Abstract
Osteoarthritis (OA) is a prevalent global health concern, posing a significant and increasing public health challenge worldwide. Recently, nanotechnology-boosted biomaterials have emerged as a highly promising strategy for OA therapy due to their exceptional physicochemical properties and capacity to regulate pathological processes. However, there is an urgent need for a deeper understanding of the potential therapeutic applications of these biomaterials in the clinical management of diseases, particularly in the treatment of OA. In this comprehensive review, we present an extensive discussion of the current status and future prospects concerning nanotechnology-boosted biomaterials for OA therapy. Initially, we discuss the pathophysiology of OA and the constraints associated with existing treatment modalities. Subsequently, various types of nanomaterials utilized for OA therapy, including nanoparticles, nanofibers, and nanocomposites, are thoroughly discussed and summarized, elucidating their respective advantages and challenges. Furthermore, we analyze recent preclinical and clinical studies that highlight the potential of nanotechnology-boosted biomaterials in OA therapy. Additionally, future research directions in this evolving field are highlighted. By establishing a link between the structural properties of nanotechnology-boosted biomaterials and their therapeutic functions in OA treatment, we aim to foster advances in designing sophisticated nanomaterials for OA, ultimately resulting in improved therapeutic efficacy of OA therapy through translation into clinical setting in the near future.
Introduction
Osteoarthritis (OA), one of the most common degenerative diseases in aging people, is estimated that more than 10% of men and 18% of women over the age of 60 have symptomatic OA worldwide.Citation1 Of note, due to the high risk of working disability, it also imposes an overwhelming economic burden on individuals, healthcare systems, and societies.Citation2 OA is distinguished by the progressive deterioration of articular cartilage in the affected joints. The pathophysiology of OA is multifactorial, involving intricate interactions among various elements, including cartilage breakdown, chronic inflammatory responses, alterations in subchondral bone structure, synovial inflammation, and aberrant joint mechanics.Citation2 The cumulative effect of these processes manifests as pain, joint stiffness, and impaired joint mobility over time. Nonetheless, the current therapeutic options for OA are not without their limitations. Pharmacological interventions, such as nonsteroidal anti-inflammatory drugs (NSAIDs), serve to provide transient pain relief but lack the ability to modify the disease trajectory.Citation1,Citation3 Additionally, a deficiency of efficacious disease-modifying therapies hampers the ability to effectively slow down or halt the progressive nature of OA. In severe cases, surgical interventions, like joint replacement, offer relief; however, they are characterized by invasiveness and inherent risks. Furthermore, achieving complete restoration of damaged cartilage remains a challenge, with current approaches yielding limited success. Compounding the complexity, the individualized presentation of OA further complicates the pursuit of universally effective treatments.Citation4,Citation5 Therefore, it is highly desirable to achieve more in-depth knowledge of OA and decipher advanced therapeutic strategies to enhance clinical outcomes of OA therapy.
Emerging studies evidenced that there is an increasing demand for precise targeting in OA therapy, and biomaterial-based precision medicine represents a promising strategy for improving the unsatisfactory outcomes of current OA therapies ().Citation6 Importantly, with the excellent stride of development in nanotechnology, nanotechnology-boosted biomaterials have shown great potential in the field of OA therapy.Citation7,Citation8 The excellent physicochemical properties of nanotechnology-boosted biomaterials, such as high surface area ratio, tunable mechanical properties, and improved biocompatibility, have attracted accumulative interest of developing advanced biomaterials-based strategies for enhancing bone and cartilage regeneration in OA.Citation9,Citation10 Furthermore, by exploiting the unique properties of nanomaterials, such as their large surface area-to-volume ratio and the ability to modulate their size and surface chemistry, nanotechnology-boosted biomaterials can reinforced with controlled drug delivery manner, pro-regeneration activity, and immunomodulatory function.Citation11 Despite extensive studies on nanotechnology-boosted biomaterials, according to our knowledge, the use of these versatile vehicles for OA therapy has not been adequately explored.
Figure 1 The application of nanotechnology-boosted biomaterials in OA therapy. Created with MedPeer (https://www.medpeer.cn).
