5 µg/L. Tumor volume decreased by >20% in 20 patients (39%).Citation64
Phase III trials with pasireotide LAR
C2305 in patients naïve to medical therapy (efficacy: biochemical control and tumor shrinkage)
The C2305 study was a multicenter, randomized, double-blinded study conducted in 358 patients with active acromegaly naïve to medical treatment, who had persistent disease despite neurosurgical intervention or were ineligible for surgery. Patients were randomized to receive pasireotide LAR 40 mg/28 days (n=176) with the option to uptitrate to 60 mg or to receive octreotide LAR 20 mg/28 days (n=182) for 12 months with the option to uptitrate to 30 mg. Key exclusion criteria included previous therapy with SRLs, dopamine agonists or GH-receptor antagonist (pegvisomant), pituitary irradiation within the past 10 years, significant cardiovascular morbidity, and glycated hemoglobin (HbA1c) >8%.Citation65 At baseline, 42% of patients had undergone at least one pituitary surgery with a median time since surgery of 7 months. GH levels were 22 and 19 µg/L in the pasireotide and octreotide groups, respectively, and IGF-1 levels were 3.1 times the upper limit of normal (ULN) in both groups. Mean tumor volume was 2,421 versus 2,259 mm3, respectively. After 12 months, biochemical control (defined earlier) was met in 31% versus 19% in patients with pasireotide LAR and octreotide LAR, respectively (P=0.007). Also, biochemical control was achieved early with pasireotide LAR (by month 3) in 30%. Dose uptitration was recommended in all uncontrolled patients but was implemented in just 51% of patients with pasireotide and 67% of patients with octreotide. Lack of uptitration has been shown to be one of the causes of reduced response in either SRLsCitation66 or pegvisomant-treated patients.Citation67 Health-related quality of life questionnaires showed similar reduction of ring size and less symptom severity score in both the treatment groups.Citation65 Interestingly, IGF-1 levels were significantly lower with pasireotide LAR than octreotide LAR (38.6% vs 23.6%, P=0.002, respectively), despite a similar GH suppression with pasireotide and octreotide. The exact mechanism of improved IGF-1 suppression is currently unknown. Biochemical control was higher in patients who had surgery in both groups; however, remarkably, ~60% of patients in this study did not undergo surgery.
A significant tumor reduction was achieved in 80% and 77% of patients with pasireotide LAR and octreotide LAR, respectively. Mean tumor volume reduction was 40% in both groups. Tumor reduction or no change in tumor volume was seen in 98% of patients with pasireotide LAR at month 12. Contrary to other studies, no differences in tumor shrinkage were observed between postsurgery or de novo patients.Citation65
C2305: extension study
Results of a 12-month, double-blinded, multicenter extension phase study demonstrated that pasireotide LAR provided sustained biochemical control in patients with acromegaly.Citation68 After month 12, patients who achieved biochemical control continued their study treatment. At month 26, 49% and 46% of patients in each group, respectively, achieved biochemical control.Citation68 Eighty-one (45%) patients who had not achieved disease control after 12 months switched from octreotide LAR to pasireotide LAR, and 38 (22%) patients switched from pasireotide LAR to octreotide LAR at month 13. After a further year of treatment, biochemical control was successful in an additional 17% (n=14) of patients who switched to pasireotide LAR and 0% of patients who switched to octreotide LAR. Also, tumor volume decreased to 25% in the pasireotide group and 18% in the octreotide group. In each group, 54% and 42% of patients, respectively, achieved >20% tumor volume reduction.Citation69,Citation70
C2402 (PAOLA study): patients inadequately controlled
The pasireotide versus continued treatment with octreotide or lanreotide in patients with inadequately controlled acromegaly (PAOLA) study was a randomized study evaluating the efficacy and safety of double-blind pasireotide LAR (40 and 60 mg) versus continued open-label pretrial octreotide or lanreotide.Citation61 Patients under combination therapy with GH-receptor antagonist (14%) or dopamine agonist (32%) were eligible for inclusion if such agents were discontinued at least 8 weeks before receiving the study drug. Patients with a positive history of pituitary irradiation within the past 10 years, significant cardiovascular morbidity, or HbA1c >8% were excluded. Inadequate control was defined by a five-point 2-hour mean GH levels of >2.5 µg/L and IGF-1 levels >1.3 times sex- and age-adjusted ULN. Biochemical control at 6 months with pasireotide LAR 40 or 60 mg versus continued pretrial octreotide or lanreotide therapy was met for both pasireotide LAR dose groups. Specifically, 15% and 20% of patients treated with pasireotide LAR 40 and 60 mg, respectively, achieved full GH and