Abstract
Major advances have been achieved recently in the treatment of metastatic castration-resistant prostate cancer, resulting in significant improvements in quality of life and survival with the use of several new agents, including the next-generation androgen receptor (AR)-targeted drugs abiraterone and enzalutamide. However, virtually all patients will eventually progress on these therapies and most will ultimately die of treatment-refractory metastatic disease. Recently, several mechanisms of resistance to AR-directed therapies have been uncovered, including the AR splice variant 7 (AR-V7), which is a ligand-independent constitutionally-active form of the AR that has been associated with poor outcomes to abiraterone and enzalutamide. Galeterone, a potent anti-androgen with three modes of action (CYP17 lyase inhibition, AR antagonism, and AR degradation), is a novel agent under clinical development that could potentially target both full-length AR and aberrant AR, including AR-V7. In this manuscript, we will first discuss the biological mechanisms of action of galeterone and then review the safety and efficacy data from Phase I and II clinical studies of galeterone in patients with metastatic castration-resistant prostate cancer. A Phase III study of galeterone (compared against enzalutamide) in AR-V7-positive patients is currently underway, and represents the first pivotal trial using a biomarker-selection design in this disease.
Introduction
Despite significant recent advances in the treatment of advanced prostate cancer, most patients with metastatic spread will ultimately die of progressive disease, and prostate cancer remains one of the leading causes of cancer-related deaths in men worldwide.Citation1,Citation2
Over the last decade, there has been an increasing understanding of mechanisms of disease progression after androgen deprivation therapy, which has led to the development of multiple new androgen receptor (AR)-directed drugs with promising activity for patients with metastatic castration-resistant prostate cancer (mCRPC).Citation3–Citation5 Of those, abiraterone (an oral CYP17 inhibitor), and enzalutamide (an oral AR-antagonist), have demonstrated significant clinical activity and have been approved by the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) based on notable improvements in median overall survival (OS), radiographic progression-free survival (rPFS), and quality of life.Citation6,Citation7 Nonetheless, these trials also demonstrate that about 10% to 20% of all patients with mCRPC show primary resistance to abiraterone or enzalutamide, and most of the remaining patients who have an initial clinical benefit will eventually develop disease progression later.Citation8–Citation11 Since patients progressing on abiraterone or enzalutamide often present with a rising PSA, it has become evident that many resistance mechanisms are related to persistent androgen/AR signaling, including AR and CYP17 upregulation, activating AR mutations, AR splice variants, glucocorticoid-receptor upregulation, and others.Citation12–Citation20
Among these, AR splicing variants have received a lot of attention recently. In particular, AR splice variant 7 (AR-V7) is a constitutionally-active variant of the AR that lacks the ligand-binding domain (LBD) and has emerged as an important mechanism of primary and acquired resistance to AR-targeted agents targeting the LBD of the AR.Citation18,Citation21 One potentially promising strategy would be to identify agents that could overcome this resistance mechanism by inhibiting the AR outside of the androgen-binding site. In this manuscript, we will review the preclinical and clinical data related to galeterone, a multi-targeted AR-signaling inhibitor for the treatment of CRPC.
Overview of approved agents for CRPC
Over the last decade, a major paradigm shift has been observed in the treatment of mCRPC, with approval of several new agents with different mechanisms of action, including cytotoxic chemotherapy (cabazitaxel) after docetaxel failure,Citation22,Citation23 next-generation AR-directed therapies (abiraterone and enzalutamide),Citation8–Citation11,Citation24,Citation25 an autologous immunotherapy agent (sipuleucel-T),Citation26 and the α-emitting radiopharmaceutical radium-223.Citation27 All of these approved agents have demonstrated significant clinical activity with OS improvement and benefits in several secondary endpoints included in each trial (). In daily medical practice, these agents continue to provide clinically meaningful benefits including pain relief and improvements in quality of life. The major challenge that clinicians face when treating patients with mCRPC is how to best sequence these agents in order to maximize benefits for an individual patient.Citation28–Citation30 Currently, oncologists often use clinical parameters such as pathology, symptoms, volume and location of metastasis, and proportion of PSA level to disease volume to help define the most appropriate agent to be used, since there are no predictive biomarkers to date that help in selecting the best treatment strategy.Citation29 Many trials are currently underway evaluating different treatment sequences as well as various drug combinations in order to maximize outcomes.Citation30 Hopefully in the future, with a better understanding of the mechanisms of drug resistance and response, predictive biomarkers will be identified to determine the use of agents most likely to benefit each patient (ie, treatment-selection biomarkers).
