Abstract
Constipation is an important health burden that reduces the quality of life for countless millions of people. Symptom-centric therapeutics are often used to treat constipation due to unknown etiology, but in many cases, these drugs are either inadequate or have significant side effects. More recently, synthetic peptide agonists for epithelial guanylyl cyclase C (GC-C) have been developed which are effective at treating constipation in a sub-population of adult constipation patients. The first to market was linaclotide that is structurally related to the diarrheagenic enterotoxin, but this was followed by plecanatide, which more closely resembles endogenous uroguanylin. Both the drugs exhibit almost identical clinical efficacy in about 20% of patients, with diarrhea being a common side effect. Despite the potential for reduced side effects with plecanatide, detailed analysis suggests that clinically, they are very similar. Ongoing clinical and preclinical studies with these drugs suggest that treating constipation might be the tip of the iceberg in terms of clinical utility. The expression of cGMP signaling components could be diagnostic for functional bowel disorders, and increasing cGMP using GC-C agonists or phosphodiesterase inhibitors has huge potential for treating enteric pain, ulcerative colitis, and for the chemoprevention of colorectal cancer.
Introduction
Chronic constipation is one of the most common gastrointestinal complaints in the United States, affecting about 30% of Americans per year.Citation1 While not a life-threatening condition, chronic constipation can have a profound negative effect on quality of life. Constipation is characterized by a variety of symptoms including lumpy or hard stools, infrequent bowel movements, abdominal cramping and bloating, excessive straining, and the sensation of incomplete defecation.Citation2 Chronic constipation is often due to dietary factors (ie, poor fiber intake), lifestyle factors (ie, reduced activity and mobility), or disorders in colonic propulsion or rectal emptying. Secondary causes of chronic constipation are mediated by medications (ie, opioids, antihypertensives, tricyclic antidepressants, etc) or result from organic disease processes (ie, diabetes, colorectal cancer, polyps, strictures, etc).Citation2
Constipation disorders: chronic idiopathic constipation (CIC), irritable bowel syndrome with constipation (IBS-C), and opioid-induced constipation (OIC)
Functional bowel disorders (FBDs) are distinguished from other gastrointestinal illnesses based on symptom duration (>6 months), symptoms at current presentation (at least 3 months), and frequency (symptoms on average, at least 1 day/week). Constipation is associated with three of the six FBDs highlighted in the 2016 Rome IV guidelines: IBS (C1), functional constipation/CIC (C2), and OIC (C6).Citation3 The largest group of chronic constipation sufferers largely fall under functional constipation where no identifiable structural or biochemical etiology is known to be the cause. As defined in the Rome IV guidelines, the diagnosis of functional constipation (CIC) is made when the patient’s symptomatology does not meet the criteria for IBS. In the new guidelines, bloating and pain are not considered predominant symptoms of functional constipation and may not be present. The diagnosis is made when patients experience two of the following symptoms in the past 3 months: fewer than three bowel movements in a week, straining, lumpy or hard stool, abdominal symptoms such as bloating and abdominal discomfort, and sensation of incomplete defecation.Citation4,Citation5 The diagnosis of IBS is made when a patient experiences recurrent abdominal pain, on average, at least 1 day/week in the last 3 months, and is associated with two or more of the following criteria related to defecation, associated with a change in frequency of stool, and lastly associated with a change in form (appearance) of stool. Furthermore, to classify the IBS-C subtype, more than 25% of bowel movements are with Bristol stool form type 1 or 2 and less than 25% of bowel movements are with Bristol stool form type 6 or 7.Citation3
OIC was recently added to the Rome IV guidelines under the FBDs category, but it is uniquely distinct in that the etiology is known. It is widely accepted that OIC shares similar characteristics of functional constipation, and in recent years, it has become more prevalent with the rapidly growing health problem of opioid abuse in the United States. Stimulation of opioid receptors throughout the enteric neuronal system affects gastrointestinal function by delaying colonic transit, stimulating non-propulsive motility, and increasing intestinal absorption, thus causing constipation.
