Abstract
This paper discusses extracorporeal removal of viral particles and their antigens from the blood as an auxiliary therapy. This hypothesis has not been reported before. In some chronic blood-borne viral infections, the virus remains systemic and persistent for extended periods of time, with adverse effects that weaken the immune system. Blood titers of virus and its toxins are proportional to the severity of the disease, and their reduction can alleviate symptoms, leading to improved health. Several blood-borne viral infections can be overcome by the young, but are life-threatening in the elderly. It is known that some older people have extreme difficulty tolerating viral infections such as influenza and the common cold. Further, several types of viral infection persist throughout the life of the individual and cannot be eliminated by conventional treatments. Well-known infections of this type include HIV and hepatitis B. In the case of Ebola virus, patients remain infectious as long as their blood contains the virus. According to the present hypothesis, an extracorporeal viral antibody column (EVAC) is proposed for elimination or reduction of the blood viral titer when treating blood-borne viral infection. EVAC would selectively trap viral antigens and toxins in the blood into an extracorporeal circuit, while returning detoxified blood back to the patient’s body. It is anticipated that EVAC would reduce mortality caused by blood-borne viral infections in the elderly since reduction of blood virus titers would improve health, leading to improved overall patient performance. Such enhancement would also make conventional therapies even more effective. EVAC could have a lifesaving role in treatment of viral illness, especially those involving lethal viruses such as Ebola, where the patient’s recovery to a large extent depends on their general health status. EVAC would be for single use and appropriately disposed of after each detoxification procedure. When sufficient research has yielded positive results in animal models, EVAC could be used as a supportive treatment in humans along with conventional antiviral therapies. EVAC would not be suitable for all viral infections, but could be expected to decrease the casualties resulting from blood-borne viral infections. The EVAC approach would be efficient in terms of time, effort, and expenditure in the research and treatment of blood-borne viral infections.
Introduction
Blood is the main route for dissemination of virus in systemic viral infections.Citation1 Some viral infections, such as the common cold in adults, can be eliminated from the body via the body’s physiological defense mechanisms without any special treatment needed, while others need intensive care and attention. Influenza viruses disseminate through the blood,Citation2 and older adults and those with chronic diseases are at high risk of complications associated with seasonal influenza. Individuals older than 84 years have the highest risk of dying from these complications.Citation3 Ebola virus accumulates specifically in the bloodstream, and patients remain infectious as long as their blood contains the virus;Citation4 transport of viral antigens and toxins via the blood to the liver, muscles, spleen, kidneys, lungs, heart, and brain produces hemorrhages that may lead to death.Citation5,Citation6 Generally, blood titers of viruses and their toxins are proportional to disease severity.Citation7,Citation8 Ebola virus RNA levels in the blood increase logarithmically during the acute phase of the illness,Citation9 and RNA copy levels in blood from patients who have died of the disease have been found to be on average 2 log10 higher than levels in patients who have survived.Citation10 Evolving immune deficiency in HIV-1 infection in humans or simian immunodeficiency virus infection in macaques has a positive correlation with the blood virus titer and progressive loss of CD4+ T lymphocytes.Citation11
Ambrus and Scamurra proposed a method for removal of HIV and other viruses from the blood, describing a method for reducing the viral load that involved removal of viruses or their fragments by circulating blood through hollow fibers containing a porous exterior surface on which affinity molecules having specificity for viral components are immobilized. These authors hypothesized that passage of the fluid through the hollow fibers would cause the viral particles to bind to the affinity molecules, thereby reducing the viral load in the effluent.Citation12
According to the present hypothesis, minimizing viral antigens and toxins in the bloodstream would ameliorate symptoms and thereby improve the health of the patient. Treatment with extracorporeal viral antibody column (EVAC) would be expected to reduce side effects and the mortality rate in high-risk patients, such as those with viral septicemia.
Proposed hypothesis
The proposed hypothesis consists of application of EVAC in patients to trap viral antigens and toxins in the blood in a safe and non-aggressive manner. Although essentially intracellular, at some stage of their life cycle, viruses and their antigens come into contact with the blood stream, with some causing viral sepsis.Citation13–Citation15 At this point, viral antigens can be removed effectively by EVAC. EVAC detoxifies the circulating blood via specific polyvalent antibodies that are effective against specific viral antigens and toxins. In the course of detoxification, EVAC behaves as the key unit in the machine circulating the blood. EVAC acts as a biological trap in “out of body detoxification” for agglutination of “blood viral and toxin antibodies” to “viral antigens and viral toxins”. Such trapping would remove viral toxins and antigens from the blood and not allow them to return to the body.