Table 1 Phase III clinical trials of FDA-approved drugs for metastatic castration-resistant prostate cancer
Mechanisms of resistance to AR-directed therapies
Despite the significant success achieved with the next-generation AR-directed therapies, eventually all patients will progress despite these therapies and will ultimately die of progressive metastatic disease. Therefore, it is key to uncover these mechanisms to allow the development of newer and more active agents, which could overcome the known resistance mechanisms. In addition, this could aid in the development of predictive biomarkers that could aid in treatment selection choices. Several mechanisms of resistance to AR-directed therapies have been described, and broadly divided into 1) AR-dependent mechanisms and 2) AR-independent mechanisms.
AR-dependent mechanisms of resistance
The main AR-dependent mechanisms of therapeutic resistance include CYP17 and AR upregulation, AR splice variants, activating AR mutations, and GR upregulation (ie, bypass pathways).Citation20,Citation31 Ultimately, these mechanisms will allow AR nuclear translocation and activation of nuclear transcriptional programs, or activation of androgen-response elements by alternative steroid receptors, leading to proliferation and disease progression despite testosterone-depleting agents (eg, androgen deprivation, abiraterone) or AR antagonists (eg, enzalutamide, apalutamide).
The role of CYP17 and AR overexpression was demonstrated by three studies with similar designs conducted by Efstathiou et al. In these studies, patients treated with abiraterone,Citation12 enzalutamide,Citation13 or bothCitation32 underwent trans-iliac bone marrow biopsies: AR and CYP17 expression was assessed by immunohistochemistry. A pre-treatment androgen-signaling signature was proposed based on CYP17 expression ([+], >10%) and AR N-terminal domain expression ([+++], >75%), which predicted benefit with AR-directed therapies in these studies. Moreover, in two of these studies, the presence of AR-V7 was assessed by immunohistochemistry at baseline, and was associated with primary resistance to enzalutamideCitation13 or the combination of abiraterone and enzalutamide.Citation32
One potential predictive biomarker for resistance to the currently approved AR-directed therapies is the AR splice variants, particularly AR-V7, which is a constitutionally active form of the AR that lacks the LBD, the target of enzalutamide and abiraterone. Amongst the AR splice variants (22 have been described to date),Citation33 AR-V7 is the most prevalent and its expression increases about 20-fold in the setting of AR-full length (FL) suppression by castration, suggesting that AR-V7 is biologically important and may be associated with ligand-independent AR-dependent progression of disease.Citation17,Citation34 The clinical importance of AR-V7 in the setting of treatment with AR-directed agents was demonstrated in a recent pilot study of patients with mCRPC treated with abiraterone or enzalutamide.Citation18 In this prospective study, AR-V7 status was determined by a circulating tumor cell (CTC)-based real-time polymerase chain reaction (RT-PCR) assay in 62 patients and was associated with clinical outcomes for abiraterone (n=31) or enzalutamide (n=31). In both cohorts, the rate of PSA decline ≥50% (PSA50) was high in the AR-V7 negative patients (52.6% and 68% for enzalutamide and abiraterone, respectively), but no AR-V7-positive patients achieved a PSA50 response. Moreover, in both cohorts, the AR-V7-negative group demonstrated a significantly higher disease-free survival and OS.Citation18 Although these results are preliminary and need to be validated in larger prospective studies,Citation35 the preclinical rationale and the magnitude of results observed strongly suggest that patients with AR-V7-positive CTCs may show primary resistance to abiraterone and enzalutamide. In addition, the CTC-based assay for AR-V7 detection requires further biomarker validation before incorporation into clinical practice.Citation36 One notable finding is that the AR-V7 status was not associated with resistance to taxane-based chemotherapy in recent studies,Citation37,Citation38 highlighting the importance of this biomarker for future treatment selection for patients with CRPC if these results are confirmed. Preliminary data on patients with serial “liquid biopsies” for CTC-based AR-V7 analysis suggest that some patients develop AR-V7 CTCs upon disease progression and in some patients the AR-V7 status may be converted from positive to negative when treated with taxane-based chemotherapy.Citation39 The clinical significance of this remains unknown.
AR point mutations are another mechanism of resistance increasingly being described, particularly in the setting of acquired resistance to abiraterone and next-generation AR antagonists (enzalutamide and apalutamide).Citation40 The AR mutations described may convert these AR-antagonists into pure agonists (AR F877L),Citation15,Citation16 or may allow the AR to be activated by progesterone (AR T878A) or glucocorticoids (AR L702H) in abiraterone/prednisone-treated patients.Citation14,Citation41,Citation42 These studies have shown that the presence of these activating mutations in AR are associated with inferior PFS and OS outcomes compared to men that harbor neither mutated nor amplified AR.
Therefore, targeting the AR-V7 and activating AR mutations is an area of priority for development of novel therapies to overcome resistance to drugs targeting the LBD of the AR, such as abiraterone, enzalutamide, and even newer drugs currently being tested in clinical trials such as apalutamide (ARN-509) and ODM-201. Some of these resistance mechanisms may perhaps be overcome with the use of galeterone, which has the ability to degrade various forms of the AR at the protein level.
