Abstract
T-helper 17 (Th17) pathway plays an important and distinct role in autoimmunity and inflammation. A growing body of evidence demonstrates that interleukin-17 (IL-17) is also synthesized in inflammatory arthritis tissues and exerts potent proinflammatory and joint-destructive activities. Clinical studies have been performed to evaluate the therapeutic efficacy of antibodies blocking the IL-17 signaling pathway in patients with rheumatoid arthritis (RA). In this study, we performed a meta-analysis to systematically evaluate the clinical effects of IL-17 antibodies in RA patients. By searching PubMed, five randomized, placebo-controlled randomized controlled clinical trials that tested three antibodies against IL-17A (LY2439821 and secukinumab/AIN457) and the IL-17A receptor (brodalumab) were identified. The primary outcomes that were analyzed include American College of Rheumatology (ACR) Improvement Criteria and Disease Activity Score in 28 joints (DAS28). Meanwhile, the safety and adverse effects were also systematically analyzed. The results of the meta-analysis demonstrated that IL-17 antibody is effective in ameliorating the RA symptoms. Specifically, IL-17-blocking antibody significantly reduced ACR20 and ACR50. It also dramatically reduced DAS28, an index that measures tenderness and swelling severity of joints. The side effects of and intolerance to the antibody treatment were higher than those in the placebo control. The analysis result provides evidence-based information for clinical use of these agents in the treatment of inflammatory arthritis.
Introduction
Rheumatoid arthritis (RA) is a chronic, inflammatory, and systemic autoimmune disease that affects ~1% of the population all over the world.Citation1 In RA patients, the affected joints contain autoreactive T- and B-cells that produce proinflammatory cytokines, resulting in cartilage and bone damage.Citation1 Targeting these cytokines provides a strategy for treatment, including disease-modifying antirheumatic drugs (DMARDs).Citation2,Citation3 such as methotrexate (MTX). However, these DMARDs only work for a small proportion of patients. New medicines are urgently in demand.
In 1995, Yao et alCitation4 discovered that human T-cells could produce a proinflammatory cytokine, interleukin (IL)-17. These IL-17-producing cells are mainly a subset of cluster of differentiation 4 (CD4+) T-cells, a type of CD4+ “helper” lymphocytes named Th17 cells.Citation5,Citation6 IL-17 levels are extremely low or undetectable in normal human peripheral blood, while the levels are elevated in peripheral blood or synovial fluid in RA patients.Citation7–Citation10 Immunohistochemistry techniques led to the identification of a subset of IL-17-expressing T-cells in the synovium of RA patients.Citation11,Citation12 Moreover, the number of IL-17A-positive cells was also increased in children with juvenile inflammatory arthritis joints.Citation13 Blocking IL-17A can reduce IL-6 expression and formation of collagen breakdown products.Citation14
Th17 cells mediate the inflammation process by stimulating production of cytokines, chemokines, and matrix metalloproteinases.Citation15 Many human autoimmune diseases, including RA and psoriatic arthritis, are associated with abnormal Th17 activity.Citation16,Citation17 Inhibition of IL-17 signaling through a ligand or its receptor could reduce inflammation and bone erosion in animal arthritis models.Citation18 Meanwhile, clinical investigations have also been carried out to target IL-17A signaling for alleviating the symptoms of RA. This meta-analysis was undertaken to evaluate the results of clinical trials and to provide evidence-based information for using these agents in clinical treatment of inflammatory arthritis.