Non-prescription treatment options for constipation
Since the underlying cause of CIC and IBS-C is not fully understood, the treatment strategies often focus on controlling disease symptoms. The first line of treatment for constipation includes recommendations for lifestyle modification, with a focus on dietary change and increasing exercise.Citation6 Diets low in fermentable oligosaccharides, disaccharides, monosaccharides, and polyols can reduce some symptoms of IBS, but there is little evidence that this diet can benefit patients with CIC. A large body of evidence supports the use of soluble fiber supplements to alleviate symptoms of both CIC and IBS-C.Citation7,Citation8 Fiber alleviates constipation by creating bulky stool that stimulates the peristaltic reflex to increase motility. While fiber provides relief for some patients, it can exacerbate symptoms of constipation such as cramping, bloating, and flatulence.Citation9
For patients who are unable to alter lifestyle appropriately and whose diet modifications do not alleviate constipation, the secondary recommendation is non-prescription osmotic and stimulant laxatives. Osmotic laxatives are hypertonic solutions that draw fluid into the intestinal lumen by osmosis to create softer stool.Citation10 The most studied osmotic laxative is PEG3350 which has been shown to improve stool frequency and consistency, while also alleviating abdominal pain.Citation11,Citation12 Stimulant laxatives, such as Ex-Lax (senna, GlaxoSmithKline, Philadelphia PA) and Dulcolax (bisacodyl, Chattem, Inc., Chattanooga, TN), induce propagated colonic contractions to accelerate colonic transit.Citation13,Citation14 Earlier, stimulant laxatives were not routinely prescribed due to reports that senna and bisacodyl could damage the enteric nervous system. However, subsequent studies have discounted that notion, and stimulant laxatives are routinely used as part of a long-term treatment strategy.Citation15,Citation16 These non-prescription laxatives only alleviate constipation and do not treat abdominal pain or discomfort which is often associated with CIC and IBS-C.
Prescription treatment options for constipation
As the cause of OIC is known, peripherally-acting-mu-opioid-receptor-antagonists such as methylnaltrexone and naldemedine have been developed. These drugs are used to treat OIC patients, but have been associated with significant side effects such as abdominal pain and gastroenteritis that have been attributed to opioid withdrawal.Citation17 While the etiology of CIC is unknown, there is growing evidence for mis-regulation of serotonin pathways in association with IBS.Citation18 This led to the development of 5HT agonists as potential therapeutic options. The first such drug, Tegaserod (Novartis, Basel, Switzerland), was associated with adverse cardiovascular events and was withdrawn from the market.Citation19 Prucalopride (Shire Pharmaceuticals Ltd, London England), was developed as a more specific high-affinity 5HT4 agonist.Citation20–Citation22 In clinical trials, Prucalopride had prokinetic gastrointestinal properties and increased the number of spontaneous bowel movements in CIC patients.Citation21–Citation23 Although no adverse cardiovascular events have been reported, Prucalopride has significant gastrointestinal side effects including nausea and diarrhea.Citation19 While approved for CIC in Europe, concerns about this class of drugs resulting from Tegaserod has prevented the Food and Drug Administration (FDA)-approval in the United States.Citation24
Intestinal secretagogues are another promising class of drugs to treat constipation. Lubiprostone is a bicyclic fatty acid derived from prostaglandin E1 that activates CLCN2 voltage-gated chloride channels in the intestinal epithelium.Citation25 The chloride-rich secretions soften stool, increase intestinal peristalsis, and decrease intestinal transit time.Citation26,Citation27 It is FDA approved to treat OIC and CIC in men and women, but IBS-C only in women.Citation27 In clinical trials, patients reported increased numbers of bowel movements, improvements in straining, and improvements in stool consistency.Citation28–Citation30 The most common side effects of lubiprostone include nausea, headache, and diarrhea, but cardiovascular effects have also been reported.Citation31 In summary, there are diverse treatment options for constipation, but none are universally effective for all forms of constipation or in all patients. Moreover, all of the drugs have disruptive side effects such as gastrointestinal complications or severe headaches that reduce patient compliance ().