AR-independent mechanisms of resistance
The AR-independent mechanisms of resistance have been increasingly recognized over the past few years due to a significant proportion of patients progressing after more active AR-directed drugs such as abiraterone and enzalutamide, often with a very aggressive clinical phenotype, described as anaplastic/small cell variant, characterized by a low PSA, osteolytic bone metastasis, visceral disease, refractoriness to available therapies, and short survival.Citation43 In addition, intermediate phenotypes (such as the recently coined histology: intermediate atypical carcinoma)Citation44 can be recognized in patients progressing after multiple lines of hormonal therapy. These patients often develop AR-independent mechanisms of resistance, including activation of PI3K/AKT pathway (often resulting from PTEN loss), Rb loss, inactivating TP53 mutations, expression of AURKA and N-Myc, alteration in DNA-repair genes, among others. Currently, several strategies are being developed to target these alterations and more comprehensive reviews on this topic can be found elsewhere.Citation45–Citation48
Galeterone: evidence to date
Preclinical studies
During the effort to identify potent CYP17 inhibitors in the 1990s and 2000s, 3β-(hydroxy)-17-(1H-benzimidazole-1-yl) androstane-5,16-dione (galeterone, formerly TOK-001 or VN/124-1) was identified as a multi-targeted AR inhibitor candidate. Initially identified as a potent CYP17 inhibitor, multiple subsequent preclinical studies have demonstrated that galeterone also acts as an AR antagonist to both FL and mutant AR, in addition to its ability to degrade the AR, including AR splice variants ().Citation49–Citation54 It has also been demonstrated that galeterone inhibits enzalutamide-resistant cells in vitro, and also blocks AR nuclear translocation and activation of androgen-dependent genes such as PSA and TMPRSS2.Citation55 The discovery, chemical properties and preclinical development of galeterone have been comprehensively reviewed elsewhere,Citation56 although the current understanding of its degradation of AR will be reviewed briefly here.
Figure 1 Three mechanisms of action of galeterone.
Abbreviation: Enz, enzalutamide.
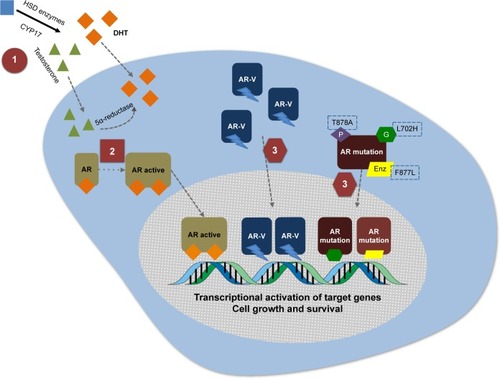
AR degradation
One of the described mechanisms of AR regulation involves the E3 ubiquitin ligase pathway.Citation57,Citation58 It has been described that the AR, MDM2, and AKT form a complex in vivo that results in ubiquitination and proteasome degradation of the AR. Specifically, it is thought that AKT phosphorylates MDM2 and the AR itself leading to MDM2 binding to the AR as a required step for AR degradation, and it has been shown that specific MDM2 binding site mutations inhibit AR ubiquitination and degradation.Citation59,Citation60 In addition, other E3 ubiquitin ligases are probably involved in the AR regulation process, such as CHIPCitation61 and SKP2Citation62 which also promote AR degradation, and Siah2,Citation63 RNF6,Citation64 and USP12Citation65,Citation66 which promote deubiquitination and AR activation.Citation57 To this end, it has been shown that galeterone-induced AR degradation is blocked by co-administration of proteasome inhibitors and also by E3 ligases MDM2 and CHIP selective knockdown, suggesting that galeterone disrupts the AR through protea-some degradation.Citation67 Notably, a recent study that screened a panel with 22 deubiquitinating enzymes in vitro showed that galeterone inhibits the enzymatic activity of USP12 and also binds directly to the USP12 and USP12/UAF1 complexes, which dephosphorylate AKT.Citation68 Therefore, the summation of data suggests that galeterone induces AR proteasomal degradation through the E3 ubiquitin ligase pathway, by changing the balance between ubiquitination and deubiquitination, both with respect to the FL AR and AR splice variants.Citation67,Citation68
Clinical studies: ARMOR1 and ARMOR2
The Androgen Receptor Modulation Optimized for Response (ARMOR) Phase I and Phase II studies conducted with galeterone were recently published and demonstrated that galeterone is a well tolerated drug with promising clinical activity for patients with CRPC.Citation69 summarizes the main characteristics and outcomes of the ARMOR trials.
