Abstract
Introduction
Information regarding the clinical course of COVID-19 patients with liver injury is very limited, especially in severe and critical patients. The objective of this study was to describe the characteristics and clinical course of liver function in patients admitted with severe and/or critical SARS-CoV-2 infection, as well as explore the risk factors that affect liver function in the enrolled COVID-19 patients.
Methods
Information on clinical characteristics of 63 severe and critical patients with confirmed COVID-19 was collected. Data on patients’ demographics, laboratory characteristics, laboratory examination, SARS-CoV-2 RNA results and liver test parameters were acquired and analyzed.
Results
The incidence of abnormal aspartate aminotransferase, alanine aminotransferase, and total bilirubin in the critical group was significantly higher than in the severe group (respectively 81.48%, 81.49%, 62.67%, and 45.71%, 63.88%, 22.86%, p < 0.05). The time for liver function parameters to reach their extremes was approximately 2–3 weeks after admission. The independent factors associated with liver injury were patients with invasive ventilators, decreased percentages of neutrophils, lymphocytes and monocytes, and sequential organ failure assessment (SOFA) score ≥2 (p < 0.05).
Conclusion
Abnormal liver tests are commonly observed in severe and critical patients with COVID-19. Severe patients infected by SARS-CoV-2 should be closely observed and monitored the liver function parameters, particularly when they present with independent risk factors for liver injury.
Introduction
The coronavirus disease 2019 (COVID-19) pandemic, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), poses an unprecedented threat to public health.Citation1,Citation2 Patients with COVID-19 frequently present with pulmonary lesions; however, increasing data reveal that COVID-19 has systemic manifestations affecting multiorgan systems including liver injury, myocarditis, thrombosis, and coagulation.Citation3–6 While the impact of COVID-19 on the liver remains unclear, a considerable proportion of patients with elevated liver enzyme have been reported.Citation6–8 Some studies found a mild [1–2 times the upper limit of normal (ULN)] increase in transaminases, while severe liver injury has also been reported.Citation3,Citation9,Citation10
However, information related to the clinical course of COVID-19 patients with liver injury is very limited, especially in severe and critical patients. Knowledge of the clinical characteristics of liver injury in this disease is vital to answering questions about the therapy and management for patients infected with SARS-CoV-2. To this end, we present detailed characteristics and the clinical course of liver function in patients admitted with severe or critical COVID-19 illness. Additionally, we further explore the independent risk factors for liver damage in these patients.
Methods
Study Design and Participants
This retrospective study included 63 severe and critical patients with confirmed COVID-19 hospitalized in Beijing Ditan Hospital from January 20, 2020 to April 06, 2020. SARS-CoV-2 infection was diagnosed by RT-PCR assays of respiratory tract samples from nasopharyngeal swabs performed by the local Center for Disease Control or by our institutional laboratory. The severity of COVID-19 was defined in accordance with the Chinese management guidelines for COVID-19 (Trial version 9.0) released by the National Health Commission of China. Patients meeting any one of the following should be considered severe cases: respiratory rate ≥30 breaths/min, oxygen saturation at rest ≤93% on room air, PaO2/FiO2 ≤300mmHg, or the clinical symptoms progressively aggravated, and the lung imaging lesions progressed more than 50% within 24 ~ 48 hours. Critical cases are any patients who have respiratory failure, shock, or any organ failure that requires mechanical ventilation or intensive care management. Acute respiratory distress syndrome (ARDS) was defined according to the Berlin definition.Citation11 Cases were excluded for patients who were younger than 18 years, accompanied by underlying liver disease, as well as mild and moderately ill patients. This study was approved by the Institutional Review Board of the Capital Medical University affiliated Beijing Ditan Hospital, and patient-level informed consent was waived owing to its retrospective nature.
Data Collection
The medical records of patients with COVID-19 were collected by the research team. Data on patients’ demographics, comorbidities, vital signs, laboratory characteristics and treatment were acquired by the hospital information system.