Table 1 Pharmaceutical agents available for the treatment of constipation
Guanylyl cyclase C (GC-C) agonists as secretagogues
Secretion of solutes and water into the intestinal lumen is essential for lubrication, and dysregulation of solute/fluid balance in the intestine is likely to underlie many cases of diarrhea and constipation.Citation32 Cyclic guanosine monophosphate (cGMP) is a second messenger and is a potent regulator of secretion in the intestine. It stimulates chloride secretion by activating type 2 cGMP-dependent protein kinase (PKG2), which in turn phosphorylates the cystic fibrosis transmembrane conductance regulator (CFTR).Citation33,Citation34 cGMP directly inhibits the NHE3 Na+/H+ exchanger to reduce sodium reabsorption, and in a CFTR-dependent manner, cGMP also promotes bicarbonate secretion by stimulating CI−/HCO3− exchanger.Citation35,Citation36 The effect of increasing cGMP in the intestinal epithelium is a net movement of water into the lumen resulting from solute secretion (). The concentration of cGMP in the intestinal epithelium is tightly controlled by the activity of GC-C receptors that convert GTP into cGMP upon stimulation by the endogenous peptide hormones uroguanylin and guanylin.Citation37 Activating mutations in the GC-C gene (GUCY2C) causes a familial diarrhea syndrome, whereas inactivating mutations result in heritable constipation.Citation38,Citation39 This genetic evidence underscores the importance of the cGMP signaling axis in FBDs.
Figure 1 The cyclic guanosine monophosphate (cGMP) signaling axis in the intestinal epithelium. Guanylyl cyclase C (GC-C) receptors expressed on the apical surface of intestinal epithelial cells produce cGMP from guanosine triphosphate (GTP) when bound by endogenous hormones uroguanylin (Uro) and guanylin (Gn). The synthetic peptide agonist plecanatide (Ple) mimics Uro, whereas linaclotide (Lin) mimics the heat-stable toxin from enterotoxigenic bacteria (STa). Elevated cGMP levels increase luminal solutes by blocking Na+ uptake through the sodium-hydrogen exchanger (NHE), and by activating type 2 cGMP-dependent protein kinase (PKG2), which in turn activates the cystic fibrosis transmembrane conductance regulator (CFTR). Several mechanisms restore equilibrium, including cGMP-specific phosphodiesterase 5 (PDE5) that hydrolyzes cGMP to inactive GMP, and by export of cGMP by multidrug resistance protein 4 (MDR4). Blockade of H+ exchange and secretion of HCO3− by the chloride-bicarbonate exchanger (Cl−/HCO3) increases luminal pH and reduces affinity of uroguanylin and plecanatide for GC-C (but not guanylin, linaclotide, or STa). Taken together, the stimulation of GC-C results in water secretion and reduced enteric nociception.
