Abstract
Purpose
This study was conducted to investigate antibody immune responses induced by BNT162b2 and AZD1222 human COVID-19 vaccines in Riyadh city, Saudi Arabia.
Patients and Methods
ELISA was used to evaluate antibodies, against the SARS-CoV-2 spike S1 protein, in serum samples from 432 vaccinated individuals at six time points: pre-vaccination (baseline), post-prime, post-boost, 6-months, and 1 year post-vaccination, and 3 weeks post a third dose. Virus microneutralization assay was used to confirm antibody responses in a subset of samples.
Results
Anti-SARS-CoV-2 spike IgG were detected in most subjects post-prime, reached a peak level post-boost, and remained at high level at the 6-month follow-up. At 1 year post-vaccine, the antibody levels were low but increased to a significant level higher than the peak following a third dose. The third dose was given at an average of 250 days after the second dose. The virus microneutralization assay confirmed the neutralization activity of the induced SARS-CoV-2 IgG antibodies. The vaccines induced higher IgG titres at post-prime (p=0.0001) and 6 months (p=0.006) in previously infected individuals. An increased interval between prime and boost, more than recommended time, appeared to enhance the IgG levels (p=0004). Moreover, the vaccines induced higher IgG levels in younger subjects (p=0.01).
Conclusion
These data provide insights and build on the current understanding of immune responses induced by these two vaccines; and support a third boosting dose for these COVID-19 vaccines.
Introduction
The severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) is a virus that caused the coronavirus disease 2019 (COVID-19) pandemic.Citation1,Citation2 Infection with this coronavirus was first reported on December 31, 2019 in Wuhan, China.Citation2,Citation3 The World Health Organization (WHO) had reported more than 517 million infected individuals and more than 6 million deaths in the world as of May 16, 2022.Citation4 There are currently a number of approved COVID-19 vaccines that have been developed using different biotechnological platforms and are available worldwide. These vaccines can vary in terms of side-effects, immunogenicity, efficacy, and duration of protection.Citation5
The Pfizer-BioNTech COVID-19 vaccine (BNT162b2) is based on nanoparticles encapsulated mRNA; it was approved for emergency use in adults and adolescentsCitation6 by the US Food and Drug Administration (FDA) on December 11, 2020.Citation7 The vaccine should be administered as two doses with a 21-day interval,Citation8 and has high efficacy of 95% against symptomatic COVID-19 in adults.Citation9 Although reports of an allergic reaction (anaphylaxis) after receiving the first dose of the vaccine have emerged, the vaccine's adverse events were acceptable and similar to those following previously implemented vaccines.Citation10
The Oxford-AstraZeneca COVID-19 vaccine (AZD1222) is a chimpanzee adenoviral vectored vaccine, which has been developed by Oxford University in collaboration with AstraZeneca.Citation5 Reports of safety and efficacy of the vaccine showed an acceptable safety profile in adults aged 18 years and older, with an efficacy of 70.4% against symptomatic COVID-19.Citation11 Thromboembolic events have been initially reported following the use of AZD1222 vaccineCitation12,Citation13 although this has also been reported for mRNA based COVID-19 vaccines.Citation14
These COVID-19 vaccines, in addition to others, are recommended by the WHO and other health agencies in order to provide active acquired immunity against SARS-CoV-2 and control the COVID-19 pandemic by blocking the virus transmission and reducing the number of symptomatic cases.Citation15 They induce immune responses against SARS-CoV-2, leading to protection from COVID-19 pathology and clinical manifestations.Citation16 Immune responses measured post-vaccination are mainly serum IgG antibodies (Ab) and vaccine-specific effector T cells, indicative of humoral and cellular immune responses, respectively.Citation17 IgG antibodies were induced to high levels post-COVID-19 vaccines although there is no defined level as a correlate of protection.Citation18
Several studies have reported immune responses post-COVID-19 vaccination; a large cohort study of 4,868 subjects was performed to measure the levels of IgG and neutralizing antibodies at 6 months post the second dose of BNT162b2 vaccine. The levels of IgG and neutralizing antibodies decreased within the six-month follow-up by 18-times (Ratio of antibodies was 29.3 at the peak and 1.6 at 6 months). IgG and neutralizing antibody levels were lower among males, older age, and participants with immunosuppression during the peak time, which is 4–30 days post-vaccination.Citation19 A significant increase in IgG responses to the BNT162b2 vaccine after the second dose followed by a decrease at 6-months post-vaccination were reported in another study of 122 participants. Older age was found to correlate with lower responses to COVID-19 vaccines and fewer side-effects among vaccinated individuals.Citation20 Pre-existing immunity from previous natural SARS-CoV-2 infections contribute to higher levels of IgG antibodies than immune responses in vaccinated subjects who did not acquire the infection prior to vaccination.Citation21 This was significant and much evident after one dose of either BNT162b2 or AZ1222. This observation was not related to gender and was mainly due to pre-existing immunity that was boosted by single doses.Citation21
Therefore, this study was conducted in order to explore IgG antibody responses following BNT162b2 and AZD1222 vaccines in a cohort in Saudi Arabia. Here, serum antibody levels were measured in a group of vaccinated individuals up to a year post-vaccination. We report IgG antibody levels in relation to the time points: pre-vaccination (baseline) or post-vaccination (post-prime, post-boost, 6-months and 1-year post-boost, and post-third dose). Antibody responses in relation to vaccine type, interval between prime and boost vaccination, age, gender, chronic diseases or co-morbidities, and prior COVID-19 infection were evaluated. This study provides real-world data that could add to clinical trial data of the two vaccines. It also provides understanding of the vaccine induced immune responses to better control the COVID-19 pandemic.