IGF-1 biochemical control at 6 months compared with 0% in the pretrial therapy control arm. Biochemical control was achieved by month 3 in 15% and 18% of 40 and 60 mg pasireotide LAR, respectively; 81% and 70% of patients treated with pasireotide LAR 40 and 60 mg had either a reduction or no change in tumor volume from baseline (as assessed by magnetic resonance imaging) at month 6, respectively.Citation61 A reduction of 25% or more in tumor volume by week 24 was achieved in more patients receiving pasireotide than active control (18% for 40 mg, 11% for 60 mg of pasireotide LAR vs 1.5% with active control). Clinically, Acromegaly Quality of Life Questionnaire scores of patients significantly improved; greater improvements were recorded with pasireotide LAR treatment.Citation61,Citation71
During the 28-week extension of PAOLA study, biochemical control was maintained with pasireotide LAR and was generally well tolerated in patients inadequately controlled by first-generation SST analogs. This was consistent with data from the core study, where ~20% of patients in the active control group achieved biochemical control after switching to pasireotide LAR in the extension study.Citation72
Safety and tolerability of pasireotide LAR
Most adverse events associated with pasireotide LAR are mild to moderate in severity.Citation61,Citation68
Pasireotide-induced hyperglycemia
Hyperglycemia was more common with pasireotide LAR (57%) than octreotide LAR (22%) in the C2305 Phase III trial with the higher incidence of diabetes mellitus (26% vs 4%, respectively).Citation50 Eight (4.5%) patients in the pasireotide LAR group discontinued treatment due to serious drug-adverse event, the most common of which was elevated blood glucose.Citation65 After 26 months of treatment in the C2305 study (core and extension), hyperglycemia-related events with pasireotide LAR 40 mg/month were 63% compared with 25% with octreotide LAR 20 mg/month (68).Citation68 Similarly, in the PAOLA study (C2402 trial), pasireotide-induced hyperglycemia was more common (~30%) versus active control (14%) as well as diabetes mellitus (~25% vs 9%, respectively).Citation61 Five patients discontinued pasireotide LAR treatment because of serious adverse events, all of which were related to pasireotide-induced hyperglycemia or diabetes. HbA1c levels, which increased in the first 3 months of pasireotide LAR treatment, were stable by month 26. Mean glucose and HbA1c levels slightly increased in the octreotide LAR group.Citation61,Citation68
The exact mechanism of pasireotide-induced hyperglycemia in patients with acromegaly is unknown. Data from a dose–response study in healthy volunteers suggested that pasireotide-associated hyperglycemia was due to insulin suppression. Also, mild inhibition of glucagon was reported.Citation73 Further clinical research using a hyperglycemic clamp test in healthy volunteers showed that hyperglycemia associated with pasireotide treatment was not due to changes in hepatic/peripheral insulin sensitivity. Decreased gastrointestinal incretin hormones with the subsequent reduction on insulin secretion would seem to explain the increment in glucose levels. During an oral glucose tolerance test, glucagon-like peptide 1 and glucose-dependent insulinotropic polypeptide were significantly decreased compared with baseline.Citation74
Dipeptidyl peptidase-4 inhibitors (vildagliptin), glucagon-like peptide 1 analogs (liraglutide), and insulin secretagogues (nateglinide) have been also tested to treat pasireotide-related hyperglycemia in 90 healthy male volunteers. Comparison between vildagliptin 50 mg bid, nateglinide 60 mg tid, or liraglutide 0.6 mg SC qd for 7 days showed that glucose increment after pasireotide treatment was lower in the groups treated with metformin (60%), nateglinide (49%), vildagliptin (38%), and liraglutide (19%), suggesting a benefit of antihyperglycemic drugs to mitigate pasireotide-related hyperglycemia.Citation75 Clinical trials comparing antihyperglycemic drugs or insulin are warranted to establish optimal treatment in patients with pasireotide-induced hyperglycemia.
Although the treatment of pasireotide-induced hyperglycemia in healthy volunteers has been focused on increasing incretin levels or using incretin analogs, optimal therapy in patients with acromegaly remains to be elucidated.Citation65 Metformin-based antidiabetic therapy appears to effectively control pasireotide-associated hyperglycemia in selected patients with acromegaly. During the 12-month study Phase III clinical trial (C2305), 24 subjects received metformin alone, 19 subjects received metformin plus another oral antidiabetic therapy, and ten subjects were treated with insulin alone or in addition to an oral agent.Citation76 Results showed lower mean levels of HbA1c at month 12 in groups with metformin (6.6%) or metformin plus another oral antidiabetic agent (6.7%) than insulin with or without other therapy (7.2%).