Table 2 Main characteristics and outcomes of the ARMOR1 and ARMOR2 clinical trials
The Phase I ARMOR1 trial included 49 patients with progressive CRPC, including patients with metastatic (M1) and non-metastatic (M0) disease, and excluded patients who had previously received taxane chemotherapy, ketoconazole, abiraterone, or enzalutamide. This was a multicenter, dose-escalation study, which included eight dose-cohorts to assess the tolerability, safety, and efficacy of oral galeterone capsules (micronized powder, 325 mg), with dose-levels ranging from 650 mg to 2,600 mg daily. After a healthy volunteer study demonstrated a significant food effect with the capsule formulation used in the ARMOR1 study, a new formulation with no food effect – the galeterone spray dry dispersion (SDD) – was developed and was included in the dose-escalation component of the Phase II trial of galeterone in CRPC (ARMOR2, part 1). This Phase II study included 28 patients in three dosing cohorts using the 425 mg SDD tablets (1,700 mg, 2,550 mg and 3,400 mg daily) and allowed inclusion of abiraterone-resistant patients. Results of the healthy volunteer study also demonstrated equivalent serum concentrations using either 2,600 mg capsules or 1,700 mg SDD tablet formulations.Citation69 Therefore, the ARMOR2, part 1 trial was designed to evaluate the safety of escalating doses of galeterone in the SDD tablet formulation to determine the recommended dose for the Phase II ARMOR2, part 2 and Phase III (ARMOR3-SV) trials.
In ARMOR1 and ARMOR2 part 1, 12/49 and 6/28 patients discontinued galeterone therapy before 12 weeks due to treatment-related adverse events (AEs). In these trials, 21 and 19 patients were eligible for the expansion phase of ARMOR1 and ARMOR2 part 1, respectively. Overall the treatment was well tolerated in this case and, although most patients in both studies experienced at least one AE, no action was required in most cases. The most common treatment-related AEs were nausea, pruritus, fatigue, anorexia, diarrhea, and increased ALT levels (). Of note, no apparent mineralocorticoid excess syndrome was observed despite concomitant corticosteroid use not being required. In terms of overall clinical activity across all dose levels, 22% and 48% of patients achieved PSA50 in ARMOR1 and ARMOR2 part 1, respectively. In the 2,550 mg dose cohort of ARMOR2 part 1, 72.7% and 54.5% achieved a PSA decline ≥30% and ≥50%, respectively. The pharmacokinetic results of this study demonstrated that there was no increase in plasma exposure to doses higher than 2,550 mg, which was the recommended dose for subsequent Phase II and Phase III trials based on pharmacokinetics, safety, and PSA response data.Citation69
Table 3 Main adverse events in the ARMOR1 and ARMOR2 clinical trials
ARMOR2 part 2 is an ongoing study designed to assess the safety and efficacy of the 2,550 mg SDD tablet formulation of galeterone once a day in four distinct mCRPC cohorts: non-metastatic treatment-naïve (TN) CRPC (M0-TN, seven patients), metastatic TN CRPC (M1-TN, 24 patients), abiraterone-refractory mCRPC (eleven patients), and enzalutamide-refractory mCRPC (two patients).Citation70 Preliminary results of the first three patient cohorts were presented at the 2014 American Society of Clinical Oncology (ASCO) Annual Meeting and demonstrated significant clinical activity with PSA declines of ≥30% and ≥50% in 83% and 67% in the M0-TN group, and 92% and 83% in the M1-TN cohort, respectively. Two out of five (40%) patients in the abiraterone-refractory cohort achieved a PSA decline ≥30%. In terms of toxicity, 94% of all AEs were grade 1 or 2 and the most common events were nausea, diarrhea, fatigue, and pruritus. No mineralocorticoid excess symptoms or seizures were recorded in the study.Citation70 These results were updated at the 2014 European Society of Medical Oncology (ESMO) meeting, with safety data of the 107 patients included and efficacy results demonstrating a PSA decline ≥30% and ≥50% in 83% and 70% of patients in the M0 and M1 TN cohorts, respectively (). In the abiraterone-refractory cohort, among the 15 evaluable patients to date, 13% achieved a PSA decline ≥30% and 27% had some degree of PSA decline.Citation71
Table 4 ARMOR2: galeterone efficacy summary
In an exploratory analysis of circulating tumor cells in patients included in the ARMOR2 trial, 90% (64/71) of enrolled patients had ≥1 CTC detected.Citation71 In the M0 and M1 cohorts, the C-terminal AR immunohistochemical assay using the Epic Sciences platform was performed and seven of these patients were found to have AR C-terminal loss, implying the presence of AR splice variants (perhaps including AR-V7 as well as others). Notably, six of these seven patients (86%) with C-terminal loss achieved a PSA50, suggesting that galeterone may be active in patients harboring AR splice variants (eg, AR-V7). Four patients continued on galeterone in the optional extension phase, with time on treatment ranging from 155 to >334 days. Another interesting finding suggests that post-galeterone therapies with docetaxel or other AR-directed therapies (such as abiraterone and enzalutamide) may confer PSA declines, although this retrospective analysis included only nine patients, five of whom had discontinued galeterone due to AEs.Citation72