Using this procedure, the blood of the infected patient would be processed during passage through EVAC. The device used to implement EVAC would be similar to that already hypothesized by Shahidi Bonjar,Citation16–Citation20 ie, like the renal dialysis machineCitation21 used in patients with renal failure, but instead of using a dialysis membrane, the machine would be equipped with EVAC. EVAC has a vast surface area for direct contact with the blood to immobilize viral antigens and their toxins. Polyvalent viral antibodies (PVAs) are attached to the contact surface of the reaction platform of the column. PVAs would be prepared for specific viral antigens and toxins, but not as a general antiviral therapeutic device.
Passage of blood through EVAC immobilizes viral antigens and their toxins on stationary PVAs. Similar to a renal dialysis machine, blood circulates from the patient to EVAC and from there back to the patient in a closed circuit until an adequate number of viral antigens and toxins are depleted from the blood. The level of viral antigens returning via the blood to the body would be monitored by sampling and evaluated using appropriate serological tests. The treatment outcome would be improved in two ways: EVAC would remove viral antigens and toxins from the blood and minimize their contact with the vital organs; and can be used in combination with conventional therapeutics, the efficacy of which would be enhanced since the population of viral antigens would be depleted and kept at a steady minimal level. Such dual therapy would decrease the symptoms of the disease and have fewer side effects. This therapy may be lifesaving in the future, especially in patients with lethal viral illnesses, in whom recovery is largely dependent on enhanced health and performance. EVAC would be for single use and would be appropriately disposed of after each detoxification treatment. When findings are sufficiently positive in animal models, EVAC could be used as an auxiliary procedure alongside conventional antiviral therapies in humans.
Present status of antiviral therapies
Some prevalent viral infections, in particular the common cold, which remains localized in the respiratory tract, rarely warrant treatment. Such viruses are eliminated from the body after a short period with no need for therapy in normal healthy people because they are controlled by the immune system.Citation22 In the case of persistent viruses, no measures have been developed to eradicate them. Vaccination, interferon, and antiviral drugs can reduce the frequency of clinical recurrences and ameliorate symptoms, but the virus continues to remain associated with the host.Citation23 To date, slow progress has been made in antiviral therapy because of the toxicity of most antiviral agents, the need for intracellular penetration of the antiviral drug to act on viral replication, difficulty in evaluation of the selective activity of antiviral therapy, and no highly efficient and clinically tested therapeutics as synthetic antivirals (analogs of nucleoside, amantadine, photodynamic dyes, and thiosemicarbazones), immunoglobulins and interferon, have gained high efficacy ranks in antiviral therapies.Citation24 In the case of viral vaccines, even though they afford protection, not everybody gets vaccinated and not all vaccines are effective against new viral strains.
Why do antiviral treatments not include antibody injections?
If antibodies against viral antigens and their toxins are injected directly into bloodstream, in many cases the immune system would be sensitized and such antibodies would be recognized as foreign antigens. Production of anti-antibodies, allergic effects, and phagocytosis by macrophages would counteract the effectiveness of the administered antiviral antibodies, and the efficacy of subsequent injections would be less because of the elevated titer of anti-antibodies in the blood, whereas extracorporeal removal of viral antigens by EVAC would not sensitize the immune system and will not have such ineffective sequences.