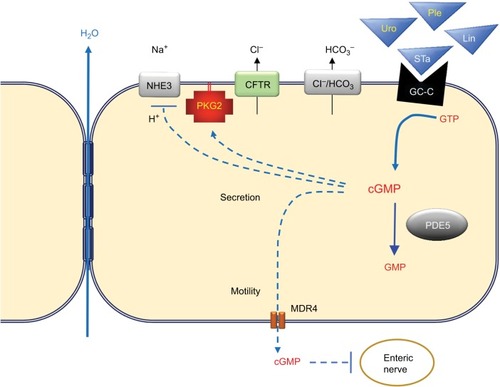
The structure and physiological roles of guanylin, uroguanylin, and the heat-stable enterotoxin (STa) that is a molecular mimic of these GC-agonists have been reviewed previously.Citation40 Uroguanylin and guanylin exhibit differential expression in the intestinal tract, with the former predominantly in the intestinal villi, and the latter more prominent in the colonic crypt.Citation41 In addition, uroguanylin is more active in acidic environments such as in the duodenum, whereas guanylin is more active in alkaline environments.Citation36,Citation42 The pH-dependent binding of uroguanylin to GC-C confers some degree of autoregulation because the increased pH resulting from cGMP-dependent inhibition of H+ secretion and stimulation of HCO3− exchange antagonizes uroguanylin efficacy. STa enterotoxin differs from uroguanylin in the amino-terminal residues than confer pH-dependent binding, resulting in excessive secretion and diarrhea. Based upon the secretagogue effect of GC-C agonists, synthetic peptides mimicking uroguanylin (plecanatide) and STa (linaclotide) have been developed for the treatment of constipation. Linaclotide was the first GC-C agonist approved and is closer in sequence to STa; with more potent GC-C activity that is less sensitive to pH.Citation43 Plecanatide was modeled after uroguanylin except for an amino-terminal substitution of one pH sensing residue. Plecanatide therefore retains some pH sensitivity, but is more potent than uroguanylin.Citation44
Clinical efficacy of plecanatide and linaclotide GC-C agonists
In August 2012, linaclotide was approved for treatment of adult patients with IBS-C (290 μg) and CIC (145 μg). More recently, 72 μg was also approved in order to help physicians better treat the heterogeneous population of adult patients suffering from CIC. In January 2017, another intestinal secretagogue and GC-C agonist plecanatide was approved for CIC (3 mg), and within a year, it was also approved for IBS-C at the same 3 mg dose. Plecanatide is administered orally in a water-soluble tablet and its mechanism of action is on the luminal epithelium, with minimal absorption and systemic availability.Citation44
Two 12-week clinical studies were conducted to determine the efficacy of plecanatide for the treatment of symptoms of CIC.Citation45 These were double-blind, placebo-controlled, randomized, multi-center trials including about 2000 adult patients. Patients were required to meet modified Rome III criteria for CIC for at least 3 months prior to the study, with symptoms being present for at least 6 months prior to diagnosis. These modified criteria included patients reporting less than three bowel movements per week, rarely reporting loose stool without the use of laxatives, and the patients could not meet the criteria for IBS-C. In addition, patients were required to report at least two of the following symptoms in at least 25% of defecations: straining, lumpy or hard stool, sensation of incomplete evacuations, or sensation of anorectal obstruction or blockage.
Patients self-reported daily spontaneous bowel movements (SBM) defined as a bowel movement that occurs without the use of laxatives. They were also instructed to record complete spontaneous bowel movements (CSBM) which are SBMs that give the patients a sense of complete evacuation. The primary efficacy endpoint of this study was the percentage of patients who were CSBM responders over the 12-week period. A CSBM responder was defined as a patient who had more than three CSBMs in a week. Plecanatide exhibited a rapid onset of efficacy, increasing the frequency of CSBMs, often as early as 1 week of treatment. Both the doses of plecanatide used in the study (3 and 6 mg) resulted in a greater percentage of overall CSBM responders than placebo. Throughout the treatment period, improvements were also observed in stool frequency and consistency and straining during bowel movements. During a 2-week follow-up period with no drug treatment, CSBMs of patients who had received plecanatide returned to placebo group. The results of the Phase III trial for plecanatide were nearly identical to the earlier clinical trial of linaclotide,Citation46 including similar diagnostic criteria, exclusion criteria, and primary endpoints. Linaclotide and plecanatide both had around a 20% response rate at all doses, and both the drugs significantly improved secondary endpoints such as stool consistency and frequency, severity of straining, and abdominal discomfort ().