Methods
Database search, selection criteria, and quality assessment
Database search was performed in PubMed using the keywords “interleukin-17A” and “rheumatoid arthritis”. Eligible studies were selected based on the following criteria: 1) study design: randomized, double-blinded, placebo-controlled clinical trials (RCTs); 2) subjects: patients with RA; 3) intervention: administration of antibodies for blocking IL-17A signaling, including LY2439821 used by Genovese et al,Citation19 a humanized anti-IL-17 monoclonal antibody; secukinumab (AIN457), a fully human monoclonal anti-IL-17A antibody used by Genovese et al,Citation20 Hueber et al,Citation21 and Patel et al;Citation22 brodalumab, a human anti-IL-17 receptor monoclonal antibody used by Martin et al.Citation23 The quality of included trials was assessed using the Jadad scale score (zero to five), with a score of ≥3 indicating high quality.Citation24
Outcomes, data extraction, and statistical analysis
Effects of treatment were measured by the improvement in the percentage of patients achieving American College of Rheumatology (ACR) scores ACR20, ACR50, and ACR70 according to Felson’s method.Citation25 Another measurement, Disease Activity Score in 28 joints (DAS28), was also used in the studies based on standard guidelines.Citation26
All analyses were performed using the Review Manager, version 5.1.0 (Cochrane Collaboration, Oxford, UK). The χ2 Cochran Q-test was performed to detect heterogeneity. Random- or fixed-effects inverse variance-weighted method was used to test the difference in significance levels.Citation27 Mean difference and the associated 95% confidence interval (CI) for ACR20/50/70 were used to assess the significance of difference.
Results
Identification of eligible studies from database search
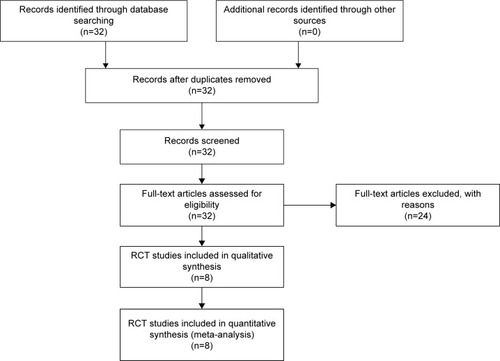
In order to obtain eligible clinical studies, we first carried out a database search. The terms “interleukin-17A” and “rheumatoid arthritis” were used as the keywords to search the PubMed database, which resulted in a total of 32 articles being retrieved. Further stringent selections were performed by using filtering criteria with the clinical trials and RCTs. We identified five eligible studies, namely, Genevese et al,Citation19,Citation20 Hueber et al,Citation21 Martin et al,Citation23 and Patel et alCitation22 (). These randomized, double-blind, and placebo-controlled studies are summarized briefly in .
Table 1 Summary of studies using IL-17A antibodies as a treatment for the rheumatoid arthritis
Three different antibodies were tested by three different research groups; the antibodies include LY2439821, a human anti-IL-17 monoclonal antibody used in Genovese et al,Citation19 secukinumab (AIN457), a human anti-IL-17A antibody tested in Genovese et al,Citation20 Hueber et al,Citation21 and Patel et al;Citation22 brodalumab (AMG827), a human anti-IL-17 receptor A monoclonal antibody tested in Martin et al.Citation23
Meta-analysis of the outcomes tested
A major end point used in these studies was the ACR score, a widely used standard to measure the improvement in symptom reduction in RA patients. We collected the ACR20, ACR50, and ACR70 values from the eligible studies identified from the search, including Genovese et al,Citation19,Citation20 Hueber et al,Citation21 Martin et al,Citation23 and Patel et al.Citation22 A meta-analysis was performed and results are presented in , showing that treatment with the IL-17-signaling blockers had a dramatic effect on ACR20 (, P=0.0005) and ACR50 (, P=0.007). ACR70 tends to change, but the effect is not significant enough (, P=0.1). Of note, brodalumab, the IL-17A receptor antibody, resulted in a lesser effect than the other three IL-17A antibodies,Citation23 suggesting that neutralizing of IL-17A ligand provides a more efficient approach for RA treatment.
Figure 2 Forest plot of meta-analysis.
Abbreviations: ACR, American College of Rheumatology; CI, confidence interval; IL, interleukin; M–H, Mantel–Haenszel method.
