?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
Nonsteroidal anti-inflammatory drugs (NSAIDs) are the most common type of medication used in the treatment of acute pain. Ketorolac trometamol (KT) is a nonnarcotic, peripherally acting nonsteroidal anti-inflammatory drug with analgesic effects comparable to certain opioids.
Objective
The aim of this study was to compare the efficacy of KT and naproxen (NA) in the treatment of acute low back pain (LBP) of moderate-to-severe intensity.
Patients and methods
In this 10-day, Phase III, randomized, double-blind, double-dummy, noninferiority trial, participants with acute LBP of moderate-to-severe intensity as determined through a visual analog scale (VAS) were randomly assigned in a 1:1 ratio to receive sublingual KT 10 mg three times daily or oral NA 250 mg three times daily. From the second to the fifth day of treatment, if patient had VAS >40 mm, increased dosage to four times per day was allowed. The primary end point was the reduction in LBP as measured by VAS. We also performed a post hoc superiority analysis.
Results
KT was not inferior to NA for the reduction in LBP over 5 days of use as measured by VAS scores (P=0.608 for equality of variance; P=0.321 for equality of means) and by the Roland–Morris Disability Questionnaire (P=0.180 for equality of variance test; P=0.446 for equality of means) using 95% confidence intervals. The percentage of participants with improved pain relief 60 minutes after receiving the first dose was higher in the KT group (24.2%) than in the NA group (6.5%; P=0.049). The most common adverse effects were heartburn, nausea, and vomiting.
Conclusion
KT is not inferior in efficacy and delivers faster pain relief than NA.
Introduction
Low back pain (LBP) has a significant impact on workplace absenteeism of ∼15.5%.Citation1 Mechanical LBP can be secondary to lumbar strain or sprain (70% of all reported cases), age-related degenerative changes (10%), herniated disks (4%), osteoporotic fractures (4%), or spinal stenosis (3%), with all other causes accounting for <1% of cases. For individuals <45 years old, mechanical LBP is the most common cause of disability, and it is generally attributed to an acute traumatic event. However, cumulative trauma must also be considered in the etiology, such as occupational overuse injury.Citation2,Citation3
The best approach to treat LBP appears to be a combination of pharmacological and nonpharmacological strategies.Citation4 There is a great variability, possibly genetically related, in the individual response to painkillers.Citation5 Nonsteroidal anti-inflammatory drugs (NSAIDs) are the most common type of medication used in the treatment of acute pain, exerting their effect by interfering in the inflammatory response.Citation6 They inhibit the cyclooxygenase (COX) enzyme, reducing the synthesis of prostaglandins. The traditional NSAIDs inhibit both COX-1 and COX-2, and inhibiting COX-1 decreases platelet aggregation, irritates the gastric mucosa, and alters renal flow.Citation7
Ketorolac trometamol (KT; Toragesic®, EMS Sigma Pharma Ltd., Campinas, Brazil) is an NSAID, which is a racemic mixture of the S- and R-enantiomeric forms. KT inhibits COX that results in reduced synthesis of prostaglandins, thromboxanes, and prostacyclin as well as diminished platelet aggregation.Citation8 Compared with aspirin, which produces prolonged and irreversible antiplatelet effects that persist beyond drug administration, the anti-platelet activity of KT is not apparent after elimination from the plasma and is reversible. In addition, the platelet effects of KT are not related to dose.Citation8 The S-enantiomer of KT is a rapid-acting and potent analgesic that possesses no anesthetic, sedative, or antianxiety effects and has no effect on gut motility.Citation8 The effectiveness, safety, and analgesic efficacy and potency of KT are considered higher than those of ketoprofen in postoperative ear–nose–throatCitation9 and dental surgeries.Citation10
The aim of the current clinical trial (protocol number NCT01471886) was to test the hypothesis that KT is not inferior to naproxen (NA) in its analgesic efficacy and incidence of adverse effects for the treatment of moderate-to-severe acute LBP.