After pasireotide is stopped, hyperglycemia appears to be reversible since patients (n=38) who switched from pasireotide to octreotide after 13 months of treatment showed improvement of their glucose levels (from 127 to 104 mg/dL) and HbA1c (from 6.7% to 6.1%). In contrast, those patients who switched from octreotide to pasireotide at month 13 (n=81) showed an increment in mean glucose levels (from 104 to 141 mg/dL) and HbA1c (6.1%–7% at month 3 and stabilizing to 6.7% at month 12).Citation55 In the C2402 (PAOLA) study, glycemic control improved when an early treatment adjustment was established in the first 2 weeks of pasireotide, regardless of diabetes status.Citation77
Pasireotide: other adverse events
Mild gastrointestinal disturbances were reported in the Phase II randomized, open-label, cross-over, multicenter pasireotide (SOM230) study.Citation64 Regardless of causality, the most common adverse events related with pasireotide in clinical Phase III trials were nausea (25%), diarrhea (22%), abdominal pain (12%), and flatulence (10%). Headache and nasopharyngitis were also more common with pasireotide LAR. Interestingly, cholelithiasis was more common with octreotide LAR (36%) than pasireotide LAR (26%).Citation61,Citation65 As expected, the frequency of adverse events was higher in patients naïve to medical treatment with SRLs (40%–45%) versus patients previously treated with SRLs (5%–20%).Citation61,Citation65 All pituitary hormones should be monitored (thyroid, adrenal, and gonadal) in patients taking pasireotide LAR, due to potential hormonal inhibition. In clinical trials, pasireotide LAR has been rarely associated with adrenal insufficiency.Citation61,Citation65 Pasireotide may induce bradycardia and QT interval prolongation. Bradycardia-related adverse events in the Phase III clinical trials were 3%–8% for pasireotide LAR and 0% for active controls.Citation61,Citation65 One patient receiving pasireotide LAR 40 mg required dose interruption due to liver injury.Citation61,Citation65
Potential drug–drug interactions related to cytochrome P450-3A4 metabolism may cause QT prolongation or cardiac arrhythmias, which should be assessed in all patients ().
Place in therapeutic armamentarium
As mentioned previously, pasireotide LAR was approved in 2014 by the US FDACitation31 and the European Medicines Agency in Europe,Citation59 as an injectable suspension, for intramuscular use, for the treatment of patients with acromegaly who have had an inadequate response to surgery and/or for whom surgery is not an option. Regulatory approval of pasireotide LAR for this indication in several other countries is awaited.
Surgical removal of the GH-secreting pituitary adenoma is the treatment of choice for acromegaly if the tumor is considered resectable, and the surgery is performed by an experienced surgeon.Citation49 Current treatment guidelines recommend normal serum IGF-1 for age- and sex-matched and serum GH <1.0 µg/L as treatment targets.Citation49 If active disease persists following surgery, patients have a tumor that cannot be resected, or in those cases whereby a patient chooses not to undergo surgery, medical management is recommended ().
Occasionally, medical therapy is used as a primary treatment before surgery.Citation78
Radiation of an adenoma as a third-line therapy might be necessary in selected cases, such as with aggressive tumors.
Pharmacotherapy includes SRLs, pegvisomant, and dopamine agonists. When uncontrolled acromegaly persists, a pharmaceutical combination may improve the efficacy and minimize the potential adverse effects with increased doses of individual agents.Citation49,Citation79
In pre- and clinical trials, pasireotide LAR was superior for biochemical disease control in patients with active acromegaly naïve to medial therapy or uncontrolled on a maximum dose of first-generation SRLs treatment. Biochemical control was rapid in the first 3 months, and control was sustained up to 26 months. Tumor volume was also decreased.
In a subset of patients with acromegaly, remission can be achieved with pasireotide LAR treatment if patients were previously uncontrolled on SRLs.Citation61,Citation80 Tumors that are large, aggressive, have less SSTR2 expression, or are sparsely granulated are more resistant to first-generation SRLs.Citation55,Citation56 Additional studies are needed to define which patients will likely benefit the most from pasireotide LAR treatment. Similarly, the use of pasireotide in combination with other drug therapies requires further investigation.
Pasireotide LAR should be administered via deep intramuscular injection with a recommended initial dose of 40 mg every 28 days. If after 3 months the patients’ disease remains active, the dose may be increased to 60 mg/28 days. Adverse reactions should be carefully monitored. Patients with mild liver or kidney failure do not require dose adjustment; however, pasireotide LAR should be avoided in cases with severe hepatic or renal failure. Glycemic status with fasting plasma glucose and HbA1c levels should be assessed prior to starting treatment and then every 1–3 months.