Laboratory Examination and Liver Test Parameters
On admission, laboratory parameters including peripheral blood leukocyte count, neutrophils, lymphocytes, monocytes, platelets, hemoglobin, hematocrit, C-reactive protein (CRP), international normalized ratio (INR), D-dimer, creatine kinase, aspartate aminotransferase (AST), alanine transaminase (ALT), total bilirubin (TBIL), serum albumin, and A/G (albumin/globulin) ratio were collected. Since COVID-19 is an emerging infectious disease, consensus or guidance on the classifications of liver injury are lacking, so we classified the pattern of liver tests into three groups: normal liver, abnormal liver, and liver injury. Abnormal liver test was defined as the elevation of serum liver enzymes exceeding the upper limit of normal (ULN), that is, AST > 40 U/L, ALT > 40 U/L, and TBIL >17.1 µmol/L. Liver injury was defined when ALT and/or AST were over 3 × ULN, and/or TBIL was over 3 × UL. Furthermore, the dynamic changes of liver enzymes, serum albumin, INR, and A/G ratio were recorded.
SARS-CoV-2 RNA Detection
COVID-19 was diagnosed according to the cycle threshold (Ct) values of open reading frame 1ab (ORF1ab) and nucleocapsid protein (N) gene by RT-PCR assay. The assay was performed by the National Center for Disease Control or the Clinical Laboratory of Beijing Ditan hospital using a commercial kit (Daan, Guangzhou, China). The SARS-CoV-2 viral loads were measured by the copy number of the N gene from sputum samples or throat swabs of the COVID-19 cases. Ct values were negatively correlated with viral RNA copy numbers.Citation12 According to the instructions of the RT-PCR kit, patients with Ct values less than 40 were considered positive.
Statistical Analysis
All statistical analyses were performed with SPSS 19.0 for Windows (IBM, USA). Continuous variables were described as mean and SD or median and IQR, and categorical variables as frequency and percentages. Normally distributed variables were analyzed using independent group t-test or one-way ANOVA, whereas the Mann–Whitney nonparametric test was used for non-normally distributed variables. Categorical variables were analyzed using the chi-square (χ2) or Fisher’s exact tests. Pearson’s correlation coefficient was used to assess the correlation between the severity of liver injury and laboratory results. Ordinal logistic regression analysis was conducted to evaluate the association of baseline characteristics with the severity of liver injury. Two-sided p-values of less than 0.05 were considered statistically significant.
Results
Baseline Characteristics of Enrolled Patients with COVID-19
The clinical characteristics of enrolled patients with COVID‑19 are shown in . A total of 63 patients were enrolled in the study. The average age of the patients was 56.75 years and 41 patients (65.08%) were male. According to the severity of the disease, subjects were classified into the severe group (36, 57.14%) or the critical group (27, 42.86%). There were significant differences between the two groups in drug use and oxygen therapy except for the requirement of high-flow oxygen or non-invasive ventilator use. In terms of laboratory results, blood indices including peripheral white blood cell count, neutrophil, C-reactive protein (CRP), blood urea nitrogen (BUN), and lactate dehydrogenase (LDH) were significantly higher in critical patients than in severe patients (p < 0.05). In contrast, lymphocytes, percentage of monocytes, hemoglobin, and hematocrit were significantly lower in critical patients than in severe patients (p < 0.05).
Table 1 Demographic, Clinical, and Laboratory Findings of Patients with COVID‑19 on Admission
Clinical Features of Enrolled Patients with COVID-19 and Liver Function Tests During Hospitalization
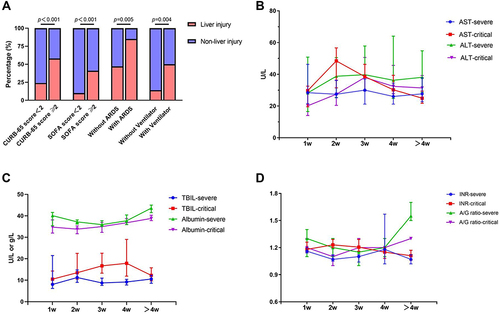
The serum liver enzyme parameters of enrolled patients were further analyzed. Results showed that there were no differences between severe and critical group in AST, ALT, TBIL, INR, albumin, and A/G ratio on admission (Supplemental Table), whereas the extreme values of these parameters had significant differences, except for ALT (). The incidence of abnormal AST, ALT, and TBIL in the critical group was significantly higher than in the severe group (81.48%, 81.49%, 62.67%, and 45.71%, 63.88%, 22.86%, respectively, p < 0.05) during hospitalization. Based on the test of liver function, subjects were further classified as normal liver (11, 17.46%), abnormal liver (32, 50.79%), and liver injury group (20, 31.75%) (). Patients in the three groups were not significantly different in distributions of gender or age. The CRP, percentage of neutrophils, BUN, and LDH in the liver injury group were significantly higher than in the normal liver group, while the percentages of lymphocytes and monocytes were significantly lower (p < 0.05). Additionally, the oxygenation index of the liver injury group was significantly lower than that of the other groups (p = 0.015), while the CURB-65 score, sequential organ failure assessment (SOFA) scores, the incidence of ARDS, and the application of an invasive ventilator were significantly higher in the liver injury group than in the non-liver group (p < 0.05) ( and ).