Materials and Methods
Samples and Data Collection
In this study, clinical data were collected from vaccinated subjects who received either BNT162b2 or AZD1222 vaccines as one or two doses, by reviewing their medical records at the King Abdulaziz Medical City (KAMC), Ministry of National Guard – Health Affairs (MNG-HA). Blood samples were collected at baseline (pre-vaccination). Further blood samples were collected from January 2021 through January 2022 at the following time-points: 3 weeks post-prime (for BNT162b2) or 1-month post-prime (for AZD1222), 1-month post-boost (for both vaccines), 6 months and 1-year post-boost, and 3 weeks post a third dose of BNT162b2. The blood samples were left in 4°C for at least 1 hour to clot before being spun, and sera were isolated and stored in −80°C freezers until the day of testing. The total number of samples collected was 666 (from 432 vaccinated individuals, ). The availability of each sample at each time point is shown in Supplementary Table S1. It should be noticed though that not all of the individuals were available for sampling at all the time points. Supplementary Table S1 shows the collection of samples from the cohort individuals at each time point. In addition, serum samples (n=119) from non-vaccinated convalescent cases who recovered from COVID-19 3–5 months prior to sample collection and before the implementation of COVID-19 vaccine program were used as comparative controls in this study. Blood samples that were collected from five healthy donors prior to the COVID-19 pandemic were used as ELISA negative control; to calculate endpoint titres of IgG. The inclusion criteria were the reception of either BNT162b2 or AZD1222 COVID-19 vaccines and willing to donate blood and consent for access to clinical data, with no exclusion criteria.
Table 1 Number of Samples Collected from Vaccinated Individuals at Different Time Points
ELISA
Enzyme-linked immunosorbent assay (ELISA) was developed to detect SARS-CoV-2 IgG for serum samples according to previously published protocols.Citation22,Citation23 A recombinant S1 subunit of the SARS-CoV-2 spike protein (Sino Biological, Beijing, China) was used to coat Nunc MaxiSorp 96-well ELISA micro-plates (Thermo Fisher, Waltham, MA) at a concentration of 1 µg/mL and incubated overnight at 4°C. The following day, the plates were washed with washing buffer (phosphate buffered saline (PBS) with 0.5% Tween20, PBS-T) using an automated Micro-plate Washer (Molecular Devices, San Jose, CA) and blocked with blocking buffer (washing buffer containing 10% skimmed milk) at 37°C for 1 hour. Plates were then washed and serum samples diluted in a 3-fold serial dilution starting from 1:100 in PBS-T were added into duplicate wells and incubated for 2 hours. Following a wash, plates were then incubated with alkaline phosphatase labeled goat anti-human IgG secondary antibody (Thermo Fisher, Waltham, MA) at 37°C for 1 hour. After a wash, the plates were incubated with PNPP (pnitrophenylphosphate, sigma) substrate at 37°C for 30 minutes. The optical density (OD) was measured at 405 nm using Micro-plate Reader (Molecular Devices, San Jose, CA). The endpoint titer for each tested serum was determined as the reciprocal value of the serum dilution with OD value converging with the cut-off. The cut-off was determined as the average OD of the negative control sera plus 3 SD as described previously.Citation24,Citation25
SARS-CoV-2 and Microneutralization Assay
SARS-CoV-2 was isolated from a confirmed COVID-19 patient’s nasopharyngeal swab, processed in a BSL-3 facility. The sample was vortexed, spun, and supernatant was filtered through a 0.22 µM filter. Then 200 µl of filtered samples was added to a monolayer of Vero cells in a 25 cm flask. Three days post-inoculation, CPE was observed under light microscope and a 200 µl of supernatant was passaged in 75 cm flask Vero cells for second and third passage. The virus was then amplified in 175 cm flasks (passage 4), then purified by regular centrifugation and filtration in total volume 180 mL. The purified virus from each passage was confirmed by RT-PCR targeting the three genes of SARS-CoV2 using the following primers and probes: RdRP-gene: RdRP_SARSr-F2 5ʹGTGARATGGTCATGTGTGGCGG’3, RdRP_SARSr-R1 5ʹCARATGTTAAASACACTATTAGCATA’3, RdRP_SARSr-P2 FAM CAGGTGGAACCTCATCAGGAGATGC-BBQ, RdRP_SARSr-P1 FAM-CCAGGTGGWACRTCATCMGGTGATGC-BBQ, E-gene: E_Sarbeco_F1 5’ ACAGGTACGTTAATAGTTAATAGCGT’3, E_Sarbeco_R2 5’ ATATTGCAGCAGTACGCACACA’3, E_Sarbeco_P1 FAM-ACACTAGCCATCCTTACTGCGCTTCG-BBQ, N-gene: N_Sarbeco_F1 5’ CACATTGGCACCCGCAATC’3, N_Sarbeco_R1 5’ GAGGAACGAGAAGAGGCTTG’3 & N_Sarbeco_P1 FAM-ACTTCCTCAAGGAACAACATTGCCA-BBQ. Virus titer was determined and a live virus neutralization assay was performed following previously published protocols.Citation26,Citation27 Briefly, heat-inactivated serum samples from vaccinated individuals were tested for their capacity to neutralize the infection of 200 TCID50/mL of SARS-CoV-2 using a 96-well plate. The samples were tested in duplicate wells and the experiments were repeated independently twice for all samples. The NAb titer was determined as the reciprocal of the highest dilution of sample that completely protected Vero cells (MN100).
