Abstract
Background
China is one of the high-burden countries for multidrug-resistant tuberculosis (MDR-TB), and pyrazinamide is one of the anti-TB drugs used for the shorter MDR-TB treatment regimen. The aim of this study was to determine the correlation between pncA gene mutations and resistance to four first-line anti-TB drugs as well as treatment history in clinical isolates of Mycobacterium tuberculosis.
Patients and methods
M. tuberculosis clinical isolates were collected from 318 in-patients with smear-positive TB between October 2008 and September 2016 at a major hospital in Zunyi, Guizhou Province of China, and used for drug susceptibility testing against four first-line anti-TB drugs. Genomic DNA extracted from clinical isolates was used for PCR amplification and DNA sequencing of the pncA gene.
Results
Among 318 clinical isolates, 129 (40.6%), 170 (53.5%), 66 (20.8%) and 109 (34.3%) were resistant to rifampicin, isoniazid, ethambutol and streptomycin respectively. In addition, 124 clinical isolates were MDR-TB and 71.8% of them were previously treated cases. Sequencing results showed that 46.8% of MDR-TB and 2.2% of drug susceptible isolates harbored a pncA mutation, and 52 types of pncA mutations were detected from 64 isolates. The prevalence of pncA mutations in isolates resistant to first-line anti-TB drugs and previously treated TB cases was significantly higher than that in drug-susceptible isolates and new cases of TB.
Conclusion
High prevalence of pncA mutations in clinical isolates of M. tuberculosis from Zunyi, Guizhou Province of China, is correlated with resistance to four first-line anti-TB drugs, MDR-TB and previously treated TB cases.
Introduction
Tuberculosis (TB), a chronic infectious disease, caused about 1.6 million deaths globally in 2017, and China is one of the high-burden countries for multidrug-resistant tuberculosis (MDR-TB).Citation1 The recommended treatment for drug-susceptible TB is a six-month first-line regimen including two months of isoniazid, rifampicin, pyrazinamide and ethambutol followed by four months of isoniazid and rifampicin.Citation1 Pyrazinamide (PZA or Z) is also one of the antituberculosis drugs used for the shorter MDR-TB treatment regimen with sterilizing and bactericidal activity. Pyrazinamide, a synthetic nicotinamide analog, has to be converted from a pro-drug to its active form pyrazinoic acid (POA) in the cytoplasm by the nicotinamidase/pyrazinamidase (PZase). Both PZA and POA have been shown to have different enzyme targets interfering with diverse biochemical pathways involved in the mycobacterial energy metabolism, lipid synthesis and membrane transport.Citation2
Previous studies have shown that PZA resistance in Mycobacterium tuberculosis is mainly caused by various mutations in the PZase coding gene, pncA, or its promoter region leading to a decrease/loss of PZase activity or reduced expression of pncA gene.Citation3 Drug susceptibility testing (DST) against PZA is not routinely performed in most resource-limited regions due to its complexity, inconsistency and high cost. Therefore, an alternative pncA gene-sequencing method was developed to detect mutations in the pncA gene and to rapidly screen for PZA susceptibility with 90.9% of sensitivity and 100% of specificity.Citation4 Currently, DNA sequencing of the pncA gene is the proposed reference method for DST against PZA because there is no WHO recommended rapid method.Citation5 In a systematic review of mutations reported in 61 studies, 641 unique mutations in 171 out of 187 codons of the pncA gene (561 bp) and its promoter region from 2,760 PZA-resistant isolates and 96 mutations from 3,329 PZA-susceptible isolates were recognized.Citation3 In another study, more than 300 mutations were identified through in vitro saturating mutagenesis of the pncA gene which mapped throughout the entire pncA coding region and conferred resistance to PZA.Citation6
It was reported from a multicountry surveillance project involving the detection of pncA mutations that PZA resistance was significantly associated with rifampicin-resistant TB (RR-TB) cases.Citation7 Other studies showed that 74% of PZA-resistant TB isolates from Pakistan, more than half of MDR/RR-TB isolates from Sub-Sahara Africa countries and Georgia, and 37.5% of PZA-resistant TB isolates from Yunnan, China, had a mutation in the pncA geneCitation8–Citation12 However, it was unclear whether similar correlations could be observed in clinical isolates of M. tuberculosis from Zunyi, China. The aim of this study was to determine the correlation between pncA gene mutations and resistance to four first-line anti-TB drugs, MDR-TB as well as treatment history in clinical isolates of M. tuberculosis from a major hospital in Zunyi, Guizhou Province of China.