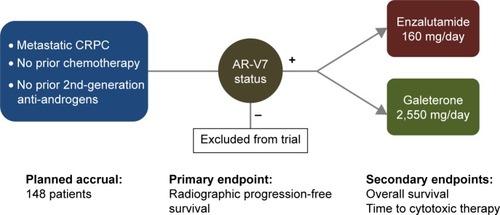
Based on the promising clinical results from the Phase II galeterone program, a registrational Phase III trial was recently launched (; ARMOR3-SV, NCT02438007).Citation73 To this end, ARMOR3-SV is a 148-patient randomized study in men with M1 CRPC who have not previously received abiraterone, enzalutamide or taxane chemotherapy (except docetaxel for hormone-sensitive metastatic prostate cancer). This trial has achieved a notable “first”, in that it is the first pivotal trial in prostate cancer to base its enrollment on a biomarker: in this case, AR-V7. Patients who appear eligible for this trial are pre-screened for AR-V7 using a CTC-based RT-PCR assay developed by Qiagen and performed by LabCorp in a Clinical Laboratory Improvement Amendments-certified environment. The prevalence of CTC-positive AR-V7-positive patients in this setting is expected to be 8%–12% (about ten patients will have to be screened to identify one AR-V7-positive case). Subsequently, only AR-V7-positive men are allowed to proceed to formal randomization, and these patients are allocated equally to receive enzalutamide 160 mg daily or galeterone 2,550 mg daily (). Patients are removed from study only if they develop confirmed radiographic progression of their disease (according to Prostate Cancer Working Group 2 criteria), or if they develop unmanageable toxicity. The primary endpoint of ARMOR3-SV is rPFS, with OS being the key secondary endpoint. Enrollment is expected to finish in early 2017, and primary results on the rPFS endpoint are expected by late 2017. If successful, this trial would be the first study to result in drug approval using an rPFS endpoint, and the first trial to employ a biomarker-selection design in prostate cancer. Moreover, if this trial demonstrates a superiority of galeterone over enzalutamide, it could represent a new option for patients with disease progression after available therapies who develop AR-V7 positive CTCs during the course of their disease.
Figure 2 ARMOR3-SV: Phase III randomized trial design and study endpoints.
Abbreviations: CRPC, castration-resistant prostate cancer; AR-V7, androgen receptor splice variant 7.
Perspectives
Despite the many advances achieved thus far in the understanding of the biology of mCRPC, it is clear that with more active AR-directed drugs the complexity of the mechanisms of resistance and progression is increasing, and novel approaches are needed to improve patient outcomes. Galeterone is a unique AR-directed drug with multiple mechanisms of action including CYP17 inhibition while also acting as an AR antagonist and AR degrader. Therefore, galeterone could potentially overcome aberrant AR signaling mediated by mutant-activated AR as well as constitutively-active AR splice variants, which have been recognized as potential escape mechanisms to abiraterone and enzalutamide, by virtue of its protein degradation mechanism. It should be highlighted that galeterone will not address the non-AR-dependent mechanisms of castration-resistance.
In order to evaluate galeterone’s efficacy for AR-V7 positive patients, the first biomarker-driven Phase III randomized trial in mCRPC (; ARMOR3-SV, NCT02438007) has been launched and will compare the efficacy of galeterone against enzalutamide in mCRPC patients harboring AR-V7, and hopefully will demonstrate activity in this refractory patient population. Meanwhile, additional Phase II trials are being planned using galeterone in the post-abiraterone and post-enzalutamide populations. Finally, if galeterone is truly capable of targeting and degrading AR-V7 and other splice variants, it remains possible that this agent may theoretically sensitize (or even re-sensitize) CRPC patients to treatment with abiraterone and enzalutamide, although this hypothesis remains to be tested.
Acknowledgments
ESA has received funding from the Prostate Cancer Foundation, the Patrick C. Walsh Fund, and NIH grants R01 CA185297 and P30 CA006973.
Disclosure
ESA has served as a paid consultant/advisor for Janssen, Astellas, Sanofi, Dendreon, Merck, Essa, and Medivation; has received research funding to his institution from Janssen, Johnson & Johnson, Medivation, Sanofi, Dendreon, Bristol Myers Squibb, Genentech, Novartis, Bayer and Tokai; and is a co-inventor of a biomarker technology that has been licensed to Tokai. DAB declares no conflicts of interest in this work.