EVAC approach
The procedure by which EVAC would remove the antigens and toxins of a known virus from the blood is a relatively simple one. Detoxification can be performed aseptically under the supervision of trained personnel. The procedure uses simple blood-circulating machines via which blood circulates from the patient to EVAC and back to the patient. This devise would act as a biological filter, selectively immobilizing all viral antigens and toxins in the infected blood. EVAC has a vast surface area available for contact with the blood, so would allow effective filtration. The reactive sites of EVAC consist of carbon nanotubes to which viral antibodies become bound. In this extracorporeal detoxification process, viral antigens and toxins conjugate with their stationary antibodies and become immobilized, so their blood titers in infected patients would be minimized. Like the dialysis machine used in patients with kidney failure, EVAC would be expected to minimize blood viral antigen and toxin levels in a relatively short period of time at each session. It should take 2–3 hours for the titers of antigens of interest in the blood to reach acceptable levels, which can be confirmed by sampling of blood and appropriate serological evaluation. Enzyme immunoassays are simple tests that can be done at the point of care, with results available in less than 15 minutes.Citation25 EVAC devices can be biotechnologically engineered against specific viruses and their strains and toxins; thus, as a polyvalent column, EVAC can contain a sufficient amount of stationary antibodies to trap the relevant antigens it is designed for. Time is a key factor in lifesaving treatment for elderly patients. EVAC may be particularly useful in high-risk patients with viral septicemia, such as those with Ebola infection or the elderly with influenza. Research on timely use of EVAC in emergency ambulances should receive special attention. Detoxification started in the ambulance setting would provide faster resuscitations in patients under critical conditions.
Limitations to use of EVAC
The EVAC approach seems to be suitable only for viruses with antigens and toxins that come into contact with the bloodstream and can be trapped by their stabilized antibodies inside the EVAC device. The EVAC approach may not be an effective strategy for cytoplasmic viral episomes (eg, Pox virus infection), herpesvirus, the viral DNA for which is maintained by the cell as an extra chromosome, several cancer-associated viruses, including Epstein–Barr virus and Kaposi’s sarcoma-associated herpesvirus which are maintained as latent, chromosomal episomes in cancer cells, chronic persistent virus infection (eg, hepatitis C virus), and a combination of latent and persistent virus infection (for which the prototype is HIV).Citation23
EVAC specifications
The antibody-bearing plates of EVAC that would act as antibody-antigen reaction platforms would be designed to be porous, made of an organic biocompatible polymer approximately 200 μm in thickness; these lattice membranes would be stacked in the column to provide an extended contact surface on both sides for blood to pass through. The PVAs would be engineered with the ability to remain firmly on both sides of the membrane and act as retention platforms to trap viral antigens and toxins in the circulation. The approximate internal volume of the column could be 100 mL; including connection tubes, the total volume of blood involved in the process would be approximately 200 mL at any given point of time. However, the settings should be optimized by appropriate investigations.
EVAC treatment setup
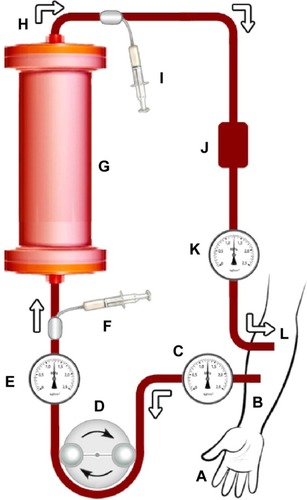
The setup for EVAC treatment would be similar to that for other devices already reported by Shahidi Bonjar.Citation16–Citation20 shows the proposed treatment setup. It indicates the path of blood from and to the patient. Blood passes through the EVAC device with the aid of a peristaltic blood pump. Pressure monitors, heparin injection, and an air trap in the line would provide safe and smooth circulation. Before blood enters the column and after leaving it, blood samples would be taken at specified intervals to detect viral antigens and toxin titers with the aid of sero-logical assays such as the enzyme-linked immunosorbent assay. All tubing and EVAC would be applied aseptically to prevent further infection; however, EVAC would be intended for single use and would be appropriately disposed of after treatment.
Figure 1 Schematic diagram of proposed extracorporeal viral antibody column (EVAC), an auxiliary therapy to reduce blood viral titers when treating viral infections in the blood by removing viral antigens and toxins from the bloodstream.
Clinical significance
Selective removal of viral antigens and toxins from the blood would slow down adverse effects and alleviate related health problems in patients with certain viral infections. EVAC is an auxiliary treatment that would immobilize the viral antigens it is designed for. The treatment is performed with the aid of a pump circulating blood. As with the renal dialysis machine, blood would circulate from the body to EVAC and from there back to the body in a closed circuit until the target antigens are sufficiently depleted from the blood. The optimal application criteria such as type of viral infection, patient’s condition for implementation, period of application, dosage, ideal performance, and similar concerns are to be revealed by appropriate studies. If EVAC technology gains approval, it would hold promise as a strategy for reducing mortality caused by blood-borne viral infection in the elderly and ameliorating symptoms of illness, leading to improved overall patient performance and enhanced viral recovery, with reduced public health expenditure.