Patients And Methods
Patients And Clinical Isolates Of M. tuberculosis
This study was conducted at the Affiliated Hospital of Zunyi Medical University, a tertiary general hospital in Zunyi, Guizhou Province of China. A total of 318 in-patients with smear-positive TB were registered at the Tuberculosis Division of Respiratory and Critical Care Medicine of the hospital. Clinical isolates of M. tuberculosis were collected from the sputum and bronchoscope brush specimens of 318 TB patients as a part of routing hospital laboratory procedures in a period from October 2008 to September 2016. These clinical isolates were not specifically collected for this study; however, patient identifiers were removed from clinical isolates prior to the initiation of this study. Collected clinical specimens were cultured on Löwenstein–Jensen solid slants by following the procedures recommended by WHOCitation13 and grown colonies were identified to the species level using 2-thiophene carboxylic acid and para-nitrobenzoic acid selective media.
Drug Susceptibility Testing
Drug susceptibility testing (DST) of M. tuberculosis clinical isolates was conducted at the Laboratory of Respiratory Medicine in the hospital, which was certified by the Chinese Center for Disease Control and Prevention (CCDC). The proportion method on Löwenstein–Jensen solid slants was used for DST against four first-line anti-TB drugs purchased from Sigma-Aldrich (St. Louis, MO, USA). The critical drug concentrations were: 40 μg/mL of rifampicin (RIF), 0.2 μg/mL of isoniazid (INH), 4 μg/mL of streptomycin (SM), and 2 μg/mL of ethambutol (EMB). The standard M. tuberculosis strain H37Rv was obtained from the China CDC and used as a control for all the tests. The critical proportion of four first-line anti-TB drugs was one percent and multidrug resistance was defined as resistance to both RIF and INH.
DNA Extraction And PCR Amplification
Genomic DNAs were extracted from 318 clinical isolates of M. tuberculosis and the standard H37Rv strain using the cetyltrimethylammonium bromide (CTAB) method. Two oligonucleotide primers, pncA-F (5ʹ-GCTGGTCATGTTCGCGATCG-3ʹ) and pncA-R (5ʹ-GCTTGCGGCGAGCGCTCCA-3ʹ), were designed by using the Web Primer website (http://seq.yeastgenome.org/cgi-bin/web-primer), and used for PCR amplification of the pncA gene with purified genomic DNA as a template. The PCR reaction mixture (25 μL) contained 12.5 μL of 2×Taq Master Mix (Beijing TIANGEN Biotech Co., Ltd., China), 0.5 μL of DNA template, and 0.5 μL of each primer pair at a concentration of 20 mM. The thermal cycling conditions were 5 min at 94°C for denaturation followed by 30 cycles of 94°C for 1 min for denaturation, 60°C for 1 min for annealing, and 72°C for 1 min for amplification; and a final extension of 10 min at 70°C. The amplified PCR products (719bp, genome sequences from 2289345 to 2288626) were verified by agarose gel electrophoresis and sent to Shanghai Invitrogen for sequencing using primers pncA-F and pncA-R. The sequencing results were analyzed and mutations in the pncA gene were identified by aligning them with the wild-type pncA gene (GeneID: 887497) of the reference strain H37Rv using the BLAST (bl2seq) program at the NCBI website (https://blast.ncbi.nlm.nih.gov/Blast.cgi).
Data Analysis
The chi-square (χ2) test was used to evaluate the correlation between pncA gene mutations in clinical isolates of M. tuberculosis and resistance to each of the first-line anti-TB drugs as well as treatment history of TB patients. Differences with a P value less than 0.05 were determined to be statistically significant.