Table 2 GC-C agonist clinical trial data for chronic idiopathic constipation
The most notable difference between the clinical trials was that patients taking linaclotide reported incidence of diarrhea three times higher than patients taking plecanatide (16% vs 6%, respectively) (). A reasonable explanation for this observation is that plecanatide retains pH sensitivity and is therefore subject to autoregulation, in contrast to linaclotide which was modeled after the diarrhea-causing STa enterotoxin. However, differences in the method of reporting adverse events might also account for the differences, since the definition of an adverse event was more stringent in the plecanatide trials. Although there have been no head-to-head comparisons of the effects of linaclotide and plecanatide in either IBS-C and CIC, a recent meta-analysis of the efficacy and tolerability of both GC-C agonists concluded that the treatment effects of each drug relative to placebo were comparable and that there was no significant difference in the odds of diarrhea for either drug.Citation47
Conclusion and future directions
Despite the plethora of potential underlying causes of CIC, patient responsiveness to plecanatide and linaclotide was similar in the independent Phase III studies. A reasonable explanation is that deficiency in the expression of endogenous GC-C agonists or in the GC-C receptor was the underlying cause of constipation in the responding patients and that treatment was essentially “hormone replacement therapy.” Although differences in relative severity were noted, the incidence of diarrhea was also similar for each drug (as a function of placebo). Both linaclotide and plecanatide have clear warnings against using either drug in pediatric patients because it can result in severe dehydrating diarrhea. The cause of this has been suggested (in the prescribing information for linaclotide) to involve overexpression of GC-C in the pediatric intestine relative to the adult. It is therefore plausible that the subset of patients who responded to the GC-C agonists with severe diarrhea might also express higher levels of GC-C (eg, a pediatric gut). This concept highlights the notion that the relative expression of all cGMP signaling components is important, and imbalances could cause either constipation or diarrhea. The cGMP phosphodiesterase (eg, PDE5) is expressed in the intestinal epithelium and is likely to play an essential role in antagonizing the effects of GC-C agonists.Citation48,Citation49 It is therefore possible that overexpression of PDE5 could be an underlying cause of constipation in some patients. Such patients would likely be refractory to linaclotide and plecanatide, but might be treated with PDE5 inhibitors. It is important to note that diarrhea is not a common side effect of PDE5 inhibitors at levels prescribed for erectile dysfunction. A likely explanation is that PDE5 inhibitors amplify endogenous cGMP levels that are generated when the GC-C agonists are released. The increased cGMP level resulting from PDE5 inhibition would therefore be subject to the same autoregulatory circuit described above for uroguanylin. A recent study has shown that PDE5 inhibition is as effective at correcting constipation as linaclotide in preclinical mouse models, suggesting that these drugs might also be clinically relevant for treating FBDs.Citation50
Although the impetus for developing synthetic GC-C agonists was the established role of the endogenous hormones in secretion, novel preclinical and clinical observations suggest that their therapeutic potential extends well beyond constipation. Both linaclotide and plecanatide (more recently) are also FDA-approved to treat constipation associated with IBS-C. The clinical response in this cohort was similar to CIC patients, but an unexpected analgesic effect was reported that was ostensibly independent of fluid secretion.Citation47,Citation51 While the underlying mechanism is poorly understood, preclinical evidence suggests that secreted epithelial cGMP has a dampening effect on neural afferents in the lamina propria.Citation52–Citation54 Increasing cGMP in the intestinal epithelium has also been shown by extensive preclinical studies to suppress intestinal carcinogenesisCitation55–Citation58 and promote barrier function in the colon.Citation59–Citation61 These intriguing findings underscore the clinical potential of GC-C agonists for treating post-infectious IBS, ulcerative colitis, and for chemoprevention of colorectal cancer.Citation61–Citation63 Although this may be counterintuitive, a drug formulation designed for delivery to the colon might avoid the intestinal secretagogue effect while maintaining barrier support and/or neuromuscular benefit.
Acknowledgments
This work was supported by the National Cancer Institute grant no. CA17262701A1.