Statistical Analysis
Data were plotted and analyzed using GraphPad Prism V8 software (GraphPad Software, San Diego, CA). Statistical analysis was performed using Kruskal–Wallis test followed by Dunn’s Multiple Comparison Test for the figures and Student’s t-test ().
Table 2 Demographic and Clinical Factors Influencing Vaccine-Induced Immune Responses
Results
IgG Antibody Responses Induced by COVID-19 Vaccines
Immune responses measured by serum anti-SARS-CoV-2 spike IgG antibodies post-COVID-19 vaccination were evaluated in 666 samples, collected from 432 vaccinated individuals over multiple time-points. COVID-19 was documented by PCR prior to vaccination in 31 individuals; with total samples of 44. Considering only the samples from non-infected individuals (622 samples), anti-spike antibodies were induced in 11% (26 out 217) at the baseline, 93% (199 out of 214 samples) post-prime, and 96% (115 out of 120 samples) post-second dose. IgG responses in convalescent cases (n=119) were included as comparisons (). The Ab levels were maintained in most individuals at 6 months (42 samples), although they waned to the levels observed post-prime (). At 1 year post-second dose, Ab levels in three samples were low while a fourth sample from a subject who is suspected of having exposure to a COVID-19 case showed a high Ab level.
Figure 1 Antibody responses post-COVID-19 vaccines.
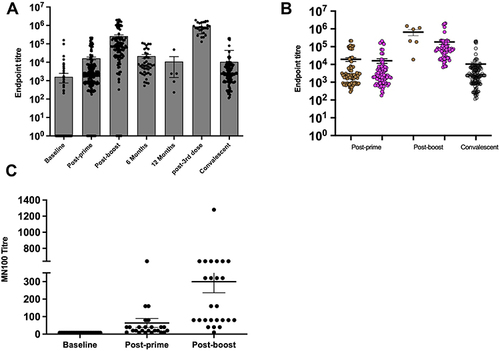
Twenty-five samples from subjects who received their third dose of BNT162b2 at 250 days on average (ranged from 117–339 days) after the second dose of either AZ1222 or BNT162b2 were tested. As expected, Ab levels post-third dose were higher than the peak (1 month post-second dose) as shown in .
To investigate whether vaccine type would impact on the level of immune responses, IgG titres were determined in the non-infected individuals who have received either AZ1222 or BNT162b2. Only samples from individuals who were primed and boosted with the same vaccine were included in this analysis. Post-prime, no significant difference between AZ1222 (n=85) and BNT162b2 (n=83) was observed (p=0.61) as they induced similar levels of antibodies with IgG mean titer of 19,443 for AZ1222 vaccine and 16,145 for BNT162b2 vaccine (). These levels were not statistically different (p=0.3) from those found in non-vaccinated convalescent cases (10,533). However, AZ1222 (n=6) induced a significantly higher level of antibodies post-second dose (p=0.01) as compared to BNT162b2 (n=69) with mean IgG titers of 660,158 and 184,270, respectively (); but the number of samples of AZ1222 vaccinees at this time-point (n=6) was small. Furthermore, IgG titers post-second dose were statistically higher (p<0.0001) than the levels post-prime or the levels in recovered cases ().
In order to confirm anti-SARS-CoV-2 antibodies using a separate assay and to confirm that these are neutralizing antibodies (NAb), matching serum samples collected at three time points (baseline, post-prime, and post-second dose) from 25 individuals were tested in a live virus microneutralization assay. All samples showed NAb titers at post-prime that increased significantly (p<0.0001) at post-boost (). This data indicates a strong correlation between NAb responses and IgG response measured by ELISA.