Table 2 Peak Values of Serum Liver Enzyme Parameters in Severe and Critical Patients with COVID‑19
Table 3 Clinical Characteristics of COVID-19 Patients in Different Liver Test Groups at Admission
Figure 1 The percentage of liver injury in different groups and dynamic changes of liver function indicators between the severe group and the critical group. (A) The percentage of liver injury in CURB-65 score, sequential organ failure assessment (SOFA) score, acute respiratory distress syndrome (ARDS) and ventilator groups. (B) Dynamic changes of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) between the severe group and the critical group. (C) Dynamic changes of total bilirubin (TBIL) and albumin between the severe group and the critical group. (D) Dynamic changes of INR and albumin/globulin (A/G) ratio between the severe group and the critical group.
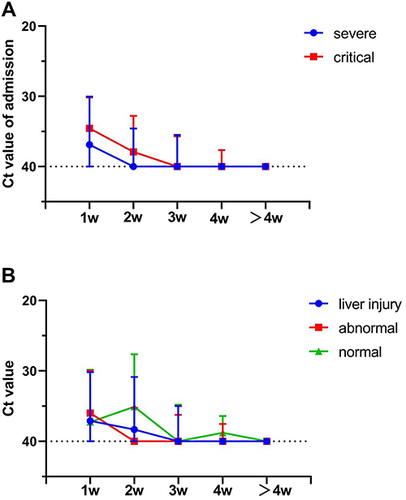
Dynamic Profile of Liver Function Indicators and Viral Clearance
To explore the correlation of dynamic changes in liver function parameters and virus clearance in enrolled patients, data of liver enzymes and Ct values were monitored during hospitalization. Results showed that the plasma levels of AST, TBIL, and INR were significantly higher in the critical group than in the severe group, and the time for these indicators to reach their peak was approximately 2–3 weeks after admission (). Compared with AST, the plasma TBIL level more slowly reached its peak at about 4 weeks, then gradually decreased. Conversely, the levels of ALT, albumin and A/G ratio were significantly lower in the critical group than in the severe group, and the time for these parameters to reach their extremes was also approximately 2–3 weeks (). The liver injury group had similar characteristics (Supplemental Figure 1A–C). The level of Ct values in the critical group was higher than in the severe group, but there was no significant difference either on admission or at the peak level (, Supplemental Figure 2A). In addition, there was no significant difference in the level of Ct values among liver injury group, abnormal group and normal group (, Supplemental Figure 2B). The negative rates at 1 week after admission in the critical group (74.1%) were lower than in the severe group (80.6%) if binarized the tested patients into positive (Ct < 40) and negative (Ct = 40) in spite of no significant difference. The Ct values of PCR increased gradually during hospitalization and the time of virus clearance was approximately 2–3 weeks after admission ().