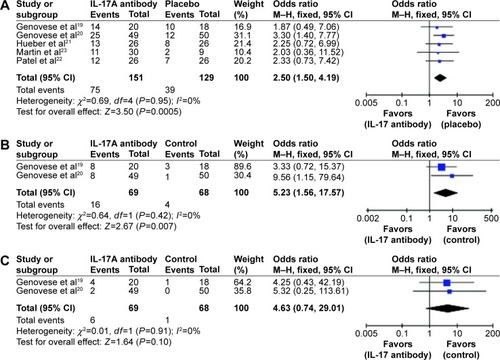
Another readout, DAS28, was derived from tender 28-joint and swollen 28-joint counts.Citation26 All three studies using IL-17-neutralizing antibodies found marked reduction in the joint swollen points relative to the placebo control treatment, supporting the efficacy of these antibodies in alleviating the RA symptoms. In Genovese et al,Citation19 greater decrease in DAS28 was observed after treatment with the antibody LY2439821 (P<0.05): −2.3, −2.4, and −2.3 in 0.2 mg/kg, 2.0 mg/kg, and all-combined groups, respectively, compared to that in the placebo control group (−1.7). Similar findings were achieved by antibody treatment using secukinumab (AIN457) in Genovese et al,Citation20 Hueber et al,Citation21 and Patel et al.Citation22
Safety and adverse effects
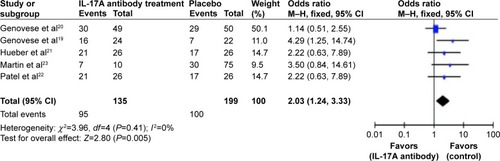
Adverse effects were investigated in these studies, and we performed a meta-analysis for the same. The median doses of IL-17A antibodies were chosen for analysis: 75 mg secukinumab in Genovese et alCitation20 (doses tested ranged from 25 mg to 300 mg) and 0.6 mg/kg LY249821 in Genovese et alCitation19 (doses tested ranged from 0.6 mg/kg to 2.0 mg/kg). Meta-analysis results demonstrated the total events of adverse effects were significantly higher in IL-17A-treated patients than in placebo control-treated patients (odds ratio [OR] =2.03; 95% CI: 1.24–3.33; P=0.005; ). Hueber et alCitation21 reported one severe adverse effect (SAE) (laryngeal abscess due to RA of the cricoarytenoid joint) in AIN457-treated patients. Two SAEs (interstitial lung disease and brachial plexopathy) were recorded in the placebo-treated group. AIN457 treatment slightly increased the overall AE incidence. Headache, diarrhea, leukemia, and vertigo were reported in patients treated with LY2439821. However, these side effects occurred equally in placebo-treated patients.
Assessment for publication bias
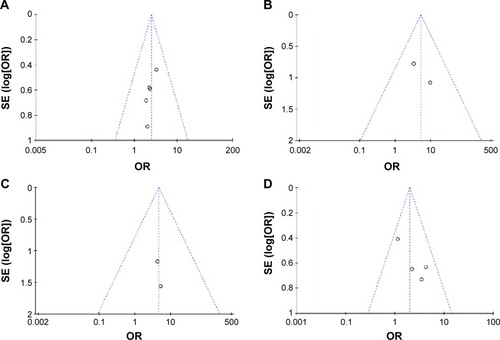
In order to assess the publication bias, Begg’s funnel plots testCitation28 was performed. As shown in , the symmetrical patterns of these plots indicate that there is no publication bias in the studies selected for meta-analysis of ACR20 (), ACR50 (), and ACR70 (), There is also no publication bias in the studies selected for meta-analysis of AEs ().
Discussion
In this study, we performed a meta-analysis on five clinical trials investigating therapeutic antibodies blocking the IL-17A signaling pathway of RA. The results demonstrated the beneficial role of IL-17A antibody in the alleviation of RA symptoms, as measured by ACR20/50/70 and DAS28, two widely used outcomes for assessment of recovery from this disease. The reduced tender joint count, swollen joint count, and pain after the antibody treatment might have resulted from the blockage of some step in the immune response pathway, such as production of cytokines or chemokines and neutrophil recruitment.Citation7
IL-17A signaling plays a key role in this inflammatory disease.Citation29–Citation33 In the mouse model, IL-17 overexpression gave rise to joint inflammation, as well as bone and cartilage damage.Citation34 By contrast, disruption of IL-17 signaling in the mouse can protect the bone cartilage from arthritis induction.Citation35–Citation37 Murine Th17 cells play critical roles in chronic, erosive diseases,Citation38,Citation39 with Toll-like receptor 4 having been proven to be critical in Th17-mediated inflammatory arthritis.Citation40,Citation41
Moreover, IL-17A antibody has also beneficial effects on other immune-mediated diseases, such as psoriasis, a skin autoimmune disease. Psoriatic arthritis also affects many people in the world. The IL-17A antibody secukinumab has also been shown to have beneficial effects on patients with this disease. IL-17A expression is increased in patients with psoriatic arthritis,Citation42–Citation44 along with increased IL-17A receptor levels,Citation42 in these patients.