References
- TorreLABrayFSiegelRLFerlayJLortet-TieulentJJemalAGlobal cancer statistics, 2012CA Cancer J Clin20156528710825651787
- SiegelRLMillerKDJemalACancer statistics, 2016CA Cancer J Clin201666173026742998
- BalkSPAndrogen receptor as a target in androgen-independent prostate cancerUrology2002603 Suppl 1132138 discussion 138–13912231070
- ScherHISawyersCLBiology of progressive, castration-resistant prostate cancer: directed therapies targeting the androgen-receptor signaling axisJ Clin Oncol200523328253826116278481
- RyanCJTindallDJAndrogen receptor rediscovered: the new biology and targeting the androgen receptor therapeuticallyJ Clin Oncol201129273651365821859989
- KluetzPGNingYMMaherVEAbiraterone acetate in combination with prednisone for the treatment of patients with metastatic castration-resistant prostate cancer: U.S. Food and Drug Administration drug approval summaryClin Cancer Res201319246650665624150234
- NingYMBraveMMaherVEU.S. Food and Drug Administration Approval Summary: Enzalutamide for the Treatment of Patients With Chemotherapy-Naive Metastatic Castration-Resistant Prostate CancerOncologist201520896096626070917
- de BonoJSLogothetisCJMolinaAAbiraterone and increased survival in metastatic prostate cancerN Engl J Med2011364211995200521612468
- RyanCJSmithMRde BonoJSAbiraterone in metastatic prostate cancer without previous chemotherapyN Engl J Med2013368213814823228172
- ScherHIFizaziKSaadFIncreased survival with enzalutamide in prostate cancer after chemotherapyN Engl J Med2012367131187119722894553
- BeerTMArmstrongAJRathkopfDEEnzalutamide in metastatic prostate cancer before chemotherapyN Engl J Med2014371542443324881730
- EfstathiouETitusMTsavachidouDEffects of abiraterone acetate on androgen signaling in castrate-resistant prostate cancer in boneJ Clin Oncol201230663764322184395
- EfstathiouETitusMWenSMolecular characterization of enzalutamide-treated bone metastatic castration-resistant prostate cancerEur Urol2015671536024882673
- CarreiraSRomanelAGoodallJTumor clone dynamics in lethal prostate cancerSci Transl Med20146254254ra125
- JosephJDLuNQianJA clinically relevant androgen receptor mutation confers resistance to second-generation antiandrogens enzalutamide and ARN-509Cancer Dis20133910201029
- AzadAAVolikSVWyattAWAndrogen Receptor Gene Aberrations in Circulating Cell-Free DNA: Biomarkers of Therapeutic Resistance in Castration-Resistant Prostate CancerClin Cancer Res201521102315232425712683
- NakazawaMAntonarakisESLuoJAndrogen receptor splice variants in the era of enzalutamide and abirateroneHorm Cancer20145526527325048254
- AntonarakisESLuCWangHAR-V7 and resistance to enzalutamide and abiraterone in prostate cancerN Engl J Med2014371111028103825184630
- AroraVKSchenkeinEMuraliRGlucocorticoid receptor confers resistance to antiandrogens by bypassing androgen receptor blockadeCell201315561309132224315100
- SilbersteinJLTaylorMNAntonarakisESNovel Insights into Molecular Indicators of Response and Resistance to Modern Androgen-Axis Therapies in Prostate CancerCurr Urol Rep20161742926902623
- MaughanBLAntonarakisESClinical Relevance of Androgen Receptor Splice Variants in Castration-Resistant Prostate CancerCurr Treat Options Oncol201516125726537882
- de BonoJSOudardSOzgurogluMPrednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trialLancet201037697471147115420888992
- TannockIFde WitRBerryWRDocetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancerN Engl J Med2004351151502151215470213
- LogothetisCJBaschEMolinaAEffect of abiraterone acetate and prednisone compared with placebo and prednisone on pain control and skeletal-related events in patients with metastatic castration-resistant prostate cancer: exploratory analysis of data from the COU-AA-301 randomised trialLancet Oncol201213121210121723142059
- RyanCJSmithMRFizaziKAbiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 studyLancet Oncol201516215216025601341
- KantoffPWHiganoCSShoreNDSipuleucel-T immunotherapy for castration-resistant prostate cancerN Engl J Med2010363541142220818862
- ParkerCNilssonSHeinrichDAlpha emitter radium-223 and survival in metastatic prostate cancerN Engl J Med2013369321322323863050