Results
Demographic Information About TB Patients
M. tuberculosis clinical isolates were collected consecutively from patients with active pulmonary TB at the hospital between October 2008 and September 2016. Among 318 TB patients, 196 (61.6%) were male and 122 (38.4%) were female; 187 (58.8%) were new cases and 131 (41.2%) were previously treated cases. Patients were divided into three age groups: <35 years (104, 32.7%), 35–55 years (104, 32.7%) and >55 years (110, 34.6%). The average age of patients was 49.3 years, the youngest patient was 13 years old and the oldest patient was 95 years old ().
Table 1 Demographic Characteristics And Treatment History Of 318 TB Patients
Drug Susceptibility Testing Against First-Line Anti-TB Drugs
M. tuberculosis clinical isolates were analyzed for their drug susceptibility profiles against four first-line anti-TB drugs (RIF, INH, EMB, and SM). Among 318 collected clinical isolates, 129 (40.6%), 170 (53.5%), 66 (20.8%) and 109 (34.3%) were resistant to RIF, INH, EMB and SM respectively; 124 (39%) were identified as MDR-TB; 58 (18.2%) were mono-resistant/poly-drug resistant TB (MR/PDR-TB); and 136 clinical isolates (42.8%) were determined to be pan-susceptible to four first-line anti-TB drugs (Pan-S-TB) (). In addition, 35 out of 124 MDR-TB cases (28.2%), 43 out of 58 MR/PDR-TB cases (74.1%) and 109 out of 136 Pan-S-TB cases (80.1%) were new cases. Whereas, 89 out of 124 MDR-TB cases (71.8%), 15 out of 58 MR/PDR-TB cases (25.9%) and 27 out of 136 Pan-S-TB cases (19.9%) were previously treated cases ().
Detection Of pncA Mutations In M. tuberculosis Clinical Isolates
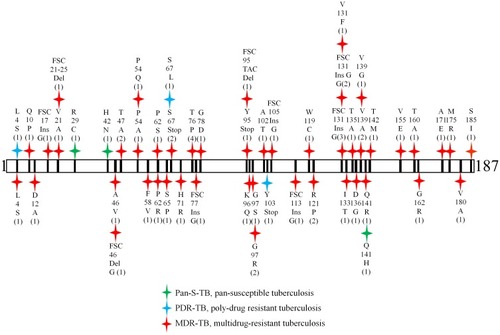
A total of 136 Pan-S-TB, 124 MDR-TB and 58 MR/PDR-TB isolates were used for DNA extraction and sequencing of the pncA gene (). Two primers, pncA-F and pncA-R, were designed to amplify a genomic DNA fragment covering the entire pncA gene plus extra 104 bp at the 5ʹ end and extra 54 bp at the 3ʹ end. Mutations in the pncA gene should not cause possible PCR negatives because both primers were located outside of the pncA coding region. The same primer set was also used for sequencing of the amplified PCR products (719bp). Analysis of sequencing results showed that 64 out of 318 clinical isolates (20.1%), including 58 MDR, three PDR and three Pan-S TB isolates had a mutation in the pncA gene (). Among 64 clinical isolates, 52 different types of pncA mutations were detected which included point mutations, insertions and deletions. The codon 131 was mutated most frequently (6 out of 64 isolates, five insertions and one point mutation Lys131Phe) followed by codon 76 with 4 isolates and codon 67 with three isolates ( and ).
Table 2 pncA Gene Mutations Detected In 64 Clinical Isolates Of M. tuberculosis
Figure 1 Distribution, mutant type and frequency of 64 pncA mutations detected in this study. Numbers are amino acid positions in the pncA coding region and numbers in brackets represent the number of isolates with the same mutation. Red symbol, MDR-TB; blue symbol, PDR-TB; and green symbol, Pan-S-TB.