Impact of Pre-Infection and Interval Duration on Antibody Responses to COVID-19 Vaccines
To evaluate the effect of prior infection on vaccine-induced antibody responses, samples (n=44) from previously infected individuals, who were confirmed by PCR, were compared to samples (n=622) of non-infected individuals. No significant difference in IgG levels was found between the two groups at baseline (p=0.65) and post-second dose (p=0.35), as shown in . In contrast, previously infected individuals had higher IgG titers at post-prime (p=0.0001) and 6 months (p=0.006; ), indicating strong priming and maintenance of immune responses in these individuals.
Figure 2 Impact of pre-infection and interval duration on antibody responses to COVID-19 vaccines.
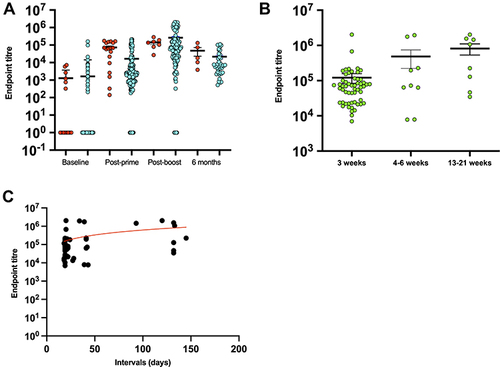
While the guidelines for BNT162b2 COVID-19 vaccination state a 3-week interval between prime and boost, not all vaccinated individuals in our cohort adhered to this interval due to supply issues. Therefore, subjects with no prior COVID-19 who received BNT162b2 vaccine for both the first and second doses (n=69) were divided into three groups according to the interval duration: 3 weeks, 4–6 weeks, and 13–21 weeks. The analysis showed that IgG titers varied significantly in the three groups at post-second dose (p=0004; ), the titers were higher in the longest interval duration. Similarly, there was a hint of correlation between the IgG titer and the length of interval duration where longer intervals slightly contributed to higher titers (Spearman r=0.3; ).
Impact of Age, Gender, and Comorbidities on Antibody Responses to COVID-19 Vaccines
The impact of demographic and clinical factors such as gender, age, and co-morbidities in influencing the immune responses induced by COVID-19 vaccines was analyzed for all subjects regardless of the vaccine type (). Males appeared to have higher IgG titers at post-prime as compared to females, but this did not reach statistical significance (p=0.06). Similarly, age appeared to play a role in immune responses to the vaccines; individuals who were younger than 38.5 years showed an increased level of IgG as compared to those older than 38.5 years (p=0.01). The age 38.5 was selected in this comparison as it represents the median age of the cohort. Clinical factors such as diabetes, hypertension, increased BMI, and asthma did not affect the anti-spike antibody responses post-vaccination in our cohort. Other co-morbidities such as auto-immune diseases were not available for analysis.
Discussion
In this study, the dynamic of antibody responses to COVID-19 vaccines, the AZD1222 and BNT162b2, was evaluated pre-vaccination and up to 1 year post-vaccination. A robust immune response that lasted for 6-months declining to low, but detectable, levels at 1 year was reported. The presence of neutralization activity of these detected antibodies in a subset of individuals was determined, and these samples showed significantly higher NAb titers at post-second dose than at post-prime (p<0.0001). These antibodies confirm the strong neutralization activity of the vaccine-induced immune responses and confirm the utility of the ELISA used in this study.