- BaschELoblawDAOliverTKSystemic therapy in men with metastatic castration-resistant prostate cancer: American Society of Clinical Oncology and Cancer Care Ontario clinical practice guidelineJ Clin Oncol201432303436344825199761
- GillessenSOmlinAAttardGManagement of patients with advanced prostate cancer: recommendations of the St Gallen Advanced Prostate Cancer Consensus Conference (APCCC) 2015Ann Oncol20152681589160426041764
- LorenteDMateoJPerez-LopezRde BonoJSAttardGSequencing of agents in castration-resistant prostate cancerLancet Oncol2015166e279e29226065613
- WatsonPAAroraVKSawyersCLEmerging mechanisms of resistance to androgen receptor inhibitors in prostate cancerNat Rev Cancer2015151270171126563462
- EfstathiouETitusMWenSEnzalutamide (ENZA) in combination with abiraterone acetate (AA) in bone metastatic castration resistant prostate cancer (mCRPC)J Clin Oncol201432:5s suppl abstr 5000
- RobinsonDVan AllenEMWuYMIntegrative clinical genomics of advanced prostate cancerCell201516151215122826000489
- YuZChenSSowalskyAGRapid induction of androgen receptor splice variants by androgen deprivation in prostate cancerClin Cancer Res20142061590160024449822
- ScherHILuDSchreiberNAAssociation of AR-V7 on circulating tumor cells as a treatment-specific biomarker with outcomes and survival in castration-resistant prostate cancerJAMA Oncol Epub201664
- SprengerCUoTPlymateSAndrogen receptor splice variant V7 (AR-V7) in circulating tumor cells: a coming of age for AR splice variants?Ann Oncol20152691805180726199394
- AntonarakisESLuCLuberBAndrogen Receptor Splice Variant 7 and Efficacy of Taxane Chemotherapy in Patients With Metastatic Castration-Resistant Prostate CancerJAMA Oncol20151558259126181238
- OnstenkWSieuwertsAMKraanJEfficacy of Cabazitaxel in Castration-resistant Prostate Cancer Is Independent of the Presence of AR-V7 in Circulating Tumor CellsEur Urol201568693994526188394
- NakazawaMLuCChenYSerial blood-based analysis of AR-V7 in men with advanced prostate cancerAnn Oncol20152691859186526117829
- SmithMRAntonarakisESRyanCJPhase 2 Study of the Safety and Antitumor Activity of Apalutamide (ARN-509), a Potent Androgen Receptor Antagonist, in the High-risk Nonmetastatic Castration-resistant Prostate Cancer CohortEur Urol Epub201656
- RomanelAGasi TandefeltDConteducaVPlasma AR and abiraterone-resistant prostate cancerSci Transl Med20157312312re310
- WyattAWAzadAAVolikSVGenomic Alterations in Cell-Free DNA and Enzalutamide Resistance in Castration-Resistant Prostate CancerJAMA Oncol Epub201655
- BeltranHTomlinsSAparicioAAggressive variants of castration-resistant prostate cancerClin Cancer Res201420112846285024727321
- SmallEJHuangJYoungrenJCharacterization of neuroendocrine prostate cancer (NEPC) in patients with metastatic castration resistant prostate cancer (mCRPC) resistant to abiraterone (Abi) or enzalutamide (Enz): Preliminary results from the SU2C/PCF/AACR West Coast Prostate Cancer Dream Team (WCDT)J Clin Oncol201533suppl abstr 5003
- BeltranHJendrisakALandersMThe Initial Detection and Partial Characterization of Circulating Tumor Cells in Neuroendocrine Prostate CancerClin Cancer Res20162261510151926671992
- BoudadiKAntonarakisESResistance to Novel Antiandrogen Therapies in Metastatic Castration-Resistant Prostate CancerClin Med Insights Oncol201610Suppl 11927013902
- BeltranHPrandiDMosqueraJMDivergent clonal evolution of castration-resistant neuroendocrine prostate cancerNat Med201622329830526855148
- AparicioAMShenLTapiaELCombined Tumor Suppressor Defects Characterize Clinically Defined Aggressive Variant Prostate CancersClin Cancer Res20162261520153026546618
- NjarVCKatoKNnaneIPGrigoryevDNLongBJBrodieAMNovel 17-azolyl steroids, potent inhibitors of human cytochrome 17 alpha-hydroxylase-C17,20-lyase (P450(17) alpha): potential agents for the treatment of prostate cancerJ Med Chem19984169029129526564
- HandrattaVDVasaitisTSNjarVCNovel C-17-heteroaryl steroidal CYP17 inhibitors/antiandrogens: synthesis, in vitro biological activity, pharmacokinetics, and antitumor activity in the LAPC4 human prostate cancer xenograft modelJ Med Chem20054882972298415828836