Disclosure
The authors report no conflicts of interest in this work.
References
- Pinto SanchezMIBercikPEpidemiology and burden of chronic constipationCan J Gastroenterol20112511B15B
- CamilleriMFordACMaweGMChronic constipationNat Rev Dis Primers201731709529239347
- MearinFLacyBEChangLBowel disordersGastroenterology Epub2016218
- DrossmanDAFunctional gastrointestinal disorders: history, pathophysiology, clinical features, and Rome IVGastroenterology201615012621279.e1262
- SchmulsonMJDrossmanDAWhat is new in Rome IVJ Neurogastroenterol Moti201723151163
- ChangLLemboASultanSAmerican Gastroenterological Association Institute Technical Review on the pharmacological management of irritable bowel syndromeGastroenterology201414711491172.e114225224525
- EswaranSMuirJCheyWDFiber and functional gastrointestinal disordersAm J Gastroenterol201310871872723545709
- McRorieJWDaggyBPMorelJGDiersingPSMinerPBRobinsonMPsyllium is superior to docusate sodium for treatment of chronic constipationAliment Pharmacol Ther1998124914979663731
- RaoSSRattanakovitKPatcharatrakulTDiagnosis and management of chronic constipation in adultsNat Rev Gastroenterol Hepatol20161329530527033126
- FordACSuaresNCEffect of laxatives and pharmacological therapies in chronic idiopathic constipation: systematic review and meta-analysisGut20116020921821205879
- DiPalmaJADeRidderPHOrlandoRCKoltsBEClevelandMBA randomized, placebo-controlled, multicenter study of the safety and efficacy of a new polyethylene glycol laxativeAm J Gastroenterol20009544645010685748
- DipalmaJAClevelandMVMcGowanJHerreraJLA randomized, multicenter, placebo-controlled trial of polyethylene glycol laxative for chronic treatment of chronic constipationAm J Gastroenterol20071021436144117403074
- ParePFedorakRNSystematic review of stimulant and nonstimulant laxatives for the treatment of functional constipationCan J Gastroenterol Hepatol20142854955725390617
- Mueller-LissnerSKammMAWaldAMulticenter, 4-week, double-blind, randomized, placebo-controlled trial of sodium picosulfate in patients with chronic constipationAm J Gastroenterol2010105489790320179697
- KammMAMueller-LissnerSWaldARichterESwallowRGessenerUOral bisacodyl is effective and well-tolerated in patients with chronic constipationClin Gastroenterol Hepatol2011957758321440672
- KiemanJAHeinickeEASennosides do not kill myenteric neurons in the colon of the rat or mouseNeuroscience1989308378422570374
- CamilleriMLemboAKatzkaDAOpioids in gastroenterology: treating adverse effects and creating therapeutic benefitsClin Gastroenterol Hepatol2017151338134928529168
- Coss-AdameERaoSSBrain and gut interactions in irritable bowel syndrome: new paradigms and new understandingsCurr Gastroenterol Rep20141637924595616
- ShinACamilleriMKolarGErwinPWestCPMuradMHSystematic review with meta-analysis: highly selective 5-HT4 agonists (prucalopride, velusetrag or naronapride) in chronic constipationAliment Pharmacol Ther20143923925324308797
- MinerPBJrCamilleriMBurtonDPrucalopride induces high-amplitude propagating contractions in the colon of patients with chronic constipation: a randomized studyNeurogastroenterol Motil2016281341134827270968
- CamilleriMPiessevauxHYiannakouYEfficacy and safety of prucalopride in chronic constipation: an integrated analysis of six randomized, controlled clinical trialsDig Dis Sci2016612357237227056037
- EmmanuelACoolsMVandeplasscheLKerstensRPrucalopride improves bowel function and colonic transit time in patients with chronic constipation: an integrated analysisAm J Gastroenterol201410988789424732867