In a Phase I trial, there were similar robust immune responses at 6 months after the second dose of BNT162b2 vaccineCitation28 that started to decline 3 and 6 months after vaccination. This indicates a weaning immunityCitation20 and potentially increases the risk of breakthrough infections overtime.Citation29 Reduction in antibody titers was reported to occur by 50% every 108 days post-vaccination,Citation30 with weaning of anti-RBD antibody titer over time starting from day 45 post-vaccination.Citation31 Waning immune responses can be a result of vaccine immunogenicity; however, multiple other factors can play roles such as demographics, comorbidities, and induction and maintenance of long-term memory plasma cells or T cells.Citation32–34
The current study showed that the level of antibodies was similar among vaccinated and convalescent patients, especially at prime time. A previous study showed that anti-SARS-CoV-2 antibody levels after two doses were similar to one dose in convalescent patients,Citation20 which is supported by our findings. Recovered individuals developed higher antibody titers with a geometric mean titer (GMT) of 9,461 U/mL as compared to uninfected vaccinated participants who had GMT of 1,613 U/mL (p<0.001)Citation35 although these observations may only apply to neutralizing antibody titers, not the total binding antibodies, especially following a BNT162b2 vaccine.Citation36 The difference in antibody responses might also be affected by memory cell stimulation and severity of the disease. Symptomatic patients had stronger responses to the vaccine than those who had mild COVID-19, becoming seronegative after recovering from the infection.Citation37 The time between infection and vaccination may also play a roleCitation36 and our data demonstrate that longer intervals contribute to higher antibody responses; although larger sample size is required for further evaluation. Extending the dosing intervals between the first two doses of the BNT162b2 vaccine resulted in higher antibody responses as compared to the initial 3-week interval,Citation38–42 and an additional study showed similar findings without an effect on the half-life of the antibody response among the different intervals.Citation43 This difference in response may be related to the generation and improvement in the number of CD4+ T cells expressing interleukin-2 (IL-2).Citation39
Different vaccines may induce different levels of antibody responses. When two mRNA vaccines, mRNA-1273 and BNT162b2, were compared, two doses of mRNA-1273 induced higher antibody titers (GMT, 3,836 U/mL) as compared to two doses of BNT162b2 (GMT of 1,444 U/mL).Citation35 There might also be differences in the type of antibody responses as BNT162b2 and AZ1222 both elicited IgM and IgG,Citation44 BNT162b2 vaccine was reported to induce much evident IgA responses.Citation45,Citation46 In addition, although AZ1222 vaccine elicited lower antibody responses as our data showed, it has been shown that it induces more stable antibody responses over time. This may suggest that priming with different types of COVID-19 vaccine such as viral vectored vaccine, in a heterologous regimen, would be an efficient approach for durable immune response.
There was no significant correlation between demographics and the levels of antibodies in the current study. Males appeared to have higher IgG titers at post-prime as compared to females (p=0.06). In contrast, a previous study showed that males had much lower titers as compared to females 6 months post-vaccination.Citation19 In this study, subjects who were older than 38.5 years showed a higher level of IgG responses at post-prime as compared to younger subjects (p=0.01); the age cut-off was selected based on the median age of the current cohort. A significantly inverse relation between antibody responses and age was previously reported, and age was the biggest predictor of immune responses.Citation20,Citation47–52 However, this does not mean lack of antibodies as it was previously shown that anti-SARS-CoV-2 spike antibodies could be detected in individuals older than 80 years old after vaccination with BNT162b2 or AZ1222 vaccines.Citation53
There was no clear relationship between antibody levels and comorbidities in our study. Previously, obese and hemodialysis patients had lower anti-SARS-CoV-2 S antibody titres at 3 weeks post-BNT162b2 vaccine as compared to healthy adults.Citation54,Citation55 From natural infection studies, patients with or without diabetes mellitus had strong and detectable antibody responses with no effect of hyperglycemia.Citation24,Citation56 Nevertheless, based on effectiveness studies and not only the IgG titers, COVID-19 vaccination guidelines have been updated to include booster doses for the elderly and those with comorbidities.
Overall, this study supports previous reports that show BNT162b2 and AZ1222 vaccines induced robust antibody responses. It reports data from a vaccination program in Saudi Arabia, one of the early countries to implement COVID-19 vaccination. Some factors that may impact on the levels and longevity of induced anti-SARS-CoV-2 antibodies were also presented. However, the current study has few limitations. The number of collected samples was very low for 3 weeks post-third dose as well as for 1 year. Thus, a larger study is needed to confirm the current findings, especially as it relates to the 3-weeks and 1-year data. An additional weakness is that not all subjects were available for all sampling time-points. In Saudi Arabia, the third dose of COVID-19 vaccination was given to vaccinated people as early as 8 months; therefore, it was very difficult to find a large number of participants (from the cohort) who did not receive the third dose by the 1-year time-point. Thus, it is difficult to generalize the results of the study at this time point. An additional limitation of the study is the non-availability of data on T-cell related immunity.
Conclusions
The study showed adequate IgG antibody responses are induced by COVID-19 vaccines increasing from baseline to post-prime and peaking at post-second dose. The Antibody plateaued for 6 months. The study did not include sufficient data for immune responses at 1 year to make a solid conclusion. Boosting with a third dose of BNT162b2 from 8 months onwards reinstated the peak Ab levels. The study also showed that longer intervals contribute to higher antibody responses, younger subjects had higher levels of IgG titers, and previously infected individuals had higher IgG titers at post-prime and 6 months, indicating strong priming and maintenance of immune responses in these individuals.
Ethics Approval and Informed Consent
The study was approved by the IRB at KAIMRC for projects RC20/180 and NRC21R-120-03. Vaccinated subjects, recovered cases, and healthy controls have signed informed consent forms for donating blood samples and allowing access to their clinical and demographic information. The study complies with the Declaration of Helsinki. Neutralization assay using a live SARS-CoV-2 virus was conducted in a BSL-3 facility, with highly restricted access, following the guidelines and regulations of the Saudi Ministry of Environment, Water and Agriculture (MEWA) and applying the requirement of the WHO Safety manual. All staff who worked in BSL-3 were trained in VIDO-InterVac, Saskatoon, Canada, for biosafety level 3 virology lab work.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no conflicts of interest in this work.