Probable adverse reactions
Patients may experience adverse reactions while undergoing EVAC. As in kidney dialysis,Citation26 these adverse reactions are generally attributable to the volume and speed of blood removal. Clearly, not every patient would experience adverse reactions, but as with kidney dialysis, the medical team should be familiar with such reactions and take steps to minimize them. Like with hemodialysis in patients with kidney failure,Citation27,Citation28 these adverse reactions are likely due to a sudden drop in blood pressure (hypotension) which may be accompanied with abdominal cramps, muscle cramps, shortness of breath, or vomiting. The treatment team can alleviate these adverse events by lowering the rate of blood flow, taking the prescription order of the physician in charge, and taking care to prevent new infection. Further complications can be avoided by aseptic management techniques.
Duration of treatment
Each EVAC treatment period would be determined by the physician in charge depending on the titers of viral antigens and toxins in the blood returning back to the patient. As indicated in , blood leaving the column would be sampled and examined for antigen titer by use of appropriate serological evaluations; hence, treatment would last until appropriate improvement is achieved with regard to minimization of viral antigens, recovery of the patient, or as determined by the physician; however, standardization protocols should be developed in appropriate optimization studies.
Optimization of therapy
It is not intended that EVAC should replace conventional antiviral therapy; however, as an auxiliary measure, its ability to selectively remove target antigens from blood would make conventional therapies more effective. Prior to any human application, optimized procedures should be developed from investigations in animals to ease the optimization protocol in humans. However, at this stage, any prediction about a set optimized protocol may have no validity for real treatment protocol. Appropriate research would be needed to conclude optimized performance from application of EVAC along with conventional treatments such as antiviral chemotherapeutics in the relevant host-virus interplays.
Sterilization
The EVAC setup, including the main column, silicone tubing, and its connections, would be for single use and would be appropriately disposed of after each treatment. The whole setup (except for the blood pump) would be manufactured as a ready to use “EVAC pack”. It would be sterilized by appropriate disinfection procedures such as gamma or electron-beam radiation during the manufacturing process. These EVAC packs would be marketed as vacuum packs when clinically available.
Conclusion and perspective
Some viruses cause blood poisoning or viral sepsis,13–15 while others, at least at some stage in their life cycle, enter the bloodstream, along with their antigens. In such circumstances, the titers of viral toxins and antigens can be minimized by EVAC, with reduction in symptoms of illness, improved patient status, and enhancement of the curative potential of conventional therapies, all of which improve patients’ well-being. Implementation of EVAC in this situation holds promise for improving the health of patients who are threatened by invasion of viral antigens and toxins in their blood. EVAC may also shorten the recovery period. In some diseases, EVAC may set new trends in the management of infections in the bloodstream and their treatment protocols; in this regard, EVAC may become part of the essential equipment in emergency and intensive care units for prompt management of patients with viral septic shock. It is anticipated that the EVAC approach may reduce the time, effort, and expense in some other blood toxicities. Further, judicious use of this type of blood filtration may prevent some fatal outcomes which conventional therapies may not. The EVAC approach may also be implemented in blood transfusion procedures in order to retain specific antigens from the donor for transfusion to the recipient. To realize the potential of EVAC, we will need appropriate investment and collaboration of scientists in many fields of research, including internal medicine, virology, nanotechnology, immunology, biochemistry, emergency medicine, and medical engineering.
Acknowledgments
This paper is dedicated to patients who would be resuscitated with EVAC and returned to their families and normal lives in a healthy condition and to the physicians and scientists whose attempts may bring this technology to reality. This research is also dedicated to AR Afzalipour and Mrs F Saba, the founders of universities in Kerman.
Disclosure
The author reports no financial or personal relationships with other people or organizations that could inappropriately influence this work, and there is no professional or other personal interest of any nature or kind in any product, service, and/or company that could be construed as influencing the position presented in this article.