- TackJStanghelliniVDuboisDJosephAVandeplasscheLKerstensREffect of prucalopride on symptoms of chronic constipationNeurogastroenterol Motil201426212724106924
- LayerPKellerJLoefflerHKreissATegaserod in the treatment of irritable bowel syndrome (IBS) with constipation as the prime symptomTher Clin Risk Manag2007310711818360619
- LiFFuTTongWDLubiprostone is effective in the treatment of chronic idiopathic constipation and irritable bowel syndrome: a systematic review and meta-analysis of randomized controlled trialsMayo Clin Proc20169145646827046523
- CamilleriMBharuchaAEUenoREffect of a selective chloride channel activator, lubiprostone, on gastrointestinal transit, gastric sensory, and motor functions in healthy volunteersAm J Physiol Gastrointest Liver Physiol2006290G942G94716603730
- WilsonNScheyRLubiprostone in constipation: clinical evidence and place in therapyTher Adv Chronic Dis20156405025729555
- JamalMMAdamsABJansenJPWebsterLRA randomized, placebo-controlled trial of lubiprostone for opioid-induced constipation in chronic noncancer painAm J Gastroenterol201511072573225916220
- CryerBKatzSVallejoRPopescuAUenoRA randomized study of lubiprostone for opioid-induced constipation in patients with chronic noncancer painPain Med2014151825183424716835
- BarishCFDrossmanDJohansonJFUenoREfficacy and safety of lubiprostone in patients with chronic constipationDig Dis Sci2010551090109720012484
- Amitiza [package insert]Takeda Pharmaceuticals America IncDeerfield, IL600152017
- FieldMIntestinal ion transport and the pathophysiology of diarrheaJ Clin Invest200311193194312671039
- PfeiferAAszódiASeidlerURuthPHofmannFFässlerRIntestinal secretory defects and dwarfism in mice lacking cGMP-dependent protein kinase IIScience1996274208220868953039
- VaandragerABBotAGRuthPDifferential role of cyclic GMP-dependent protein kinase II in ion transport in murine small intestine and colonGastroenterology200011810811410611159
- ChaBKimJHHutHcGMP inhibition of Na+/H+ antiporter 3 (NHE3) requires PDZ domain adapter NHERF2, a broad specificity protein kinase G-anchoring proteinJ Biol Chem2005280166421665015722341
- JooNSLondonRMKimHDForteLRClarkeLLRegulation of intestinal Cl- and HCO3-secretion by uroguanylinAm J Physiol1998274G633G6449575844
- VaandragerABStructure and function of the heat-stable enterotoxin receptor/guanylyl cyclase CMol Cell Biochem2002230738311952098
- FiskerstrandTArshadNHaukanesBIFamilial diarrhea syndrome caused by an activating GUCY2C mutationN Engl J Med20123661586159522436048
- RomiHCohenILandauDMeconium ileus caused by mutations in GUCY2C, encoding the CFTR-activating guanylate cyclase 2CAm J Hum Genet20129089389922521417
- ForteLRJrUroguanylin and guanylin peptides: pharmacology and experimental therapeuticsPharmacol Ther200410413716215518884
- WhitakerTLWitteDPScottMCCohenMBUroguanylin and guanylin: distinct but overlapping patterns of messenger RNA expression in mouse intestineGastroenterology1997113100010069287995
- HamraFKEberSLChinDTCurrieMGForteLRRegulation of intestinal uroguanylin/guanylin receptor-mediated responses by mucosal acidityProc Natl Acad Sci U S A199794270527109122260
- BryantAPBusbyRWBartoliniWPLinaclotide is a potent and selective guanylate cyclase C agonist that elicits pharmacological effects locally in the gastrointestinal tractLife Sci20108676076520307554
- ShailubhaiKComiskeySFossJAPlecanatide, an oral guanylate cyclase C agonist acting locally in the gastrointestinal tract, is safe and well-tolerated in single dosesDig Dis Sci2013582580258623625291