Acknowledgments
The authors are grateful for the assistance of the COVID-19 vaccination center at MNG-HA and the Saudi Ministry of Health. We express special thanks to all the vaccinated volunteers who participated in the study.
Additional information
Funding
References
- Sohrabi C, Alsafi Z, O’Neill N, et al. World Health Organization declares global emergency: a review of the 2019 novel coronavirus (COVID-19). Int J Surg. 2020;76:71–76. doi:10.1016/J.IJSU.2020.02.034
- Rauf A, Abu-Izneid T, Olatunde A, et al. COVID-19 Pandemic: epidemiology, Etiology, Conventional and Non-Conventional Therapies. Int J Environ Res Public Health. 2020;17(21):1–32. doi:10.3390/IJERPH17218155
- Alsagaby SA, Aljouie A, Alshammari TH, et al. Haematological and radiological-based prognostic markers of COVID-19. J Infect Public Health. 2021;14(11):1650–1657. doi:10.1016/J.JIPH.2021.09.021
- WHO Coronavirus (COVID-19) Dashboard | WHO Coronavirus (COVID-19) Dashboard With Vaccination Data. Available from: https://covid19.who.int/. Accessed November 10, 2021.
- Forni G, Mantovani A, Forni G, et al. COVID-19 vaccines: where we stand and challenges ahead. Cell Death Differ. 2021;28(2):626–639. doi:10.1038/S41418-020-00720-9
- Wallace M, Woodworth KR, Gargano JW, et al. The Advisory Committee on Immunization Practices’ Interim Recommendation for Use of Pfizer-BioNTech COVID-19 Vaccine in Adolescents Aged 12-15 Years - United States, May 2021. MMWR Morb Mortal Wkly Rep. 2021;70(20):749–752. doi:10.15585/MMWR.MM7020E1
- Oliver SE, Gargano JW, Marin M, et al. The Advisory Committee on Immunization Practices’ Interim Recommendation for Use of Pfizer-BioNTech COVID-19 Vaccine - United States, December 2020. MMWR Morb Mortal Wkly Rep. 2020;69(50):1922–1924. doi:10.15585/MMWR.MM6950E2
- Shimabukuro T, Nair N. Allergic Reactions Including Anaphylaxis After Receipt of the First Dose of Pfizer-BioNTech COVID-19 Vaccine. JAMA. 2021;325(8):780–781. doi:10.1001/JAMA.2021.0600
- Polack FP, Thomas SJ, Kitchin N, et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383(27):2603–2615. doi:10.1056/NEJMOA2034577
- Covid CD, Team R. Allergic Reactions Including Anaphylaxis After Receipt of the First Dose of Pfizer-BioNTech COVID-19 Vaccine - United States, December 14-23, 2020. MMWR Morb Mortal Wkly Rep. 2021;70(2):46–51. doi:10.15585/MMWR.MM7002E1
- Voysey M, Clemens SAC, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99–111. doi:10.1016/S0140-6736(20)32661-1
- Pottegård A, Lund LC, Karlstad Ø, et al. Arterial events, venous thromboembolism, thrombocytopenia, and bleeding after vaccination with Oxford-AstraZeneca ChAdOx1-S in Denmark and Norway: population based cohort study. BMJ. 2021:373. doi:10.1136/BMJ.N1114
- Wise J. Covid-19: european countries suspend use of Oxford-AstraZeneca vaccine after reports of blood clots. BMJ. 2021;372:n699. doi:10.1136/BMJ.N699
- Lundstrom K, Barh D, Uhal BD, et al. COVID-19 Vaccines and Thrombosis—Roadblock or Dead-End Street? Biomolecules. 2021;11(7):1020. doi:10.3390/BIOM11071020
- Ndwandwe D, Wiysonge CS. COVID-19 vaccines. Curr Opin Immunol. 2021;71:111–116. doi:10.1016/J.COI.2021.07.003
- Yang L, Liu S, Liu J, et al. COVID-19: immunopathogenesis and Immunotherapeutics. Signal Transduct Target Ther. 2020;5:1. doi:10.1038/S41392-020-00243-2
- Boudjelal M, Almajed F, Salman AM, et al. COVID-19 vaccines: global challenges and prospects forum recommendations. Int J Infectious Dis. 2021;105:448–451. doi:10.1016/J.IJID.2021.02.093
- Bosaeed M, Balkhy HH, Almaziad S, et al. Safety and immunogenicity of ChAdOx1 MERS vaccine candidate in healthy Middle Eastern adults (MERS002): an open-label, non-randomised, dose-escalation, Phase 1b trial. Lancet Microbe. 2021. doi:10.1016/S2666-5247(21)00193-2
- Levin EG, Lustig Y, Cohen C, et al. Waning Immune Humoral Response to BNT162b2 Covid-19 Vaccine over 6 Months. N Engl J Med. 2021. doi:10.1056/NEJMOA2114583
- Naaber P, Tserel L, Kangro K, et al. Dynamics of antibody response to BNT162b2 vaccine after six months: a longitudinal prospective study. Lancet Regional Health Europe. 2021;10:100208. doi:10.1016/J.LANEPE.2021.100208
- Mahallawi WH, Fakher MH, Alsarani MA, Aljohani RH, Al-Mutabgani SA, Ibrahim NA. A Single Dose of SARS-CoV-2 Vaccine Primes a Strong Humoral Immune Response in COVID-19-Recovered Patients. Viral Immunol. 2021. doi:10.1089/VIM.2021.0108/ASSET/IMAGES/LARGE/VIM.2021.0108_FIGURE6.JPEG
- Garg J, Singh V, Pandey P, et al. Evaluation of sample pooling for diagnosis of COVID-19 by real time-PCR: a resource-saving combat strategy. J Med Virol. 2021;93(3):1526–1531. doi:10.1002/JMV.26475
- Interim Guidance for Use of Pooling Procedures in SARS-CoV-2 Diagnostic and Screening Testing | CDC. Available from: https://www.cdc.gov/coronavirus/2019-ncov/lab/pooling-procedures.html. Accessed November 10, 2021.