Prevalence Of pncA Mutations And Resistance To First-Line Anti-TB Drugs And Treatment History
Among 124 MDR-TB isolates, 58 (46.8%) harbored a mutation in the pncA gene, whereas, only three out of 136 Pan-S-TB isolates (2.2%) had a mutation in the pncA gene and the difference was statistically significant (p<0.01) (). The prevalence of pncA mutations in isolates resistant to four first-line anti-TB drugs was higher than that in drug-susceptible isolates and the differences were also statistically significant (p<0.01): 46.5% verse 2.1% for RIF, 34.1% verse 4.1% for INH, 42.4% verse 14% for EMB, and 40.4% verse 9.1% for SM (). When treatment history of TB patients was considered, the prevalence of pncA mutations in previously treated TB cases (36.2%) was much higher than that in the new cases of TB (9%) and the difference was statistically significant (p<0.01) ().
Table 3 The Prevalence Of pncA Mutations In Drug-Resistant And Drug-Susceptible Isolates Of M. tuberculosis, And In Isolates From New And Previously Treated TB Cases
Discussion
Pyrazinamide is one of the major anti-TB drugs used for both the first- and second-line regimens, which plays an important role in reducing the treatment duration for drug-susceptible and drug-resistant TB.Citation1 Drug susceptibility testing against PZA is not routinely performed, and mutations in the pncA gene or the pncA promoter region have been proved to be the main molecular mechanism causing PZA resistance in clinical isolates of M. tuberculosis.Citation3 Therefore, detection of mutations in the pncA gene by DNA sequencing is the proposed reference method for rapid screening of PZA susceptibility.Citation5,Citation6 Results from a large multicenter study assessing pncA sequence variations in 1,950 clinical isolates showed that 888 (45.5%) isolates harbored 280 mutations in the pncA gene.Citation14
During the current study, M. tuberculosis clinical isolates were collected from 318 TB patients and the proportion of male to female patients was 61.6% to 38.4%, which was consistent with our previous study for male (62.8%) and female (37.2%) patients.Citation15 However, the proportion of previously treated cases in this study (41.2%) was higher than that in our previous study (31%),Citation15 probably because only in-patients were enrolled in this study. For the same reason, rates of MDR-TB in new (18.7%, 35/187) and previously treated TB cases (69.7%, 89/131) were higher than those in our previous studiesCitation15 and combined data from 53 Member States in the WHO European Region (15.7% of new and 45.3% of previously treated cases of TB were MDR-TB).Citation16 Most patients from this study (65.4%) were 55 years of age or younger and the average age of patients was 49.3 years ().
Our PCR and sequencing results showed that the prevalence of pncA mutations in clinical isolates resistant to each of the four first-line anti-TB drugs (RIF, INH, EMB and SM), MDR-TB and previously treated TB cases were significantly higher than those in drug-susceptible isolates and new cases of TB (). The prevalence of pncA mutations in MDR-TB isolates (46.8%) collected from Zunyi, Guizhou Province, is lower than that in MDR-TB isolates collected from the neighboring Chongqing Province (57.9%, 77/133),Citation17 but higher than those in MDR-TB isolates collected from Yunnan Province (21.4%, 6/28)Citation12 and Zhejiang Province (35.4%, 97/274)Citation18 of China, supporting the suggestion that pncA mutations may differ from one geographic region to another based on studies from different regions.Citation17
To compare the prevalence of pncA mutations in MDR/RR, new, previously treated and total cases of TB in different settings around the world, we listed in the published data from 32 countries in six WHO regions (Africa, Americas, Eastern Mediterranean, Europe, South-East Asia and Western Pacific). It was obvious that the prevalence of pncA mutations in M. tuberculosis isolates from different countries and different regions were quite different, consistent with the observation that levels of PZA resistance varied substantially among different settings.Citation7 For example, the prevalence of pncA mutations in MDR/RR (85%) and total cases of TB (57.9%) from GeorgeCitation11 was much higher than that in MDR/RR cases from Turkey (25%)Citation28 and in total cases of TB from Azerbaijan (12.6%),Citation7 even though these three countries were located in the same European region (). The prevalence of pncA mutations among MDR-TB cases from 32 different countries in six WHO regions varied widely ranging from 21.4% in Yunnan Province of ChinaCitation12 and 39.5% in PakistanCitation7 to 81.3% in BelarusCitation7 and 87.8% in Republic of Korea.Citation38 In addition, the prevalence of pncA mutations among previously treated TB cases from six WHO regions also varied extensively ranging from 4.7% in South Africa,Citation7 8.9% in PakistanCitation7 and 13.8% in BangladeshCitation7 to 36.2% in Zunyi, China, 66.7% in RwandaCitation10 and 70.8% in George.Citation11