- MinerPBJrKoltunWDWienerGJA randomized phase III clinical trial of plecanatide, a uroguanylin analog, in patients with chronic idiopathic constipationAm J Gastroenterol201711261362128169285
- LemboAJSchneierHAShiffSJTwo randomized trials of linaclotide for chronic constipationN Engl J Med201136552753621830967
- ShahEDKimHMSchoenfeldPEfficacy and tolerability of guanylate cyclase-C agonists for irritable bowel syndrome with constipation and chronic idiopathic constipation: a systematic review and meta-analysisAm J Gastroenterol201811332933829380823
- BakreMMSoporySVisweswariahSSExpression and regulation of the cGMP-binding, cGMP-specific phosphodiesterase (PDE5) in human colonic epithelial cells: role in the induction of cellular refractoriness to the heat-stable enterotoxin peptideJ Cell Biochem20007715916710679826
- SoporySKaurTVisweswariahSSThe cGMP-binding, cGMP-specific phosphodiesterase (PDE5): intestinal cell expression, regulation and role in fluid secretionCell Signal20041668169215093609
- SharmanSKIslamBNHouYSildenafil normalizes bowel transit in preclinical models of constipationPLoS One201712e017667328448580
- RaoSSQuigleyEMShiffSJEffect of linaclotide on severe abdominal symptoms in patients with irritable bowel syndrome with constipationClin Gastroenterol Hepatol20141261662324075889
- CastroJHarringtonAMHughesPALinaclotide inhibits colonic nociceptors and relieves abdominal pain via guanylate cyclase-C and extracellular cyclic guanosine 3′,5′-monophosphateGastroenterology2013145613341346e11123958540
- EutameneHBradesiSLaraucheMGuanylate cyclase C-mediated antinociceptive effects of linaclotide in rodent models of visceral painNeurogastroenterol Motil201022e312e384
- FengBKiyatkinMELaJHActivation of guanylate cyclase-C attenuates stretch responses and sensitization of mouse colorectal afferentsJ Neurosci2013339831983923739979
- ChangWLMasihSThadiAPlecanatide-mediated activation of guanylate cyclase-C suppresses inflammation-induced colorectal carcinogenesis in Apc+/Min-FCCC miceWorld J Gastrointest Pharmacol Ther20178475928217374
- IslamBNSharmanSKHouYSildenafil suppresses inflammation-driven colorectal cancer in miceCancer Prev Res (Phila)20171037738828468928
- ShailubhaiKYuHHKarunanandaaKUroguanylin treatment suppresses polyp formation in the Apc(Min/+) mouse and induces apoptosis in human colon adenocarcinoma cells via cyclic GMPCancer Res2000605151515711016642
- SharmanSKIslamBNHouYCyclic-GMP-elevating agents suppress polyposis in Apc(Min) mice by targeting the preneoplastic epitheliumCancer Prev Res (Phila)201811819229301746
- HanXMannEGilbertSLoss of guanylyl cyclase C (GCC) signaling leads to dysfunctional intestinal barrierPLoS One20116e1613921305056
- LinJESnookAELiPGUCY2C opposes systemic genotoxic tumorigenesis by regulating AKT-dependent intestinal barrier integrityPLoS One20127e3168622384056
- MannEAHarmel-LawsECohenMBSteinbrecherKAGuanylate cyclase C limits systemic dissemination of a murine enteric pathogenBMC Gastroenterol20131313524004613
- ShailubhaiKVaseemPKrishna PriyaAPlecanatide and dolcanatide, novel guanylate cyclase-C agonists, ameliorate gastrointestinal inflammation in experimental models of murine colitisWorld J Gastrointest Pharmacol Ther2015621322226558155
- WangRKwonIKSinghNType 2 cGMP-dependent protein kinase regulates homeostasis by blocking c-Jun N-terminal kinase in the colon epitheliumCell Death Differ20142142743724270408