One of these antibodies, brodalumab, has little effect on RA. It is a human immunoglobulin G2 monoclonal antibody against IL-17 receptor A and blocks the biological activity of IL-17A and IL-17F, as well as A/F heterodimer signaling. This antibody was tested and found to be capable of suppressing the inflammatory process in psoriasis.Citation16 This might be due to the multiple ligands involved in RA.
Tumor necrosis factor (TNF) can regulate IL-17A signaling; an anti-TNF antibody, infliximab, was tested in terms of its ability to reduce the number of IL-17-producing cells and decrease the disease activity.Citation45 Other TNF-blocking treatments had a similar effect in RA patients.Citation46,Citation47 Yue et al demonstrated a decrease in peripheral Th17 cells in RA patients treated with adalimumab/MTX.Citation48 Therefore, targeting Th17 cells provides a tool to treat inflammatory diseases such as RA.
However, antibodies targeting Th17 may have potential side effects that would adversely affect their clinical application. It has been reported that neutralization of IL-17 in mice exacerbated Candida-induced dermatitis in skin.Citation49 Moreover, it is suspected that neutralization of IL-17RA might be linked to suicidal thoughts.Citation50 However, increased plasma concentration of proinflammatory cytokines, especially IL-17a, after myocardial infarction plays an important role in the stress reaction that predisposes to depression during the next 6 months.Citation51 A recent study also demonstrated a higher rate of injection site reaction and allergy.Citation52 The exact roles of IL-17a and IL-17-neutralizing antibodies in this process remain to be elucidated, and comprehensive evaluation of these agents for clinical application will be needed.
Acknowledgments
This work was supported by the grants of National Natural Science Foundation of China (No 81473710 and No 81473710) and Beijing Natural Science Foundation (No 7152134).
Disclosure
The authors report no conflicts of interest in this work.
References
- McInnesIBSchettGThe pathogenesis of rheumatoid arthritisN Engl J Med2011365232205221922150039
- DaikhDISt ClairEWUpdated recommendations for the treatment of rheumatoid arthritis: another step on a long roadArthritis Care Res (Hoboken)201264564865122473919
- SinghJAFurstDEBharatA2012 update of the 2008 American College of Rheumatology recommendations for the use of disease-modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritisArthritis Care Res (Hoboken)201264562563922473917
- YaoZPainterSLFanslowWCHuman IL-17: a novel cytokine derived from T cellsJ Immunol199515512548354867499828
- KiklyKLiuLNaSSedgwickJDThe IL-23/Th(17) axis: therapeutic targets for autoimmune inflammationCurr Opin Immunol200618667067517010592
- MiossecPKornTKuchrooVKInterleukin-17 and type 17 helper T cellsN Engl J Med2009361988889819710487
- ZiolkowskaMKocALuszczykiewiczGHigh levels of IL-17 in rheumatoid arthritis patients: IL-15 triggers in vitro IL-17 production via cyclosporin A-sensitive mechanismJ Immunol200016452832283810679127
- ChoMLYoonCHHwangSYEffector function of type II collagen-stimulated T cells from rheumatoid arthritis patients: crosstalk between T cells and synovial fibroblastsArthritis Rheum200450377678415022319
- KotakeSUdagawaNTakahashiNIL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclasto-genesisJ Clin Invest199910391345135210225978
- RazaKFalcianiFCurnowSJEarly rheumatoid arthritis is characterized by a distinct and transient synovial fluid cytokine profile of T cell and stromal cell originArthritis Res Ther200574R784R79515987480
- ChabaudMDurandJMBuchsNHuman interleukin-17: a T cell-derived proinflammatory cytokine produced by the rheumatoid synoviumArthritis Rheum199942596397010323452