Patients and methods
This 10-day, double-dummy, randomized, prospective, noninferiority clinical trial was conducted at two research centers in São Paulo, Brazil, in accordance with the principles of the Declaration of Helsinki and under protocol number 0752/11, which was approved by the Ethics Committee of the University Hospital at São Paulo University.
Because the package insert indicates that the upper limit of use for KT is 5 days, the treatment duration was not longer than 5 days, and the study finished with safety reassessment 10 days after treatment initiation.
Participants
Eighty-three outpatients diagnosed with moderate or severe acute LBP as determined by a visual analog scale (VAS) score >40 mm were screened. Eligible participants were 18–65 years old and able to give written informed consent. Women of childbearing age had to agree to use contraceptive methods throughout the study. The exclusion criteria included the following: weight <50 kg; severe congestive heart failure; current alcoholism or illegal drug use; presence of fever or signs of infection; kidney disease; fracture; fibromyalgia; cancer; neuropsychiatric disease; rheumatologic disease; history of peptic ulcer disease, gastrointestinal bleeding, or hemorrhagic diathesis; cerebrovascular disease; hemostatic disorders or use of anticoagulants; pregnancy; lactation; postoperative patients at high risk of bleeding; history of hypersensitivity to any of the ingredients in the formula or other NSAIDs; nasal polyps; and asthma. Participants could not have participated in another experimental study in the 6 months prior to study entry. None of the 83 prospective participants were excluded; thus, these 83 volunteers were randomized.
Treatments, groups, and outcome measures
This noninferiority study compared the analgesic efficacy and incidence of adverse effects in the treatment of moderate-to-severe acute LBP for KT at a dosage of 10 mg given sublingually three times daily (TID) and NA at a dosage of 250 mg administered orally TID. Eligible participants were randomly assigned to one of these two treatment groups by a computer-generated lottery. Each participant received a numbered kit in the order of arrival. Both the medical staff and the participants were blinded to the treatment assignments.
A superiority post hoc analysis was performed using a Likert scale. This scale was used to evaluate the investigators’ global assessment of efficacy on reducing the participant’s pain through the following ratings: excellent, VAS score reduction 50 mm or greater; very good, VAS score reduction between 40 mm and 50 mm; good, VAS reduction between 30 mm and 40 mm; regular, VAS between 20 mm and 30 mm; bad, VAS score 10 mm or less.
During the initial visit (V0) on the first treatment day, the participant received two pills of either the tested or reference medication, followed by one tablet every 8 hours, for a total of 40 mg and 1,000 mg, respectively. Thereafter, a daily dosage of 10 mg TID was administered sublingually for the test medication and 250 mg TID for the reference medication. From the second to the fifth day of treatment, if the patient had VAS >40 mm, increased dosage to four times per day was allowed. Participants were reassessed after the initiation of treatment at 2 days (V1) and 4 days (V2), when the treatment was discontinued. Ten days (V3) after the initiation of the study, participants returned for a safety assessment and adverse events were recorded.
The analgesic effect was evaluated on V0 before and 60 minutes after taking the drug (V0–60) as well as on V1 and V2 through a VAS score categorized as follows: 0 mm, no pain; 0.1–40 mm, mild pain; 41–70 mm, moderate pain; and 71–100 mm, severe pain. The primary end point was the rate of pain relief (RPR) calculated by the following formula:
The secondary end point was RPR1 and was calculated for each of the three drug administrations on each day as follows:
Statistical analysis
A sample size of at least 78 participants was deemed sufficient to detect differences in the RPR at a significance level of 5%, power of 80%, noninferiority margin of −10% (as per the recommendation of the US Food and Drug Administration [FDA]), and an average difference between 5% and 10%, considering a 20% dropout.Citation11,Citation12 A total of 63 (per protocol) of the 83 (intention-to-treat, ITT) participants completed the study (there was no screening failure). The homogeneity of the demographic and clinical features between the groups was compared by χ2 tests, Fisher’s exact tests (for sex, and clinical changes), Levene’s test for variance equality, and t-test for two independent samples to compare means between two groups for the averages equality (age, weight, Rolland-Morris, and VAS).