In the C2402 (PAOLA) study, 47% of all patients treated with pasireotide LAR (40 or 60 mg) did not receive antidiabetic medication at any time during the study. It has been suggested that the effects of pasireotide LAR on glucose homeostasis may be attenuated by increased insulin sensitivity due to the compensatory increase in IGF-binding protein-2.Citation81
Metformin, if not contraindicated, should be initiated early.Citation77 Also, other antidiabetic agents or insulin should be considered to increase glycemic control. Guidelines for the treatment of hyperglycemia and diabetes should be followed when patients with acromegaly already have or develop diabetes during the follow-up.Citation82 If hyperglycemia persists despite adequate treatment, pasireotide dose should be reduced or discontinued. Liver function tests and a gallbladder ultrasound should be performed periodically with pasireotide LAR treatment. An electrocardiogram may be indicated in patients with cardiac disease or with risk factors for bradycardia or arrhythmias. Drug–drug interactions should be carefully monitored ().Citation83
Other emerging medical therapies for acromegaly
Oral octreotide
Oral octreotide appears to be an attractive option for patients with acromegaly that is biochemically controlled with injectable SRLs. A newly developed transient permeability enhancer to improve the absorption of pharmacologically active drug has been shown to increase the intestinal absorption of an oral formulation of octreotide and may represent an alternative to the parenteral formulation in patients with acromegaly. Oral octreotide is absorbed within 1 hour of administration. Equivalent pharmacokinetic and pharmacodynamics in a Phase II study were demonstrated using 20 mg of oral octreotide or 100 µg of SC octreotide.Citation84 Furthermore, a single dose of 20 mg of oral octreotide resulted in the significant suppression of both basal GH- and arginine-GH-releasing hormone stimulated levels. A large, multicenter, Phase III study in 151 patients with acromegaly during 13 months showed biochemical normalization in 62% of cases after switching from injectable SRLs (from 88.7% at the baseline visit while on injectable SRLs).Citation85 The effect was durable, and 85% of subjects initially controlled on oral octreotide maintained the response for ~1 year. GH levels were reduced compared with baseline and acromegaly-related symptoms improved.Citation85 Approximately half of the patients did require >40 mg doses to maintain response. Good baseline control with injectable SRLs (IGF-1 ≤1× ULN/GH <2.5 ng/mL) predicted responsiveness to oral octreotide.Citation85 The dose of injectable SRLs was not a good predictor of responsiveness. Over 70% of patients on low- or mid-dose injectable SRLs responded to capsules, but also half of the patients on high-dose injections were responders after switching to oral capsules. As expected, the safety profile was similar to that of other SRLs. Most adverse events occurred at the beginning of treatment. Gastrointestinal adverse events were transient and mostly resolved within 2 weeks.Citation85
Long-acting subcutaneous octreotide and octreotide implants
CAM2029 is a long-acting octreotide formulation that is based on a proprietary fluid crystal (FC) delivery system, for the treatment of acromegaly. Octreotide is delivered such that rapid onset and long-acting octreotide release is provided. CAM2029 has been evaluated in Phase I trials, specifically assessing pharmacokinetics and pharmacodynamics. In healthy volunteers, octreotide FC provided greater bioavailability with a more rapid onset and similar duration of effect compared with octreotide LAR. The FC formulation offered enhanced convenience as it is supplied in a prefilled syringe with a thin needle.Citation86 A Phase III study of octreotide FC in patients with acromegaly is planned.
Octreotide implants for long-acting continuous release have been studied in Phase II and Phase III clinical trials. In comparison with monthly octreotide LAR, biochemical control and safety parameters were maintained >24 weeks with an octreotide implant. Diarrhea and headache were more frequently reported with the octreotide implant, whereas cholecystitis and hypertension were more commonly reported with octreotide LAR.Citation87
Antisense oligonucleotide
ATL1103 is a second-generation antisense oligomer drug targeted to block the GH receptor, thereby lowering IGF-1 levels. ATL1103 is a 20mer with a phosphorothioate backbone and 2′-O-methoxyethyl modifications of the five nucleotides at either end intended to increase its plasma half-life and affinity for the target RNA to allow posthybridization RNaseH degradation of the GHr RNA strand. Animal studies showed a rapid distribution from plasma to peripheral tissues (Cmax in 2–4 hours), with a tissue clearance half-life of 2–4 weeks.
ATL1103 (COR-004) was evaluated in a Phase II randomized, open-label, multicenter, parallel group study in patients with active acromegaly. Patients were randomized to receive either ATL1103 200 mg once or twice weekly for 13 weeks (three doses in the first week). The primary endpoint was to evaluate the safety and to understand the single-dose and multiple-dose pharmacokinetic profiles. Thirty-five patients were recruited (average age 50.4 years [26–80 years]; eleven males), and all completed the treatment. There was a significant fall in serum IGF-1 of 26% by week 14 (1 week past the last dose) with 200 mg ATL1103 twice weekly (577±198 vs 411±174 ng/mL [mean ± standard deviation], P<0.0001). Once-weekly dosing did not result in a significant fall in IGF-1. The fall in IGF-1 was associated with a mean reduction in ring size and serum GH. ATL1103 was well tolerated with mild-to-moderate injection site reactions, the most common drug-related adverse event. ATL1103 offers a novel therapeutic approach for patients with active acromegaly.Citation88
Somatoprim
Somatoprim (DG3173-COR-005) is a multireceptor SRL that binds SSTR2, SSTR4, and SSTR5. Somatoprim is unique in comparison to other SRLs. In particular, in vivo studies showed an additional 40% response in patients with pituitary adenomas that were resistant to octreotide and less of a hyperglycemic effect since insulin-suppressing activity is less potent.Citation89 A Phase II study showed lower GH secretion with reduced effects on insulin secretion and glucose levels as compared with octreotide in two single ascending dose in patients with acromegaly.Citation89
Targeted secretion inhibitor
A botulinum neurotoxin-derived targeted secretion inhibitor was designed to target pituitary somatotroph cells and suppress GH secretion.Citation90 The recombinant protein was created by modifying the GH-releasing hormone domain and the endopeptidase domain of botulinum toxin. The N-ethylmaleimide-sensitive fusion attachment protein receptor (SNARE) was depleted in vitro, blocking the GH exocytosis after GH-releasing hormone binding to its receptor. Administration in vivo in rats produced a dose-dependent inhibition of GH synthesis, storage, and secretion with IGF-1 reduction.Citation90 In human samples of pituitary adenoma from 25 patients with acromegaly and 47 patients with nonfunctioning pituitary adenoma, SNARE proteins were variably expressed.Citation91 However, the targeted secretion inhibitor did not inhibit GH secretion in somatotroph cell cultures.Citation91 Novel targeted secretion inhibitors may have future potential with therapeutic benefit, but further research is needed.