- VasaitisTBelosayASchayowitzAAndrogen receptor inactivation contributes to antitumor efficacy of 17{alpha}-hydroxylase/17,20-lyase inhibitor 3beta-hydroxy-17-(1H-benzimidazole-1-yl)androsta-5,16-diene in prostate cancerMol Cancer Ther2008782348235718723482
- PurushottamacharPGodboleAMGediyaLKSystematic structure modifications of multitarget prostate cancer drug candidate galeterone to produce novel androgen receptor down-regulating agents as an approach to treatment of advanced prostate cancerJ Med Chem201356124880489823713567
- SoiferHSSouleimanianNWuSDirect regulation of androgen receptor activity by potent CYP17 inhibitors in prostate cancer cellsJ Biol Chem201228763777378722174412
- YuZCaiCGaoSSimonNIShenHCBalkSPGaleterone prevents androgen receptor binding to chromatin and enhances degradation of mutant androgen receptorClin Cancer Res201420154075408524874833
- Al NakouziNWangCJacobyDGleaveMEZoubeidiAGaleterone suppresses castration-resistant and enzalutamide-resistant prostate cancer growth in vitroMol Cancer Ther20131211 SupplC89
- NjarVCBrodieAMDiscovery and development of Galeterone (TOK-001 or VN/124-1) for the treatment of all stages of prostate cancerJ Med Chem20155852077208725591066
- LiBLuWChenZRegulation of Androgen Receptor by E3 Ubiquitin Ligases: for More or LessReceptors Clin Investig201415110
- QiJFanLHussainAImplications of ubiquitin ligases in castration-resistant prostate cancerCurr Opin Oncol201527317217625811345
- LinHKWangLHuYCAltuwaijriSChangCPhosphorylation-dependent ubiquitylation and degradation of androgen receptor by Akt require Mdm2 E3 ligaseEMBO J200221154037404812145204
- RileyMFLozanoGThe Many Faces of MDM2 Binding PartnersGenes Cancer201233–422623923150756
- MurataSMinamiYMinamiMChibaTTanakaKCHIP is a chaperone-dependent E3 ligase that ubiquitylates unfolded proteinEMBO Rep20012121133113811743028
- LiBLuWYangQYuXMatusikRJChenZSkp2 regulates androgen receptor through ubiquitin-mediated degradation independent of Akt/mTOR pathways in prostate cancerProstate201474442143224347472
- QiJTripathiMMishraRThe E3 ubiquitin ligase Siah2 contributes to castration-resistant prostate cancer by regulation of androgen receptor transcriptional activityCancer Cell201323333234623518348
- XuKShimelisHLinnDERegulation of androgen receptor transcriptional activity and specificity by RNF6-induced ubiquitinationCancer Cell200915427028219345326
- BurskaULHarleVJCoffeyKDeubiquitinating enzyme Usp12 is a novel co-activator of the androgen receptorJ Biol Chem201328845326413265024056413
- McClurgULSummerscalesEEHarleVJGaughanLRobsonCNDeubiquitinating enzyme Usp12 regulates the interaction between the androgen receptor and the Akt pathwayOncotarget20145167081709225216524
- Kwegyir-AffulAKRamalingamSPurushottamacharPRamamurthyVPNjarVCGaleterone and VNPT55 induce proteasomal degradation of AR/AR-V7, induce significant apoptosis via cytochrome c release and suppress growth of castration resistant prostate cancer xenografts in vivoOncotarget2015629274402746026196320
- DransfieldDTNamdevNJacobyDBFerranteKGaleterone-induced degradation of the androgen receptor involves inhibition of deubiquitinating enzymesAACR Annual MeetingNew OrleansApril 18, 2016 Abstract 12342016
- MontgomeryBEisenbergerMARettigMBAndrogen Receptor Modulation Optimized for Response (ARMOR) Phase I and II Studies: Galeterone for the Treatment of Castration-Resistant Prostate CancerClin Cancer Res20162261356136326527750
- MontgomeryBEisenbergerMAHeathEIGaleterone in men with CRPC: Results in four distinct patient populations from the ARMOR2 studyJ Clin Oncol2014325s suppl; abstr 5029
- TaplinMEChiKNChuFActivity of galeterone in castrate-resistant prostate cancer (CRPC) with C-terminal AR loss: Results from ARMOR2European Journal of Cancer20145068
- McKayRRWernerLFiorilloMActivity of sequential therapies (txs) in patients (pts) with castration resistant prostate cancer (CRPC) previously treated with galeteroneJ Clin Oncol201533 suppl; abstr e16072
- TaplinMEAntonarakisESDransfieldDTFerranteKJDe BonoJSAndrogen receptor modulation optimized for response: Splice variant (ARMOR3-SV)–Randomized, open-label, multicenter, controlled study of galeterone vs enzalutamide in men with metastatic castration-resistant prostate cancer (mCRPC) expressing AR-V7 splice variantJ Clin Oncol201533 suppl; abstr TPS5069