The evaluation of the effectiveness (VAS) was performed by Levene’s test for variance equality and t-tests for mean equality in the protocol population. The primary end point was the RPR between V0–60 and V0, between V1 and V0, and between V2 and V0. The secondary end point was the pain relief following each of the three drug administrations on each day (RPR1). The security assessment was conducted in the ITT population using Levene’s test. This population consisted of all participants who received at least one dose of medication.
We also conducted a post hoc superiority analysis. The primary end point for this analysis was the investigators’ global assessments of efficacy as measured using a Likert scale. We used a logistic model with a stepwise backward approach to select the dependent variables among treatment, sex, age, current smoking status, alcohol use, and clinical changes (inclusion in the model P<0.05, and exclusion P>0.1). The secondary end point in this analysis was the percentage of participants with improvement in pain relief as assessed by the VAS. For this analysis, we considered pain improvement as VAS scores from 0 to 3 after the administration of medication. We also used a logistic model with a stepwise backward approach to select the dependent variables among treatment, sex, age, current smoking status, alcohol use, and clinical changes (inclusion in the model P<0.05, and exclusion P>0.1).Citation13
Results
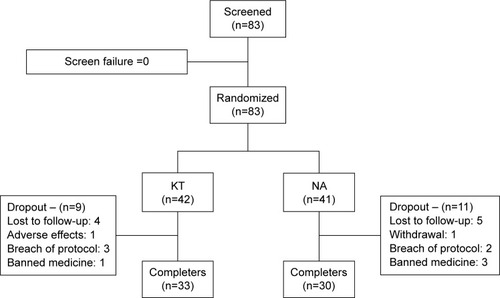
A total of 63 of the 83 participants completed the study. One participant was withdrawn due to adverse effects (acute cholecystitis, improbably associated with the test drug), four due to the use of banned medicine, and five due to a breach of protocol, while four chose to withdraw from the study, and six were withdrawn because of lack of follow-up (). Both sample groups were clinically homogeneous, and there was no significant difference between them ().
Table 1 Distribution of demographic data at the beginning of the treatment
Figure 1 Enrollment and randomization flowchart.
Abbreviations: KT, ketorolac trometamol; NA, naproxen.
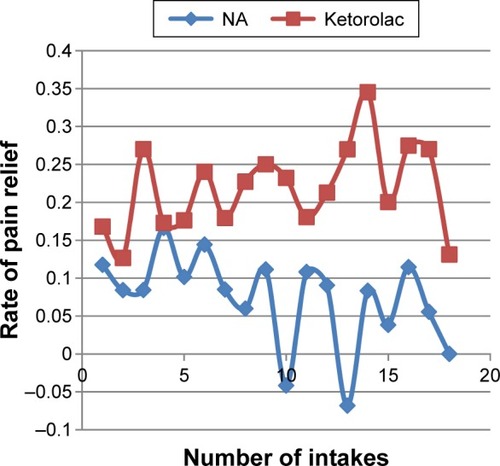
Comparison of the RPR between V0 and V0–60 showed a 5.6% gain in the RPR for KT compared with NA, which was below the 10% limit (difference =−0.056; 95% confidence interval [CI], −0.166, 0.055) and within the noninferiority margin. Comparing the RPR for V1 and V0, and for V2 and V0, the upper limit of the 95% CI was >10% (). The secondary end point showed that KT was not inferior to NA on day 1 for the first and third administrations of the drug, on day 2 for the second and third administrations, on days 3 and 4 for the first three administrations, and on day 5 for the first and third administrations ().