Table 4 The Prevalence Of pncA Mutations In M. tuberculosis Isolates From Six Different WHO Regions And 32 Countries Of The World
Sequencing results also showed that 52 different types of pncA mutations were detected from 64 clinical isolates of M. tuberculosis, mostly from MDR-TB isolates, but three from PDR-TB and three from Pan-S-TB isolates (). The distribution, type, and frequency of 64 pncA mutations detected in this study were revealed in . Five mutations were new and deposited in GenBank with accession numbers KR534845 (Ser67Stop), KR534846 (Tyr95del), KR534847 (Ser67Leu), KR534848 (Gly105FSC), and KR534849 (Gly113FSC). Based on a multicenter study, Miotto et al divided 280 genetic variants in pncA into four classes (i, ii, iii and iv), and class (i) mutations were very high confidence resistance ones that were found only in PZA-resistant strains.Citation14 Among 52 mutation types (), 19 belonged in class (i), 2 belonged in class (ii) and 3 belonged in class (iii). Through comparison with the data published by the CRyPTIC consortium on whole-genome analyses of 10,209 isolates,Citation40 we found that 25 mutations detected in MDR/PDR-TB isolates and two of three mutations detected in Pan-S isolates (R29C and H42D) fell in the “R” category, and one MDR (S65P) and one Pan-S isolates (Q141H) fell in the “S” category.
It was previously found in a systematic review of mutations reported in 61 studies that 79% of 2,760 PZA-resistant and 9% of 3,329 PZA-susceptible isolates had a mutation in the pncA gene,Citation3 suggesting that M. tuberculosis clinical isolates with a mutation in the pncA gene be more likely resistant to PZA than those without a pncA mutation. The comparison results also indicated the existence of geographical diversity of prevalence of pncA mutations among M. tuberculosis clinical isolates depending on where they were collected. Therefore, we should consider geographical diversity as an important factor when we select screening of pncA mutations as a simple method for quick diagnosis of PZA resistance in M. tuberculosis isolates, and decide whether PZA should be included in the optimized treatment regimens for TB patients, particularly those from countries with high prevalence of pncA mutations in MDR/RR and previously treated TB cases.
Limitations of this study included that we only collected smear-positive specimens to make sure the sputum specimens to be positive of bacteria at the time of collection and to grow enough bacteria for DST and DNA extraction. If we had also collected smear-negative specimens for this study, the overall prevalence of pncA mutations would have been lower but should not affect the conclusion of this study. Another limitation was that culture-based phenotypic testing for PZA was not performed on clinical isolates of M. tuberculosis due to its technical difficulties.
Conclusion
Results from this study show that high prevalence of pncA mutations in clinical isolates of M. tuberculosis from Zunyi, Guizhou Province of China, are correlated with resistance to each of the four first-line anti-TB drugs, MDR-TB and previously treated TB cases.
Author Contributions
ZC, YL, MX and ZP carried out the experiments. All authors contributed to data analysis, drafting and revising the manuscript, agreed to be accountable for all aspects of the work, and approved the final version of the manuscript.
Acknowledgments
We would like to thank the clinical/laboratory staff and graduate students from the Tuberculosis Division of Respiratory and Critical Care Medicine, Affiliated Hospital of Zunyi Medical University, for their assistance in collecting clinical data and specimens from patients, and culture of M. tuberculosis clinical isolates for drug susceptibility testing during this study. This work was supported by the National Natural Science Foundation of China (NSFC Nos. 81160003, 81360002 and 81760003). The sponsor played no role in the collection, analysis, and interpretation of data, in writing the manuscript, and in the decision to submit the article for publication.