Conclusion
Treating patients with acromegaly can be extremely challenging, and inadequate disease control may lead to serious consequences. Pegvisomant and combination therapies have been used to manage patients uncontrolled on first-generation SRLs. Novel medical therapies provide different or enhanced mechanisms to control elevated circulating GH and IGF-1 levels. Pasireotide LAR seems a promising medical therapy for patients with acromegaly that cannot be controlled with available SRLs. In recent Phase III trials, pasireotide LAR has been shown to be more efficacious than first-generation SRLs. However, hyperglycemia developed in approximately half of the patients. Interestingly, the rates of hyperglycemia were similar in responders versus nonresponders, but baseline fasting plasma glucose level could predict hyperglycemia. Proactive monitoring and early intervention in the management of pasireotide-induced hyperglycemia are recommended. Other novel pharmaceutical therapies for patients with acromegaly, as well as new potential delivery modalities, including the first oral octreotide therapy are on the horizon and show early promise. Overall, vigilant and judicious care is advised in treating individual patients with acromegaly.
Acknowledgments
The authors thank Shirley McCartney, PhD, Oregon Health & Science University, for editorial assistance with the manuscript.
Disclosure
The authors did not receive any support for writing of this manuscript. Dr Fleseriu is the principal investigator on research grants to Oregon Health & Science University from Chiasma, Cortendo, Ipsen, Novartis, and Pfizer and an ad hoc scientific consultant to Cortendo, Chiasma, and Novartis. Dr Cuevas-Ramos has no conflicts of interest in this work.
References
- AsaSLBilbaoJMKovacsKHypothalamic neuronal hamartoma associated with pituitary growth hormone cell adenoma and acromegalyActa Neuropathol1980522312347445985
- AsaSLScheithauerBWBilbaoJMA case for hypothalamic acromegaly: a clinicopathological study of six patients with hypothalamic gangliocytomas producing growth hormone-releasing factorJ Clin Endocrinol Metab1984587968036423659
- BeckersALodishMBTrivellinGX-linked acrogigantism syndrome: clinical profile and therapeutic responsesEndocr Relat Cancer20152235336725712922
- BeuschleinFStrasburgerCJSiegerstetterVAcromegaly caused by secretion of growth hormone by a non-Hodgkin’s lymphomaN Engl J Med20003421871187610861322
- MelmedSEzrinCKovacsKAcromegaly due to secretion of growth hormone by an ectopic pancreatic islet-cell tumorN Engl J Med19853129172981107
- ShimonIMelmedSGrowth hormone- and growth-hormone-releasing hormone-producing tumorsCancer Treatment and ResearchLos Angeles, CASpringer Science + Business Media1997124
- TrivellinGDalyAFFauczFRGigantism and acromegaly due to Xq26 microduplications and GPR101 mutationN Engl J Med20143712363237425470569
- DalyAFRixhonMAdamCHigh prevalence of pituitary adenomas: a cross-sectional study in the province of Liège, BelgiumJ Clin Endocrinol Metab2006914769477516968795
- FernandezAKaravitakiNWassJAHPrevalence of pituitary adenomas: a community-based, cross-sectional study in Banbury (Oxfordshire, UK)Clin Endocrinol (Oxf)20107237738219650784
- HoskuldsdottirGTFjalldalSBSigurjonsdottirHAThe incidence and prevalence of acromegaly, a nationwide study from 1955 through 2013Pituitary20151880380725893613
- MelmedSAcromegalyN Engl J Med20063552558257317167139
- MelmedSAcromegaly pathogenesis and treatmentJ Clin Invest20091193189320219884662
- MelmedSPathogenesis of pituitary tumorsNat Rev Endocrinol2011725726621423242
- HoldawayIMBollandMJGambleGDA meta-analysis of the effect of lowering serum levels of GH and IGF-I on mortality in acromegalyEur J Endocrinol2008159899518524797
- ReidTJPostKDBruceJNFeatures at diagnosis of 324 patients with acromegaly did not change from 1981 to 2006: acromegaly remains under-recognized and under-diagnosedClin Endocrinol (Oxf)20107220320819473180
- HoldawayIMRajasooryaRCGambleGDFactors influencing mortality in acromegalyJ Clin Endocrinol Metab20048966767414764779
- McCabeJAyukJSherlockMTreatment factors that influence mortality in acromegalyNeuroendocrinology Epub2015130
- AyukJClaytonRNHolderGGrowth hormone and pituitary radiotherapy, but not serum insulin-like growth factor-I concentrations, predict excess mortality in patients with acromegalyJ Clin Endocrinol Metab2004891613161715070920