- Alharbi NK, Alghnam S, Algaissi A, et al. Nationwide Seroprevalence of SARS-CoV-2 in Saudi Arabia. J Infect Public Health. 2021;14(7):832–838. doi:10.1016/J.JIPH.2021.04.006
- Chappell KJ, Mordant FL, Li Z, et al. Safety and immunogenicity of an MF59-adjuvanted spike glycoprotein-clamp vaccine for SARS-CoV-2: a randomised, double-blind, placebo-controlled, phase 1 trial. Lancet Infect Dis. 2021;21(10):1383–1394. doi:10.1016/S1473-3099(21
- Amanat F, White KM, Miorin L, et al. An In Vitro Microneutralization Assay for SARS-CoV-2 Serology and Drug Screening. Curr Protoc Microbiol. 2020;58(1):e108. doi:10.1002/cpmc.108
- Algaissi A, Hashem AM. Evaluation of MERS-CoV Neutralizing Antibodies in Sera Using Live Virus Microneutralization Assay. Methods Mol Biol. 2020;2099:107–116. doi:10.1007/978-1-0716-0211-9_9
- Doria-Rose N, Suthar MS, Makowski M, et al. Antibody Persistence through 6 Months after the Second Dose of mRNA-1273 Vaccine for Covid-19. N Engl J Med. 2021;384(23):2259–2261. doi:10.1056/NEJMC2103916
- Thomas SJ, Moreira ED, Kitchin N, et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine through 6 Months. N Engl J Med. 2021;385(19):1761–1773. doi:10.1056/NEJMOA2110345
- Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27(7):1205–1211. doi:10.1038/S41591-021-01377-8
- Wheeler SE. Differential Antibody Response to mRNA COVID-19 Vaccines in Healthy Subjects. Microbiol Spectr. 2021;9(1). doi:10.1128/SPECTRUM.00341-21
- Baumgarth N, Nikolich-žugich J, Lee FEH, Bhattacharya D. Antibody Responses to SARS-CoV-2: let’s Stick to Known Knowns. J Immunol. 2020;205(9):2342–2350. doi:10.4049/JIMMUNOL.2000839
- Khodadadi L, Cheng Q, Radbruch A, Hiepe F. The Maintenance of Memory Plasma Cells. Front Immunol. 2019;10. doi:10.3389/FIMMU.2019.00721
- Quast I, Tarlinton D. B cell memory: understanding COVID-19. Immunity. 2021;54(2):205–210. doi:10.1016/J.IMMUNI.2021.01.014
- Steensels D, Pierlet N, Penders J, Mesotten D, Heylen L. Comparison of SARS-CoV-2 Antibody Response Following Vaccination With BNT162b2 and mRNA-1273. JAMA. 2021;326(15):1533–1535. doi:10.1001/JAMA.2021.15125
- Wang Z, Muecksch F, Schaefer-Babajew D, et al. Naturally enhanced neutralizing breadth against SARS-CoV-2 one year after infection. Nature. 2021;595(7867):426–431. doi:10.1038/S41586-021-03696-9
- Angyal A, Longet S, Moore S, et al. T-Cell and Antibody Responses to First BNT162b2 Vaccine Dose in Previously SARS-CoV-2-Infected and Infection-Naive UK Healthcare Workers: a Multicentre, Prospective, Observational Cohort Study. SSRN Electronic J. 2021. doi:10.2139/SSRN.3812375
- Parry H, Bruton R, Stephens C, et al. Extended interval BNT162b2 vaccination enhances peak antibody generation. NPJ Vaccines. 2022;7(1):14. doi:10.1038/s41541-022-00432-w
- Payne RP, Longet S, Austin JA, et al. Immunogenicity of standard and extended dosing intervals of BNT162b2 mRNA vaccine. Cell. 2021;184(23):5699–5714.e11. doi:10.1016/j.cell.2021.10.011
- Amirthalingam G, Bernal JL, Andrews NJ, et al. Serological responses and vaccine effectiveness for extended COVID-19 vaccine schedules in England. Nat Commun. 2021;12(1):7217. doi:10.1038/s41467-021-27410-5
- Voysey M, Costa Clemens SA, Madhi SA, et al. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trials. Lancet. 2021;397(10277):881–891. doi:10.1016/S0140-6736(21