Independent Factors Associated with Severity of Liver Function
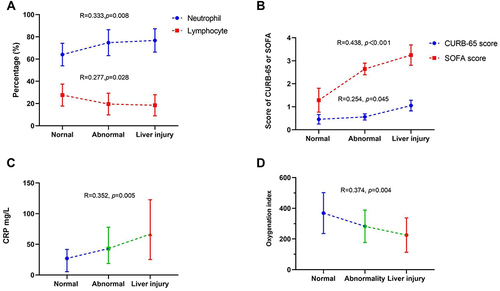
As shown in , the severity of damage to liver function was positively correlated with the percentage of neutrophils, CRP, CURB-65 score and SOFA score and negatively correlated with percentage of lymphocytes and oxygenation index. To further analyze the correlation between the severity of liver injury and clinical or laboratory index and identify independent factors associated with severity of damage to liver function, we conducted ordinal logistic regression analysis. The variables included in the ordinal logistic regression and statistical univariate analysis results are shown in . Variables with p-values of <0.05 in univariate analyses were included in the ordinal logistic analysis to identify the significant indicators affecting liver function in enrolled COVID-19 patients (). Results revealed that SOFA score ≥2 [OR = 165.41, 95% confidence interval (CI) = (1.57, 8.64); p = 0.005] was positively associated with abnormality or injury as indicated by liver tests. After adjustment for age, sex, and comorbidities, patients with invasive ventilators, decreased percentages of neutrophils, lymphocytes and monocytes, and SOFA score ≥2 were the independent factors associated with abnormal liver tests or liver tests showing injury.
Table 4 Independent Factors Associated with Severity of Liver Injury
Figure 3 The correlation between the severity of liver injury and laboratory results. (A) the correlation between the severity of liver injury and the percentage of neutrophils and lymphocytes. (B) the correlation between the severity of liver injury and CURB-65 and sequential organ failure assessment (SOFA) score. (C) the correlation between the severity of liver injury and C-reactive protein (CRP). (D) the correlation between the severity of liver injury and oxygenation index.
Discussion
In this cohort of 63 cases, we demonstrate that abnormal liver tests are common in patients with severe and critical COVID-19. Patients with critical COVID-19 should be aware of the occurrence of liver injury at 2–3 weeks after admission. Particular attention should be paid to patients with decreased ratios of neutrophils, lymphocytes and monocytes, the requirement of an invasive ventilator, and SOFA score ≥2 during hospitalization, as these are independent risk factors for the occurrence of liver injury.
The incidence of liver enzyme elevation observed here ranged from 22.86% to 81.49% in severe and critical patients during hospitalization, higher than in a previous study, which showed a range from 14% to 53% in patients including those with mild and moderate COVID-19.Citation13 The increase was mainly manifested by elevated levels of ALT, AST, and TBIL accompanied by the slightly decreased albumin levels. The increased liver enzymes were observed more commonly in the critical group than in the severe group. Interestingly, we observed that the levels of AST and TBIL were higher in the critical group than in the severe group, while no differences in ALT were observed between groups. Elevated AST could be from muscle damage rather than directly reflecting liver injury, and the levels of INR in this study were primarily within the range of 1.5. Thus, the discovery that SARS-CoV-2 virus binds to angiotensin-converting enzyme 2 (ACE2) on hepatocytes, especially on biliary epithelial cells, and then causes liver injuryCitation14 may partially explain the results in our patients. In other words, COVID-19 patients with liver injury were more likely to be a cholestatic type than a hepatocellular type.
Currently, the underlying mechanisms of COVID-19 related liver injury remain unclear. In fact, liver injury may be multifactorial and individualized. First, a hyper-inflammatory response to COVID-19 may contribute to liver injury.Citation15,Citation16 The severity of damage to liver function was positively correlated with the percentage of neutrophils and CRP and negatively correlated with percentages of lymphocytes and monocytes; decreased ratios of neutrophils, lymphocytes and monocytes are independent risk factors for the occurrence of liver injury. Hepatic inflammation involving activation of the innate immune system accompanied by a cytokine storm is a well-established driver of liver injury.Citation17 Notably, lymphopenia was commonly observed in COVID-19 studies and patients with lower counts of lymphocytes are more susceptible to fatal outcomes.Citation18 Second, the increased ACE2 expressed on hepatocytes that infected by SARS-CoV-2 virus might provide a mechanistic link between disease severity and liver injury.Citation19,Citation20 In the present study, no significant difference was observed in Ct values on admission or at the peak among the liver injury group, abnormal group and normal group. However, it is impossible to demonstrate whether the virus has an impact on liver cytopathy owing to the lack of liver biopsy and Ct values of the liver in situ. Preclinical studies demonstrated that at least in vitro hepatocytes can be directly infected, albeit at considerably lower levels than airway or endothelial cells.Citation20–22 Third, hypoxia induced by COVID-19-related complications (ie, ARDS and multiple organ failure) may also induce hepatic ischemia and hypoxia-reperfusion dysfunction.Citation23 The severity of damage to liver function was positively correlated with CURB-65 score and SOFA score and negatively correlated with oxygenation index. Moreover, the requirement of an invasive ventilator and SOFA score ≥2 are independent risk factors for the occurrence of liver injury. Lastly, drug-elicited liver injury may also account for laboratory test abnormalities.Citation3,Citation24 However, there was no significant difference in drug use among the liver injury group, abnormal group and normal group in this study, except for a vasoactive drug. However, the vasoactive drug was not an independent risk factor for the occurrence of liver injury in the logistic analysis. Thus, the enrolled drugs in this study may not directly induce liver injury.