Disclosure
HZ is employed by and has shares in Z-BioMed, Inc., which is involved in infectious disease research. The authors report no other conflicts of interest in this work.
References
- World Health Organization. Global tuberculosis report. 2018 Available from: http://www.who.int/tb/publications/global_report/en;2018. Accessed 78, 2019.
- Stehr M, Elamin AA, Singh M. Pyrazinamide: the importance of uncovering the mechanisms of action in mycobacteria. Expert Rev Anti Infect Ther. 2015;13:593–603. doi:10.1586/14787210.2015.102178425746054
- Ramirez-Busby SM, Valafar F. Systematic review of mutations in pyrazinamidase associated with pyrazinamide resistance in Mycobacterium tuberculosis clinical isolates. Antimicrob Agents Chemother. 2015;59:5267–5277. doi:10.1128/AAC.00204-1526077261
- Streicher EM, Maharaj K, York T, et al. Rapid sequencing of the Mycobacterium tuberculosis pncA gene for detection of pyrazinamide susceptibility. J Clin Microbiol. 2014;52:4056–4057. doi:10.1128/JCM.02438-1425165081
- World Health Organization. Technical manual for drug susceptibility testing of medicines used in the treatment of tuberculosis. 2018 Available from: http://apps.who.int/medicinedocs/documents/s23565en/s23565en.pdf. Accessed 78, 2019.
- Yadon AN, Maharaj K, Adamson JH, et al. A comprehensive characterization of PncA polymorphisms that confer resistance to pyrazinamide. Nat Commun. 2017;8:588. doi:10.1038/s41467-017-00721-228928454
- Zignol M, Dean AS, Alikhanova N, et al. Population-based resistance of Mycobacterium tuberculosis isolates to pyrazinamide and fluoroquinolones: results from a multicountry surveillance project. Lancet Infect Dis. 2016;16:1185–1192. doi:10.1016/S1473-3099(16)30190-627397590
- Khan MT, Malik SI, Ali S, et al. Pyrazinamide resistance and mutations in pncA among isolates of Mycobacterium tuberculosis from Khyber Pakhtunkhwa, Pakistan. BMC Infect Dis. 2019;19:116. doi:10.1186/s12879-019-3764-230728001
- Daneau G, Gumusboga M, De Rijk P, et al. The majority of patients with multidrug-resistant tuberculosis in Sub-Saharan Africa present a concomitant resistance to pyrazinamide. Int J Mycobacteriol. 2016;(Suppl 1):S46–S47. doi:10.1016/j.ijmyco.2016.10.01528043604
- Ngabonziza JCS, Diallo AB, Tagliani E, et al. Half of rifampicin-resistant Mycobacterium tuberculosis complex isolated from tuberculosis patients in Sub-Saharan Africa have concomitant resistance to pyrazinamide. PLoS One. 2017;12:e0187211. doi:10.1371/journal.pone.018721129088294
- Sengstake S, Bergval IL, Schuitema AR, et al. Pyrazinamide resistance-conferring mutations in pncA and the transmission of multidrug resistant TB in Georgia. BMC Infect Dis. 2017;17:491. doi:10.1186/s12879-017-2594-328697808
- Li D, Song Y, Zhang CL, Li X, Xia X, Zhang AM. Screening mutations in drug-resistant Mycobacterium tuberculosis strains in Yunnan, China. J Infect Public Health. 2017;10:630–636. doi:10.1016/j.jiph.2017.04.00828623123
- World Health Organization. Laboratory Services in Tuberculosis Control. Part III: Culture. Geneva: World Health Organization; 1998 Available from: http://apps.who.int/iris/bitstream/handle/10665/65942/WHO_TB_98.258_(part3).pdf;jsessionid=64EF54513AD4144A520B24B4E3DFCBA5?sequence=3. Accessed 78, 2019.