References
- BerhanuAKingDSMosierSST-246 inhibits in vivo poxvirus dissemination, virus shedding, and systemic disease manifestationAntimicrob Agents Chemother200953124999500919752270
- HourfarMKThemannAEickmannMBlood screening for influenzaEmerg Infect Dis20071371081108318214186
- WebMDFlu in older adults Available from: http://www.webmd.com/cold-and-flu/flu-guide/fact-sheet-elderly-peopleAccessed February 18, 2015
- FeldmannHEbola – a growing threat?N Engl J Med201471137524805988
- FeldmannHGeisbertTWEbola haemorrhagic feverLancet2011377976884986221084112
- BeechingNJFenechMHoulihanCFEbola virus diseaseBMJ2014349g734825497512
- VaughnDWGreenSKalayanaroojSDengue viremia titer, antibody response pattern, and virus serotype correlate with disease severityJ Infect Dis200018112910608744
- BoonACFinkelsteinDZhengMH5N1 influenza virus pathogenesis in genetically diverse mice is mediated at the level of viral loadMBio201125e00171e0021121896679
- Centers for Disease Control and PreventionReview of human-to-human transmission of Ebola virus Available from: http://www.cdc.gov/vhf/ebola/transmission/human-transmission.htmlAccessed March 22, 2015
- TownerJSRollinPEBauschDGRapid diagnosis of Ebola hemorrhagic fever by reverse transcription-PCR in an outbreak setting and assessment of patient viral load as a predictor of outcomeJ Virol20047884330434115047846
- SchenkelARUnoHPauzaCDAsymptomatic simian immunodeficiency virus infection decreases blood CD4+ T cells by accumulating recirculating lymphocytes in the lymphoid tissuesJ Virol19997316016079847365
- AmbrusJLScamurraDOMethod for removal of HIV and other viruses from bloodUS Patent6528057 B1 filed August 30, 1999 and issued March 4, 2003. Available from: https://www.lens.org/lens/patent/US_6528057_B1Accessed April 24, 2015
- Cleveland ClinicDiseases and conditionsSepsis Available from: http://my.clevelandclinic.org/health/diseases_conditions/hic_SepsisAccessed March 19, 2015
- National Health ServiceSepsis Available from: http://www.nhs.uk/Conditions/Blood-poisoning/Pages/Introduction.aspxAccessed March 24, 2015
- Healthline.comSeptic shock Available from: http://www.healthline.com/health/septic-shockAccessed March 21, 2015
- Shahidi BonjarL“Nanogold detoxifying machine” to remove idle nano-gold particles from blood stream of cancer patients treated with antibody-nanogold therapeuticsMed Hypotheses201380560160523462370
- Shahidi BonjarLDesign of a new therapy to treat snake envenomationDrug Des Dev Ther20148820824
- Shahidi BonjarMRShahidi BonjarLAntiaging therapy: a prospective hypothesisDrug Des Dev Ther20159663667
- Shahidi BonjarMRShahidi BonjarLDesign of a new therapy for patients with chronic kidney disease: use of microarrays for selective hemoadsorption of uremic wastes and toxins to improve homeostasisDrug Des Dev Ther20159625629
- Shahidi BonjarMRShahidi BonjarLA prospective treatment for sepsisDrug Des Dev Ther2015925372543
- DaVita HealthCare Partners IncHow does a dialysis machine work? Available from: http://www.davita.com/treatment-options/hemodialy-sis/in-center-hemodialysis/how-does-a-dialysis-machine-work?/t/5596Accessed March 22, 2015
- Torrey Pines Institute for Molecular StudiesPersistent viral infections Available from: http://www.tpims.org/disease-research/aids-a-other-infectious-diseases/persistent-viral-infectionsAccessed February 19, 2015
- BoldoghIAlbrechtTPorterDDPersistent viral infectionsBaronSMedical Microbiology4th edGalveston, TX, USAUniversity of Texas Medical Branch at Galveston1996
- StalderHAntiviral therapyYale J Biol Med1977505507532341538
- TalbotHKFalseyARThe diagnosis of viral respiratory disease in older adultsClin Infect Dis201050574775120121411
- Mayo Clinic StaffRisks–tests and procedures, hemodialysis Available from: http://www.mayoclinic.org/tests-procedures/hemodialysis/basics/risks/prc-20015015Accessed March 19, 2015
- National Kidney FoundationCoping with the top five side effects of dialysis Available from: https://www.kidney.org/news/ekidney/january12/top5Accessed January 29, 2015
- Kidney Dialysis Information CentreRisks and side effects during kidney dialysis Available from: http://www.kidneydialysis.org.uk/risks.htmAccessed March 20, 2015