Our results showed that the time for liver parameters to reach their extremes was approximately 2–3 weeks after admission, which is critical for clinical implications in managing patients infected with SARS-CoV-2. Thus, regular monitoring of liver function tests should be performed, particularly in patients with severe and critical COVID-19.
This study has some limitations. First, not all laboratory tests were collected in enrolled patients, including alkaline phosphatase and gamma-glutamyl transferase owing to the retrospective design. Additionally, it is impossible to demonstrate whether the virus has an impact on liver cytopathy owing to the lack of liver biopsy and viral load results of the liver in situ. Further studies should corroborate the pathogenic mechanism. The relatively small sample size is also a limitation of this study. Future studies needed to enroll larger sample sizes to strengthen the accuracy of the results.
Conclusions
In summary, abnormal liver tests are commonly observed in severe and critical patients with COVID-19. In particular, patients with critical COVID-19 should be closely monitored at 2–3 weeks after admission in case of the occurrence of liver injury. Independent risk factors for the occurrence of liver damage are decreased ratios of neutrophils, lymphocytes and monocytes, the requirement of an invasive ventilator, and SOFA score ≥2; patients with these abnormal parameters should be of particular concern during hospitalization.
Abbreviations
ARDS, acute respiratory distress syndrome; ALT, alanine aminotransferase; AST, aspartate aminotransferase; A/G, albumin/globulin; BUN, blood urea nitrogen; COVID-19, coronavirus disease 2019; CRP, C-reactive protein; Ct, cycle threshold; ECMO, extracorporeal membrane oxygenation; INR, international normalized ratio; LDH, lactate dehydrogenase; NLR, neutrophil-to-lymphocyte ratio; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SOFA, Sequential Organ Failure Assessment; TBIL, total bilirubin.
Data Sharing Statement
Data of this study can be available upon request from the author.
Ethics Approval and Consent to Participate
This clinical study was conducted in compliance with the ethical principles of the Declaration of Helsinki and its later amendments. The Ethics Committee of Beijing Ditan Hospital approved our study protocol [approval No. NA2018(005)-01]. As a de-identified retrospective study, the ethics committee did not require us to obtain written or verbal informed consent from participants.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas. All authors took part in drafting, revising or critically reviewing the article. All authors gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests in this work.
Acknowledgments
The authors gratefully acknowledge Gang Wan Ph.D. and Jun-nan Li Ph.D. for their assistance with data analysis. Thanks to all the front-line medical staff of Beijing Ditan hospital for their bravery and efforts in SARS-CoV-2 prevention and control.