- JoostenLARadstakeTRLubbertsEAssociation of interleukin-18 expression with enhanced levels of both interleukin-1beta and tumor necrosis factor alpha in knee synovial tissue of patients with rheumatoid arthritisArthritis Rheum200348233934712571842
- NistalaKMoncrieffeHNewtonKRVarsaniHHunterPWedderburnLRInterleukin-17-producing T cells are enriched in the joints of children with arthritis, but have a reciprocal relationship to regulatory T cell numbersArthritis Rheum200858387588718311821
- ChabaudMFossiezFTaupinJLMiossecPEnhancing effect of IL-17 on IL-1-induced IL-6 and leukemia inhibitory factor production by rheumatoid arthritis synoviocytes and its regulation by Th2 cytokinesJ Immunol199816114094149647250
- GaffenSLRecent advances in the IL-17 cytokine familyCurr Opin Immunol201123561361921852080
- PappKALeonardiCMenterABrodalumab, an anti-interleukin-17-receptor antibody for psoriasisN Engl J Med2012366131181118922455412
- MiossecPKollsJKTargeting IL-17 and TH17 cells in chronic inflammationNat Rev Drug Discov2012111076377623023676
- LubbertsEIL-17/Th17 targeting: on the road to prevent chronic destructive arthritis?Cytokine2008412849118039580
- GenoveseMCVan den BoschFRobersonSALY2439821, a humanized anti-interleukin-17 monoclonal antibody, in the treatment of patients with rheumatoid arthritis: a phase I randomized, double-blind, placebo-controlled, proof-of-concept studyArthritis Rheum201062492993920131262
- GenoveseMCDurezPRichardsHBEfficacy and safety of secukinumab in patients with rheumatoid arthritis: a phase II, dose-finding, double-blind, randomised, placebo controlled studyAnn Rheum Dis201372686386922730366
- HueberWPatelDDDryjaTRheumatoid Arthritis Study Group, Uveitis Study GroupEffects of AIN457, a fully human antibody to interleukin-17A, on psoriasis, rheumatoid arthritis, and uveitisSci Transl Med201025252ra72
- PatelDDLeeDMKolbingerFAntoniCEffect of IL-17A blockade with secukinumab in autoimmune diseasesAnn Rheum Dis201372Suppl 2ii116ii12323253932
- MartinDAChurchillMFlores-SuarezLA phase Ib multiple ascending dose study evaluating safety, pharmacokinetics, and early clinical response of brodalumab, a human anti-IL-17R antibody, in methotrexate-resistant rheumatoid arthritisArthritis Res Ther2013155R16424286136
- JadadARMooreRACarrollDAssessing the quality of reports of randomized clinical trials: is blinding necessary?Control Clin Trials19961711128721797
- FelsonDTAndersonJJBoersMThe American College of Rheumatology preliminary core set of disease activity measures for rheumatoid arthritis clinical trials. The committee on outcome measures in rheumatoid arthritis clinical trialsArthritis Rheum19933667297408507213
- PrevooMLvan ‘t HofMAKuperHHvan LeeuwenMAvan de PutteLBvan RielPLModified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritisArthritis Rheum199538144487818570
- HigginsJGreenSCochrane Handbook for Systematic Reviews of InterventionsChichester West Sussex, UKJohn Wiley & Sons Ltd2011
- EggerMDavey SmithGSchneiderMMinderCBias in meta-analysis detected by a simple, graphical testBMJ199731571096296349310563
- KryczekIBruceATGudjonssonJEInduction of IL-17+ T cell trafficking and development by IFN-gamma: mechanism and pathological relevance in psoriasisJ Immunol200818174733474118802076
- HarperEGGuoCRizzoHTh17 cytokines stimulate CCL20 expression in keratinocytes in vitro and in vivo: implications for psoriasis pathogenesisJ Invest Dermatol200912992175218319295614
- ZabaLCSuárez-FariñasMFuentes-DuculanJEffective treatment of psoriasis with etanercept is linked to suppression of IL-17 signaling, not immediate response TNF genesJ Allergy Clin Immunol20091245102210.e139519895991
- LinAMRubinCJKhandpurRMast cells and neutrophils release IL-17 through extracellular trap formation in psoriasisJ Immunol2011187149050021606249