Table 2 RPR as assessed using VAS scores
Table 3 Pain relief as assessed by comparing VAS scores 1 hour before and after receiving medication
Because we observed a pattern of superiority in the RPR for KT compared with that for NA (), we performed a post hoc analysis using Likert scale scores of the investigators’ global assessment of efficacy on reducing the participants’ pain as the end point. The final logistic model included age as the only important independent variable after a stepwise approach. On V1, participants in the KT group had 193.1% higher odds of pain reduction compared with those in the NA group, controlling for age (95% CI, 1.10–7.80). However, this was not observed on V2, at which no statistically significant difference in pain improvement was detected between the two treatment groups (). However, the percentage of participants who reported an improvement in pain relief at V0–60 was higher in the KT group (24.2%) than in the NA group (6.5%; P=0.049). The percentage of participants rated as excellent, very good, or good by the investigator for the KT treatment group (66.7%) was much higher than that for the NA group (40.0%), with 95% CIs for the differences of −0.493 and −0.041. These results indicated that, compared with the NA treatment, the KT treatment had a margin of superiority equal to 4.1%.
Table 4 Efficacy evaluation for investigator ratings of excellent, very good, or good
Security assessment
The assessment for adverse effects was performed in the ITT population. There were 35 adverse events in the study population treated with NA and 42 in the population treated with KT. The main adverse effects were diarrhea, stomach pain, drowsiness, nausea, and vomiting. The frequency of occurrence of each event between the two treatments was not statistically significant (). The safety evaluation for changes in the laboratory results performed in the protocol population on V2 and V3 and compared with that on V0 did not show any difference between the two treatment groups ().
Table 5 Major adverse effects: incidence as a result of treatment in the ITT population
Table 6 Percentage of change in laboratory test results for the protocol population on visits 2 (V2) and 3 (V3) compared with values obtained on the initial visit (V0)
Discussion
This study showed that the efficacy of KT was not inferior to that of NA in the treatment of acute LBP of moderate-to-severe intensity, with no significant differences in the occurrence of adverse effects between the two treatment groups. However, participants who received KT exhibited a higher percentage of response after the initial administration and had higher odds of responding according to the investigator’s assessment at the first visit, suggesting a faster pain relief in the KT group. The rate of pain relief should always be considered when choosing an analgesic in order to improve the quality of life for patients.
There is evidence that KT is more effective than other NSAIDs in pain reduction from both inflammatory and non-inflammatory etiologies.Citation9,Citation10 Its mechanism of action is to reduce prostaglandin production by blocking COX 1 and 2. It has no sedative or anxiolytic properties.Citation14 The FDA approved ketorolac in November 1989.
In studies of postoperative pain, KT showed an opioid dose-sparing effect and consequently a decrease in the adverse events related to opioids.Citation15,Citation16 In addition, for intravenous administration, KT is more cost-effective than morphine in blunt limb injury.Citation17
Only two studies have evaluated KT for the treatment of acute LBP, and both studies used opioids as comparators.Citation18,Citation19 In both studies, KT had comparable efficacy and fewer side effects. The current study is the first to compare KT to another NSAID in the management of acute LBP. The treatment of acute LBP is an unmet need in which the variability of medical options, mostly uncontrolled, includes the use of NSAIDs, opioids, corticosteroids, and invasive procedures, such as epidural blockade. The results of our study comparing KT with the gold standard NSAID NA indicated that KT is a valid option for the treatment of LBP.
Conclusion
KT is not inferior to NA in efficacy, provides faster pain relief, and is a safe acute treatment option for acute pain relief.
Acknowledgments
This study was funded by EMS. Role of the Funder/Sponsor: EMS was involved in the study design and protocol development, provided logistical support, and obtained the data, which were evaluated jointly by the authors and the sponsor. All authors interpreted the data and wrote the manuscript together with the sponsor’s medical writing services. The sponsor did not have the right to suppress or veto publications. Dr Hélio Plapler, MD for reviewing the data and text; Dr Helena Hideko Segushi Kaziyama and Adriana Pereira de Paula for data collection.
Disclosure
All authors report receiving grants and consulting fees from EMS Industry. No other disclosures are reported.