Additional information
Funding
References
- Zhu Y, Liu Y, Jiang H. Geriatric health care during the COVID-19 pandemic: managing the health crisis. Clin Interv Aging. 2022;17:1365–1378. doi:10.2147/CIA.S376519
- Siso-Almirall A, Kostov B, Sanchez E, et al. Impact of the COVID-19 pandemic on primary health care disease incidence rates: 2017 to 2020. Ann Fam Med. 2022;20(1):63–68. doi:10.1370/afm.2731
- Li P, Liu Y, Cheng Z, et al. COVID-19-associated liver injury: clinical characteristics, pathophysiological mechanisms and treatment management. Biomed Pharmacother. 2022;154:113568. doi:10.1016/j.biopha.2022.113568
- Diaz GA, Parsons GT, Gering SK, et al. Myocarditis and pericarditis after vaccination for COVID-19. JAMA. 2021;326(12):1210–1212. doi:10.1001/jama.2021.13443
- Gupta A, Satapathy AK, Bahinipati P. Delayed catastrophic thrombotic events in post-acute COVID-19. Thromb Res. 2022;220:60–64. doi:10.1016/j.thromres.2022.10.004
- Jang TY. Liver injury caused by SARS-CoV-2 delta and omicron-variant in Taiwan. J Formos Med Assoc. 2022;121(11):2367–2368. doi:10.1016/j.jfma.2022.06.004
- Yuan WZ, Fu T. Liver Dysfunction in COVID-19: from Onset to Recovery. Semin Liver Dis. 2022;42(2):151–158. doi:10.1055/s-0042-1745871
- Luo M, Ballester MP, Soffientini U, et al. SARS-CoV-2 infection and liver involvement. Hepatol Int. 2022;16(4):755–774. doi:10.1007/s12072-022-10364-1
- Gao YD, Ding M, Dong X, et al. Risk factors for severe and critically ill COVID-19 patients: a review. Allergy. 2021;76(2):428–455. doi:10.1111/all.14657
- Garrido I, Liberal R, Macedo G. Review article: COVID-19 and liver disease-what we know on 1st May 2020. Aliment Pharmacol Ther. 2020;52(2):267–275. doi:10.1111/apt.15813
- Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307(23):2526–2533. doi:10.1001/jama.2012.5669
- Zou L, Ruan F, Huang M, et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382(12):1177–1179. doi:10.1056/NEJMc2001737
- Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi:10.1056/NEJMoa2002032
- Da Silva M, De Lucena AS, Paiva SSL, et al. Cell death mechanisms involved in cell injury caused by SARS-CoV-2. Rev Med Virol. 2022;32(3):e2292. doi:10.1002/rmv.2292
- McConnell MJ, Kondo R, Kawaguchi N, et al. Covid-19 and liver injury: role of inflammatory endotheliopathy, platelet dysfunction, and thrombosis. Hepatol Commun. 2022;6(2):255–269. doi:10.1002/hep4.1843
- Zhu DD, Tan XM, Lu LQ, et al. Interplay between nuclear factor erythroid 2-related factor 2 and inflammatory mediators in COVID-19-related liver injury. World J Gastroenterol. 2021;27(22):2944–2962. doi:10.3748/wjg.v27.i22.2944
- Mehta P, McAuley DF, Brown M, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi:10.1016/S0140-6736(20)30628-0
- Henry BM. COVID-19, ECMO, and lymphopenia: a word of caution. Lancet Respir Med. 2020;8(4):e24. doi:10.1016/S2213-2600(20)30119-3
- Stebbing J, Krishnan V, de Bono S, et al. Mechanism of baricitinib supports artificial intelligence-predicted testing in COVID-19 patients. EMBO Mol Med. 2020;12(8):e12697. doi:10.15252/emmm.202012697
- Stebbing J, Sanchez NG, Falcone M, et al. JAK inhibition reduces SARS-CoV-2 liver infectivity and modulates inflammatory responses to reduce morbidity and mortality. Sci Adv. 2021;7(1). doi:10.1126/sciadv.abe4724
- Lei HY, Ding YH, Nie K, et al. Potential effects of SARS-CoV-2 on the gastrointestinal tract and liver. Biomed Pharmacother. 2021;133:111064. doi:10.1016/j.biopha.2020.111064
- Jothimani D, Venugopal R, Abedin MF, et al. COVID-19 and the liver. J Hepatol. 2020;73(5):1231–1240. doi:10.1016/j.jhep.2020.06.006
- Idalsoaga F, Ayares G, Arab JP, et al. COVID-19 and indirect liver injury: a narrative synthesis of the evidence. J Clin Transl Hepatol. 2021;9(5):760–768. doi:10.14218/JCTH.2020.00140
- Huang J, Zhang Z, Hao C, et al. Identifying drug-induced liver injury associated with inflammation-drug and drug-drug interactions in pharmacologic treatments for COVID-19 by bioinformatics and system biology analyses: the role of pregnane X receptor. Front Pharmacol. 2022;13:804189. doi:10.3389/fphar.2022.804189