- CaiYShenXDingCPivotal role of dermal IL-17-producing gammadelta T cells in skin inflammationImmunity201135459661021982596
- LubbertsEJoostenLAOppersBIL-1-independent role of IL-17 in synovial inflammation and joint destruction during collagen-induced arthritisJ Immunol200116721004101311441109
- NakaeSNambuASudoKIwakuraYSuppression of immune induction of collagen-induced arthritis in IL-17-deficient miceJ Immunol2003171116173617714634133
- BushKAFarmerKMWalkerJSKirkhamBWReduction of joint inflammation and bone erosion in rat adjuvant arthritis by treatment with interleukin-17 receptor IgG1 Fc fusion proteinArthritis Rheum200246380280511920418
- LubbertsEKoendersMIOppers-WalgreenBTreatment with a neutralizing anti-murine interleukin-17 antibody after the onset of collagen-induced arthritis reduces joint inflammation, cartilage destruction, and bone erosionArthritis Rheum200450265065914872510
- KoendersMILubbertsEOppers-WalgreenBBlocking of interleukin-17 during reactivation of experimental arthritis prevents joint inflammation and bone erosion by decreasing RANKL and interleukin-1Am J Pathol2005167114114915972960
- Abdollahi-RoodsazSJoostenLAHelsenMMShift from toll-like receptor 2 (TLR-2) toward TLR-4 dependency in the erosive stage of chronic streptococcal cell wall arthritis coincident with TLR-4-mediated interleukin-17 productionArthritis Rheum200858123753376419035506
- van LentPLGreversLCBlomABStimulation of chondrocyte-mediated cartilage destruction by S100A8 in experimental murine arthritisArthritis Rheum200858123776378719035520
- Abdollahi-RoodsazSJoostenLARoelofsMFInhibition of toll-like receptor 4 breaks the inflammatory loop in autoimmune destructive arthritisArthritis Rheum20075692957296717763416
- RaychaudhuriSPRaychaudhuriSKGenoveseMCIL-17 receptor and its functional significance in psoriatic arthritisMol Cell Biochem20123591–241942921894442
- JandusCBioleyGRivalsJPDudlerJSpeiserDRomeroPIncreased numbers of circulating polyfunctional Th17 memory cells in patients with seronegative spondylarthritidesArthritis Rheum20085882307231718668556
- NoordenbosTYeremenkoNGofitaIInterleukin-17-positive mast cells contribute to synovial inflammation in spondylarthritisArthritis Rheum20126419910921968742
- ShenHXiaLLuJXiaoWInfliximab reduces the frequency of interleukin 17-producing cells and the amounts of interleukin 17 in patients with rheumatoid arthritisJ Investig Med2010587905908
- KageyamaYKobayashiHKatoNInfliximab treatment reduces the serum levels of interleukin-23 in patients with rheumatoid arthritisMod Rheumatol200919665766219685204
- KageyamaYKobayashiHKatoNShimazuMEtanercept reduces the serum levels of macrophage chemotactic protein-1 in patients with rheumatoid arthritisMod Rheumatol200919437237819458908
- YueCYouXZhaoLThe effects of adalimumab and methotrexate treatment on peripheral Th17 cells and IL-17/IL-6 secretion in rheumatoid arthritis patientsRheumatol Int201030121553155719847432
- ZhangHLiHLiYIL-17 plays a central role in initiating experimental Candida albicans infection in mouse corneasEur J Immunol201343102671268223843112
- SchmidtCSuicidal thoughts end Amgen’s blockbuster aspirations for psoriasis drugNat Biotechnol201533989489526348946
- WilkowskaAPikułaMRynkiewiczAWdowczyk-SzulcJTrzonkowskiPLandowskiJIncreased plasma pro-inflammatory cytokine concentrations after myocardial infarction and the presence of depression during next 6-monthsPsychiatr Pol201549345546426276914
- GenoveseMCGreenwaldMChoCSA phase II randomized study of subcutaneous ixekizumab, an anti-interleukin-17 monoclonal antibody, in rheumatoid arthritis patients who were naive to biologic agents or had an inadequate response to tumor necrosis factor inhibitorsArthritis Rheumatol20146671693170424623718