- BiermaszNRPituitary gland: mortality in acromegaly reduced with multimodal therapyNat Rev Endocrinol20141070871025365921
- FleseriuMAdvances in the pharmacotherapy of patients with acromegalyDiscov Med20141732933824979253
- GiustinaAChansonPKleinbergDExpert consensus document: a consensus on the medical treatment of acromegalyNat Rev Endocrinol20141024324824566817
- JaneJAStarkeRMElzoghbyMAEndoscopic transsphenoidal surgery for acromegaly: remission using modern criteria, complications, and predictors of outcomeJ Clin Endocrinol Metab2011962732274021715544
- MelmedSKleinbergDBonertVAcromegaly: assessing the disorder and navigating therapeutic options for treatmentEndocr Pract20142071725374336
- AlbarelFCastinettiFMorangeIOutcome of multimodal therapy in operated acromegalic patients, a study in 115 patientsClin Endocrinol201378263270
- Cuevas-RamosDCarmichaelJDCooperOA structural and functional acromegaly classificationJ Clin Endocrinol Metab201510012213125250634
- MercadoMEspinosaERamirezCCurrent status and future directions of the pharmacological therapy of AcromegalyMinerva Endocrinol Epub20151020
- ColaoAAuriemmaRSPivonelloRThe effects of somatostatin analogue therapy on pituitary tumor volume in patients with acromegalyPituitary Epub2015820
- EspinosaERamirezCMercadoMThe multimodal treatment of acromegaly: current status and future perspectivesEndocr Metab Immune Disord Drug Targets20141416918124915854
- KleinbergDLPrimary therapy for acromegaly with somatostatin analogs and a discussion of novel peptide analogsRev Endocr Metab Disord20056293715711912
- US Food Drug AdministrationFDA Approves Signifor, a New Orphan Drug for Cushing’s Disease2012 Available from: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm332351.htmAccessed July 17, 2015
- Novartis PharmaceuticalsSignifor LAR Prescribing Information2014 Available from: http://www.pharma.us.novartis.com/product/pi/pdf/signifor_lar.pdfAccessed March 14, 2015
- BondanelliMAmbrosioMRdegli UbertiECPathogenesis and prevalence of hypertension in acromegalyPituitary2001423924912501974
- ColaoAFeroneDMarzulloPSystemic complications of acromegaly: epidemiology, pathogenesis, and managementEndocr Rev20042510215214769829
- GurnellMPowlsonASCardiovascular disease and sleep disordered breathing in acromegalyNeuroendocrinology Epub2015728
- MelmedSAcromegaly and cancer: not a problem?J Clin Endocrinol Metab2001862929293411443145
- OrmeSMMcNallyRJQCartwrightRAMortality and cancer incidence in acromegaly: a retrospective cohort study 1J Clin Endocrinol Metab199883273027349709939
- MestronAWebbSAstorgaREpidemiology, clinical characteristics, outcome, morbidity and mortality in acromegaly based on the Spanish Acromegaly Registry (Registro Espanol de Acromegalia, REA)Eur J Endocrinol200415143944615476442
- LoisKBukowczanJPerrosPThe role of colonoscopic screening in acromegaly revisited: review of current literature and practice guidelinesPituitary20141856857425052731
- RenehanAGBrennanBMAcromegaly, growth hormone and cancer riskBest Pract Res Clin Endocrinol Metab20082263965718971124
- ColaoAPivonelloRAuriemmaRSThe association of fasting insulin concentrations and colonic neoplasms in acromegaly: a colonos-copy-based study in 210 patientsJ Clin Endocrinol Metab2007923854386017652220
- LambertsSWvan der LelyAJde HerderWWOctreotideN Engl J Med19963342462548532003
- Ben-ShlomoAMelmedSPituitary somatostatin receptor signalingTrends Endocrinol Metab20102112313320149677
- ReubiJWaserBSchaerJ-CSomatostatin receptor sst1–sst5 expression in normal and neoplastic human tissues using receptor autoradiography with subtype-selective ligandsEur J Nucl Med20012883684611504080
- TaboadaGFLuqueRMBastosWQuantitative analysis of somatostatin receptor subtype (SSTR1-5) gene expression levels in somatotropinomas and non-functioning pituitary adenomasEur J Endocrinol2007156657417218727
- SellersLAAldertonFCarruthersAMReceptor isoforms mediate opposing proliferative effects through gbeta gamma-activated p38 or Akt pathwaysMol Cell Biol2000205974598510913180
- TheodoropoulouMStallaGKSpenglerDZAC1 target genes and pituitary tumorigenesisMol Cell Endocrinol2010326606520117169
- Cuevas-RamosDFleseriuMTreatment of Cushing’s disease: a mechanistic updateJ Endocrinol2014223R19R3925134660