- Flaxman A, Marchevsky NG, Jenkin D, et al. Reactogenicity and immunogenicity after a late second dose or a third dose of ChAdOx1 nCoV-19 in the UK: a substudy of two randomised controlled trials (COV001 and COV002). Lancet. 2021;398(10304):981–990. doi:10.1016/S0140-6736(21)01699-8
- Wei J, Pouwels KB, Stoesser N, et al. Antibody responses and correlates of protection in the general population after two doses of the ChAdOx1 or BNT162b2 vaccines. Nat Med. 2022. doi:10.1038/s41591-022-01721-6
- Müller M, Volzke J, Subin B, et al. Single-Dose SARS-CoV-2 Vaccination With BNT162b2 and AZD1222 Induce Disparate Th1 Responses and IgA Production. medRxiv. 2021;2021:21263726. doi:10.1101/2021.09.17.21263726
- Müller M, Volzke J, Subin B, et al. Single-dose SARS-CoV-2 vaccinations with either BNT162b2 or AZD1222 induce disparate Th1 responses and IgA production. BMC Med. 2022;20(1):29. doi:10.1186/s12916-022-02240-4
- Zurac S, Nichita L, Mateescu B, et al. COVID-19 vaccination and IgG and IgA antibody dynamics in healthcare workers. Mol Med Rep. 2021;24:2. doi:10.3892/mmr.2021.12217
- Madhumita Shrotri A, Fragaszy E, Geismar C, et al. Spike-antibody responses following first and second doses of ChAdOx1 and BNT162b2 vaccines by age, gender, and clinical factors - a prospective community cohort study (Virus Watch). medRxiv. 2021;2021. doi:10.1101/2021.05.12.21257102
- Krammer F, Srivastava K, Alshammary H, et al. Antibody Responses in Seropositive Persons after a Single Dose of SARS-CoV-2 mRNA Vaccine. N Engl J Med. 2021;384(14):1372–1374. doi:10.1056/NEJMC2101667
- Saadat S, Rikhtegaran Tehrani Z, Logue J, et al. Binding and Neutralization Antibody Titers After a Single Vaccine Dose in Health Care Workers Previously Infected With SARS-CoV-2. JAMA. 2021;325(14):1467–1469. doi:10.1001/JAMA.2021.3341
- Ebinger JE, Fert-Bober J, Printsev I, et al. Antibody responses to the BNT162b2 mRNA vaccine in individuals previously infected with SARS-CoV-2. Nat Med. 2021;27(6):981–984. doi:10.1038/S41591-021-01325-6
- Mazzoni A. First-dose mRNA vaccination is sufficient to reactivate immunological memory to SARS-CoV-2 in subjects who have recovered from COVID-19. J Clin Invest. 2021;131(12). doi:10.1172/JCI149150
- Gobbi F, Buonfrate D, Moro L, et al. Antibody Response to the BNT162b2 mRNA COVID-19 Vaccine in Subjects with Prior SARS-CoV-2 Infection. Viruses. 2021;13:3. doi:10.3390/V13030422
- Parry H, Bruton R, Tut G, et al. Immunogenicity of single vaccination with BNT162b2 or ChAdOx1 nCoV-19 at 5-6 weeks post vaccine in participants aged 80 years or older: an exploratory analysis. Lancet Healthy Longev. 2021;2(9):e554–e560. doi:10.1016/S2666-7568(21)00169-0
- Bernal JL, Andrews N, Gower C, et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on covid-19 related symptoms, hospital admissions, and mortality in older adults in England: test negative case-control study. BMJ. 2021;373. doi:10.1136/BMJ.N1088
- Simon B, Rubey H, Treipl A, et al. Haemodialysis patients show a highly diminished antibody response after COVID-19 mRNA vaccination compared with healthy controls. Nephrol Dialysis Transplanta. 2021;36(9):1709–1716. doi:10.1093/NDT/GFAB179
- Dispinseri S, Lampasona V, Secchi M, et al. Robust Neutralizing Antibodies to SARS-CoV-2 Develop and Persist in Subjects with Diabetes and COVID-19 Pneumonia. J Clin Endocrinol Metab. 2021;106(5):1472–1481. doi:10.1210/CLINEM/DGAB055