References
- Wynne-JonesGCowenJJordanJLAbsence from work and return to work in people with back pain: a systematic review and meta-analysisOccup Environ Med201471644845624186944
- Hills EC [webpage on the Internet]Mechanical Low Back Pain2014 [updated Aug 4, 2014]. Available from: http://emedicine.medscape.com/article/310353-followupAccessed May 13, 2016
- IjzelenbergWBurdorfARisk factors for musculoskeletal symptoms and ensuing health care use and sick leaveSpine200530131550155615990672
- VadiveluNMitraSNarayanDRecent advances in postoperative pain managementYale J Biol Med2010831112520351978
- LötschJGeisslingerGCurrent evidence for genetic modulation of the response to analgesicsPain20061211516472919
- EksterowiczNQuinlan-CowellAVanderveerBMenezJAcute Pain Management. Core Curriculum for Pain Management Nursing2nd edDubuque, IAKendall Hunt Professional2010343
- GhafoorVMarieBSOverview of pharmacologyMarieBJStCore Curriculum for Pain Management Nursing2nd edDubuque, IAKendall Hunt Professional2010236242
- Truven Health Analytics [webpage on the Internet]Ketorolac tromethamine DRUGDEXSystem (Micromedex 20)Greenwood Village, COTruven Health Analytics2016c2012c2016 Available from: http://www.micromedexsolutions.com/micromedex2/librarian/CS/128FA5/ND_PR/evidencexpert/ND_P/evidencexpert/DUPLICATIONSHIELDSYNC/DABBB0/ND_PG/evidencexpert/ND_B/evidencexpert/ND_AppProduct/evidencexpert/ND_T/evidencexpert/PFActionId/evidencexpert.DoIntegratedSearch?SearchTerm=ketorolac&UserSearchTerm=ketorolac&SearchFilter=filterNone&navitem=searchALL#Accessed April 04, 2016
- PatrocinioLGRangelMOMiziaraGSMRodriguesAMPatrocinioJAPatrocinioTGA comparative study between Ketorolac and Ketoprofen in postoperative pain after uvulopalatopharyngoplastyRev Bras Otorrinolaringol2007733339342
- OlmedoMVGálvezRVallecilloMDouble-blind parallel comparison of multiple doses of ketorolac, ketoprofen and placebo administered orally to patients with postoperative dental painPain2001901–213514111166979
- ChowSCShaoJWangHSample Size Calculations in Clinical Research2nd edBoca Raton, FLChapman & Hall/CRC2008
- FDAGuidance for Industry Non-Inferiority Clinical TrialRockville, MDCenter for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER)2010
- PaulaGAModelos de regressão com apoio computacional. [Regression models with computer support]IME-USP2004São Paulo, Brazil Portuguese
- Hoffmann-La RocheToradol® (ketorolac tromethamine): 10 mg tablets; Toradol® IM (ketorolac tromethamine injection) 10 mg/mL or 30 mg/mL intramuscular injection [product monograph]Mississauga (ON)Hoffmann-La Roche2003
- AlexanderREl-MoalemHEGanTJComparison of the morphine-sparing effects of diclofenac sodium and ketorolac tromethamine after major orthopedic surgeryJ Clin Anesth200214318719212031750
- ChenJYWuGJMokMSEffect of adding ketorolac to intravenous morphine patient-controlled analgesia on bowel function in colorectal surgery participants – a prospective, randomized, double-blind studyActa Anaesthesiol Scand200549454655115777304
- RainerTHJacobsPNgYCCost effectiveness analysis of intravenous ketorolac and morphine for treating pain after limb injury: double blind randomized controlled trialBMJ200032172711247125111082083
- VeenemaKRLeaheyNSchneiderSKetorolac versus meperidine: ED treatment of severe musculoskeletal low back painAm J Emerg Med200018440440710919528
- InnesGDCroskerryPWorthingtonJBeveridgeRJonesDKetorolac versus acetaminophen-codeine in the emergency department treatment of acute low back painJ Emerg Med19981645495569696169