- Ben-ShlomoAMelmedSSomatostatin agonists for treatment of acromegalyMol Cell Endocrinol200828619219818191325
- KatznelsonLLawsERMelmedSAcromegaly: an endocrine society clinical practice guidelineJ Clin Endocrinol Metab2014993933395125356808
- BrunsCLewisIBrinerUSOM230: a novel somatostatin peptidomimetic with broad somatotropin release inhibiting factor (SRIF) receptor binding and a unique antisecretory profileEur J Endocrinol200214670771611980628
- HoflandLJLambertsSWJThe pathophysiological consequences of somatostatin receptor internalization and resistanceEndocr Rev200324284712588807
- LescheSLehmannDNagelFDifferential effects of octreotide and pasireotide on somatostatin receptor internalization and trafficking in vitroJ Clin Endocrinol Metab20099465466119001514
- NagelFDollCPöllFStructural determinants of agonist-selective signaling at the sst 2A somatostatin receptorMol Endocrinol20112585986621330405
- BrzanaJYedinakCGGultekinSHGrowth hormone granulation pattern and somatostatin receptor subtype 2A correlate with postoperative somatostatin receptor ligand response in acromegaly: a large single center experiencePituitary20121649049823184260
- GadelhaMRKasukiLKorbonitsMNovel pathway for somatostatin analogs in patients with acromegalyTrends Endocrinol Metab20132423824623270713
- WildembergLENetoLVCostaDFLow somatostatin receptor subtype 2, but not dopamine receptor subtype 2 expression predicts the lack of biochemical response of somatotropinomas to treatment with somatostatin analogsJ Endocrinol Invest201336384322472799
- NielsenSMellemkjærSRasmussenLMExpression of somatostatin receptors on human pituitary adenomas in vivo and ex vivoJ Endocrinol Invest20012443043711434667
- RenSGTaylorJDongJFunctional association of somatostatin receptor subtypes 2 and 5 in inhibiting human growth hormone secretionJ Clin Endocrinol Metab2003884239424512970293
- European Medicines AgencySummary of Product Characteristics: Signifor Powder and Solvent for Suspension for Injection2014 Available from: www.ema.europa.eu/emaAccessed May 14, 2015
- PetersennSBollerslevJArafatAMPharmacokinetics, pharmacodynamics, and safety of pasireotide LAR in patients with acromegaly: a randomized, multicenter, open-label, phase I studyJ Clin Pharmacol2014541308131724800725
- GadelhaMRBronsteinMDBrueTPasireotide versus continued treatment with octreotide or lanreotide in patients with inadequately controlled acromegaly (PAOLA): a randomised, phase 3 trialLancet Diabetes Endocrinol2014287588425260838
- HorsmansYHuKRuffinMEffect of hepatic impairment on the pharmacokinetics of pasireotide (SOM230): results from a multicenter phase I studyJ Clin Pharmacol20125255255822282526
- van der HoekJde HerderWWFeeldersRAA single-dose comparison of the acute effects between the new somatostatin analog SOM230 and octreotide in acromegalic patientsJ Clin Endocrinol Metab20048963864514764775
- PetersennSSchopohlJBarkanAPasireotide (SOM230) demonstrates efficacy and safety in patients with acromegaly: a randomized, multicenter, phase II trialJ Clin Endocrinol Metab2010952781278920410233
- ColaoABronsteinMDFredaPPasireotide versus octreotide in acromegaly: a head-to-head superiority studyJ Clin Endocrinol Metab201499379179924423324
- FleseriuMClinical efficacy and safety results for dose escalation of somatostatin receptor ligands in patients with acromegaly: a literature reviewPituitary20101418419321161602
- van der LelyAJBillerBMKBrueTLong-term safety of pegvisomant in patients with acromegaly: comprehensive review of 1288 subjects in ACROSTUDYJ Clin Endocrinol Metab2012971589159722362824
- SheppardMBronsteinMDFredaPPasireotide LAR maintains inhibition of GH and IGF-1 in patients with acromegaly for up to 25 months: results from the blinded extension phase of a randomized, double-blind, multicenter, phase III studyPituitary20141838539425103549
- FleseriuMSheppardMBronsteinMDSwitching patients with acromegaly from octreotide LAR to pasireotide LAR improves biochemical control: crossover extension to a randomized, double-blind, multicenter, phase III studyThe Endocrine Society’s 94th Annual Meeting and Expo; June 23–26, 2012, Presentation Number: SUN-741Houston, TX, 2012