- Miotto P, Cabibbe AM, Feuerriegel S, et al. Mycobacterium tuberculosis pyrazinamide resistance determinants: a multicenter study. MBio. 2014;5:e01819–14. doi:10.1128/mBio.01819-1425336456
- Lan Y, Li Y, Chen L, Zhang J, Zhang H. Drug resistance profiles and trends in drug-resistant tuberculosis at a major hospital in Guizhou Province of China. Infect Drug Resist. 2019;12:211–219. doi:10.2147/IDR.S18853830666136
- Zignol M, Dara M, Dean AS, et al. Drug-resistant tuberculosis in the WHO European Region: an analysis of surveillance data. Drug Resist Updat. 2013;16:108–115. doi:10.1016/j.drup.2014.02.00324631052
- Pang Y, Zhu D, Zheng H, et al. Prevalence and molecular characterization of pyrazinamide resistance among multidrug-resistant Mycobacterium tuberculosis isolates from Southern China. BMC Infect Dis. 2017;17:711. doi:10.1186/s12879-017-2761-629110640
- Xia Q, Zhao LL, Li F, et al. Phenotypic and genotypic characterization of pyrazinamide resistance among multidrug-resistant Mycobacterium tuberculosis isolates in Zhejiang, China. Antimicrob Agents Chemother. 2015;59:1690–1695. doi:10.1128/AAC.04541-1425583712
- Bhuju S, Fonseca Lde S, Marsico AG, et al. Mycobacterium tuberculosis isolates from Rio de Janeiro reveal unusually low correlationbetween pyrazinamide resistance and mutations in the pncA gene. Infect Genet Evol. 2013;19:1–6. doi:10.1016/j.meegid.2013.06.00823770140
- Alexander DC, Ma JH, Guthrie JL, Blair J, Chedore P, Jamieson FB. Gene sequencing for routine verification of pyrazinamide resistance in Mycobacterium tuberculosis: a role for pncA but not rpsA. J Clin Microbiol. 2012;50:3726–3728. doi:10.1128/JCM.00620-1222895038
- Cuevas-Córdoba B, Xochihua-González SO, Cuellar A, Fuentes-Dominguez J, Zenteno-Cuevas R. Characterization of pncA gene mutations in pyrazinamide-resistant Mycobacterium tuberculosis isolates from Mexico. Infect Genet Evol. 2013;19:330–334. doi:10.1016/j.meegid.2012.12.01323321280
- Sheen P, Requena D, Gushiken E, et al. A multiple genome analysis of Mycobacterium tuberculosis reveals specific novel genes and mutations associated with pyrazinamide resistance. BMC Genomics. 2017;18:769. doi:10.1186/s12864-017-4146-z29020922
- Kahbazi M, Sarmadian H, Ahmadi A, et al. Novel mutations in pncA gene of pyrazinamide resistant clinical isolates of Mycobacterium tuberculosis. Sci Pharm. 2018;86:pii:15. doi:10.3390/scipharm86020015
- Andres S, Gröschel MI, Hillemann D, Merker M, Niemann S, Kranzer K. A diagnostic algorithm to investigate pyrazinamide and ethambutol resistance in rifampin-resistant Mycobacterium tuberculosis isolates in a low-incidence setting. Antimicrob Agents Chemother. 2019;63:e01798–18. doi:10.1128/AAC.01798-1830455227
- Akhmetova A, Kozhamkulov U, Bismilda V, et al. Mutations in the pncA and rpsA genes among 77 Mycobacterium tuberculosis isolates in Kazakhstan. Int J Tuberc Lung Dis. 2015;19:179–184. doi:10.5588/ijtld.14.030525574916
- Marttila HJ, Marjamäki M, Vyshnevskaya E, et al. pncA mutations in pyrazinamide-resistant Mycobacterium tuberculosis isolates from northwestern Russia. Antimicrob Agents Chemother. 1999;43:1764–1766.10390239