- FredaPUFleseriuMAJvdLImprovement of biochemical control in patients with acromegaly switched from octreotide LAR to pasireotide LAR: crossover extension to a double-blind, multicenter, randomized, phase III studyThe Endocrine Society’s 95th Annual Meeting and Expo; June 15–18, 2013, Poster Board SUN-95San Francisco, CA2013
- FleseriuMGadelhaMBronsteinMDOR7-7: pasireotide LAR provides superior efficacy over octreotide LAR and lanreotide ATG in patients with inadequately controlled acromegaly: a phase III, multi-center, randomized study (PAOLA)Growth Horm IGF Res201424S20S21
- GadelhaMRBronsteinMDBrueTBiochemical control is maintained with pasireotide LAR in patients with acromegaly: results from the extension of a randomized phase III study (PAOLA)The Endocrine Society’s 97th Annual Meeting and ExpoMar 5–8, 2015 Presentation Number: PP09-01San Diego, CA2015
- ShenoudaMMaldonadoMWangYAn open-label dose-escalation study of once-daily and twice-daily pasireotide in healthy volunteersAm J Ther20142116417322713526
- HenryRRCiaraldiTPArmstrongDHyperglycemia associated with pasireotide: results from a mechanistic study in healthy volunteersJ Clin Endocrinol Metab2013983446345323733372
- BreitschaftAHuKHermosillo ReséndizKManagement of hyperglycemia associated with pasireotide (SOM230): healthy volunteer studyDiabetes Res Clin Pract201410345846524461109
- ColaoAMGuFGadelhaMRMetformin-based oral antidiabetic therapy proved effective in hyperglycaemia associated with pasireotide in patients with acromegalyEndocr Abstr201537E800
- GadelhaMRBrueTFleseriuMProactive monitoring and early intervention in the management of pasireotide-induced hyperglycemia: lessons from the phase III, 24-week paola studyThe Endocrine Society’s 97th Annual Meeting and ExpoMar 5–8, 2015 Presentation Number: LBT-079San Diego, CA2015
- FleseriuMHoffmanARKatznelsonLAmerican Association of Clinical Endocrinologists and American College of Endocrinology Disease State Clinical Review: management of acromegaly patients: what is the role of pre-operative medical therapy?Endocr Pract20152166867326135961
- FleseriuMThe role of combination medical therapy in acromegalyCurr Opin Endocrinol Diabetes Obes201320432132923807604
- Ben-ShlomoAPharmacotherapy for acromegalyEndocrinol Metab Clin North Am201544354125732640
- SchmidHBrueTColaoAEffect of pasireotide on GH, IGF1, IGFBP2, IGFBP3, HbA1C and glucose in patients with inadequately controlled acromegaly: exploratory results from a multicentre, randomized, 24-week study (PAOLA)Endocr Abstr201435905
- ColaoADe BlockCGaztambideMSManaging hyperglycemia in patients with Cushing’s disease treated with pasireotide: medical expert recommendationsPituitary20131718018623564338
- McKeageKPasireotide in acromegaly: a reviewDrugs2015751039104826017304
- TuviaSAtsmonJTeichmanSLOral octreotide absorption in human subjects: comparable pharmacokinetics to parenteral octreotide and effective growth hormone suppressionJ Clin Endocrinol Metab2012972362236922539587
- MelmedSPopovicVBidlingmaierMSafety and efficacy of oral octreotide in acromegaly: results of a multicenter phase III trialJ Clin Endocrinol Metab20151001699170825664604
- RobertsJLindenMCervinCOctreotide fluid crystal provides sustained octreotide bioavailability and similar IGF1 suppression to that of octreotide LAR (Sandostatin LAR): randomized, open-label, Phase I, repeat-dose study in healthy volunteersEndocr Abstr201435914
- ChieffoCCookDXiangQEfficacy and safety of an octreotide implant in the treatment of patients with acromegalyJ Clin Endocrinol Metab2013984047405423969184
- StörmannSSchopohlJEmerging drugs for acromegalyExpert Opin Emerg Drugs201419799724400774
- PlockingerUHoffmannUGeeseMDG3173 (somatoprim), a unique somatostatin receptor subtypes 2-, 4- and 5-selective analogue, effectively reduces GH secretion in human GH-secreting pituitary adenomas even in Octreotide non-responsive tumoursEur J Endocrinol201116622323422065857
- SommEBonnetNMartinezAA botulinum toxin-derived targeted secretion inhibitor downregulates the GH/IGF1 axisJ Clin Invest20121223295330622850878
- GarciaEATrivellinGAfloreiEDCharacterization of SNARE proteins in human pituitary adenomas: targeted secretion inhibitors as a new strategy for the treatment of acromegaly?J Clin Endocrinol Metab201398E1918E192624152687
- Cuevas-RamosDFleseriuMSomatostatin receptor ligands and resistance to treatment in pituitary adenomasJ Mol Endocrinol201452R223R24024647046