- Juréen P, Werngren J, Toro JC, Hoffner S. Pyrazinamide resistance and pncA gene mutations in Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2008;52:1852–1854. doi:10.1128/AAC.00110-0818316515
- Yüksel P, Tansel O. Characterization of pncA mutations of pyrazinamide-resistant Mycobacterium tuberculosis in Turkey. New Microbiol. 2009;32:153–158.19579692
- Daum LT, Konstantynovska OS, Solodiankin OS, et al. Characterization of novel Mycobacterium tuberculosis pncA gene mutations in clinical isolates from the Ukraine. Diagn Microbiol Infect Dis. 2019;93:334–338. doi:10.1016/j.diagmicrobio.2018.10.01830583880
- Muthaiah M, Jagadeesan S, Ayalusamy N, et al. Molecular epidemiological study of pyrazinamide-resistance in clinical isolates of Mycobacterium tuberculosis from South India. Int J Mol Sci. 2010;11:2670–2680. doi:10.3390/ijms1107267020717529
- Chaidir L, Ruesen C, Dutilh BE, et al. Use of whole-genome sequencing to predict Mycobacterium tuberculosis drug resistance in Indonesia. J Glob Antimicrob Resist. 2019;16:170–177. doi:10.1016/j.jgar.2018.08.01830172045
- Aung WW, Ei PW, Nyunt WW, et al. Pyrazinamide resistance among multidrug-resistant Mycobacterium tuberculosis clinical isolates in Myanmar. Antimicrob Agents Chemother. 2018;62:e01984–17. doi:10.1128/AAC.01984-1729263056
- Jonmalung J, Prammananan T, Leechawengwongs M, Chaiprasert A. Surveillance of pyrazinamide susceptibility among multidrug-resistant Mycobacterium tuberculosis isolates from Siriraj Hospital, Thailand. BMC Microbiol. 2010;10:223. doi:10.1186/1471-2180-10-22320727143
- Gu Y, Yu X, Jiang G, et al. Pyrazinamide resistance among multidrug-resistant tuberculosis clinical isolates in a nationalreferral center of China and its correlations with pncA, rpsA, and panD gene mutations. Diagn Microbiol Infect Dis. 2016;84:207–211. doi:10.1016/j.diagmicrobio.2015.10.01726775806
- Li D, Hu Y, Werngren J, et al. Multicenter study of the emergence and genetic characteristics of pyrazinamide-resistant tuberculosis in China. Antimicrob Agents Chemother. 2016;60:5159–5166. doi:10.1128/AAC.02687-1527297481
- Cui Z, Wang J, Lu J, Huang X, Zheng R, Hu Z. Evaluation of methods for testing the susceptibility of clinical Mycobacterium tuberculosis isolates to pyrazinamide. J Clin Microbiol. 2013;51:1374–1380. doi:10.1128/JCM.03197-1223390285
- Ando H, Mitarai S, Kondo Y, et al. Pyrazinamide resistance in multidrug-resistant Mycobacterium tuberculosis isolates in Japan. Clin Microbiol Infect. 2010;16:1164–1168. doi:10.1111/j.1469-0691.2009.03078.x19832709
- Jnawali HN, Hwang SC, Park YK, et al. Characterization of mutations in multi- and extensive drug resistance among strains of Mycobacterium tuberculosis clinical isolates in Republic of Korea. Diagn Microbiol Infect Dis. 2013;76:187–196. doi:10.1016/j.diagmicrobio.2013.02.03523561273
- Huy NQ, Lucie C, Hoa TTT, et al. Molecular analysis of pyrazinamide resistance in Mycobacterium tuberculosis in Vietnam highlights the high rate of pyrazinamide resistance-associated mutations in clinical isolates. Emerg Microbes Infect. 2017;6:e86. doi:10.1038/emi.2017.7329018250
- CRyPTIC Consortium and the 100,000 Genomes Project, et al. Prediction of susceptibility to first-line tuberculosis drugs by DNA sequencing. N Engl J Med. 2018;379:1403–1415. doi:10.1056/NEJMoa180047430280646