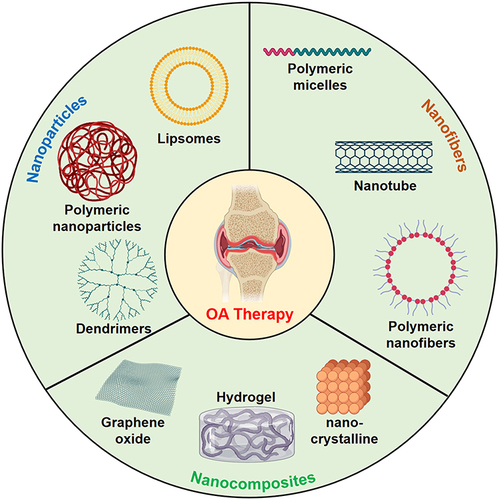
Herein, in this review, we summarize the advanced strategies developed for enhancing OA therapy based on the nanotechnology-boosted biomaterials. We conducted a comprehensive literature search using relevant databases such as PubMed, Scopus, and Web of Science. The search was focused on peer-reviewed articles and research papers published within the last ten years (from 2013 to 2023). We utilized specific keywords related to “nanotechnology-boosted biomaterials”, “nanotechnology-boosted biomaterials” and “osteoarthritis therapy” to retrieve relevant studies. First, we provide an overview of the pathophysiology of OA and the limitations of current treatment options. Second, we explore the various types of nanotechnology-boosted biomaterials which have been used for OA therapy, including nanoparticles, nanofibers, and nanocomposites. Third, we highlight the advantages and challenges associated with the use of nanotechnology-boosted biomaterials in OA therapy, such as toxicity, biodegradation, and regulatory issues. Finally, advanced biomaterials-based OA therapies with their potential for clinical translation and emerging nanotechnology-boosted biomaterials directions for OA therapy are discussed.
Characteristics of Nanotechnology-Boosted Biomaterials
Nanotechnology-boosted biomaterials have attracted considerable attention in recent years as promising candidates for revolutionizing the field of therapeutics.Citation12,Citation13 These materials combine the unique properties of nanotechnology with the versatility and biocompatibility of biomaterials, offering numerous advantages over existing therapeutic approaches. Nanotechnology enables the precise engineering of biomaterials at the nanoscale, allowing for the encapsulation and controlled release of therapeutic agents, such as drugs and growth factors.Citation14–17 This feature facilitates targeted and sustained drug delivery to specific sites within the body, reducing systemic side effects and enhancing treatment efficacy. In the context of OA, this targeted drug delivery can be utilized to deliver anti-inflammatory agents or disease-modifying drugs directly to affected joint tissues, promoting tissue repair and alleviating symptoms. Furthermore, nanotechnology-boosted biomaterials can be designed to mimic the native tissue environment, thereby enhancing their biocompatibility and reducing the risk of adverse reactions or immune responses.Citation18 This characteristic is crucial for successful integration and long-term functionality of biomaterials in biomedical applications. Moreover, nanomaterials can facilitate tissue regeneration by stimulating cellular responses and promoting tissue growth.Citation19 In the context of OA, nanotechnology-boosted biomaterials can assist in cartilage repair and regeneration, potentially slowing down disease progression and improving joint function.Citation3 In addition, nanotechnology allows for the customization of biomaterials with a wide range of physical, chemical, and biological properties.Citation13 This flexibility enables the development of multifunctional biomaterials that can simultaneously perform multiple tasks, such as drug delivery, imaging, and tissue regeneration. These advantages collectively contribute to their potential as innovative solutions in addressing various biomedical challenges and improving patient outcomes. In this section, we will discuss some of the key properties of nanotechnology-boosted biomaterials and their impact on OA treatment.
First, the size, shape, and composition of nanotechnology-boosted biomaterials are critical determinants of their biological activity. Nanoparticles with a size range of 1–100 nm have unique physical and chemical properties compared to larger particles, such as increased surface area-to-volume ratio and enhanced cellular uptake.Citation20 The size of nanoparticles can be controlled during their synthesis, and it is important to optimize the size for the specific application.Citation18 For example, nanoparticles with a size of 50–100 nm have been shown to accumulate in the inflamed joints of mice, making them ideal for targeted delivery of bioactive molecules in OA treatment.Citation3 Similarly, the shape of nanoparticles also plays a critical role in their biological activity. Nanoparticles with different shapes, such as spherical, cylindrical, and rod-like, have different cellular uptake mechanisms and bio-distribution.Citation21 For example, rod-shaped viral nanoparticles have been shown to penetrate deeper into tissues than spherical nanoparticles, making them ideal for delivery to the deeper layers of articular cartilage in OA treatment.Citation22 Moreover, the composition of nanotechnology-boosted biomaterials is also critical for their biological activity.Citation23 The choice of polymer, metal, or ceramic for the synthesis of nanoparticles can impact their biocompatibility, stability, and release kinetics.Citation24
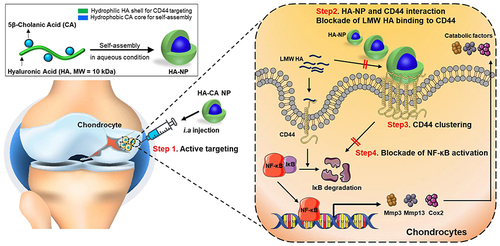
Importantly, the surface of nanotechnology-boosted biomaterials can be modified or functionalized to achieve specific biological activities, such as improved biocompatibility, targeted delivery, and enhanced cellular uptake.Citation12 Surface modification can be achieved by various techniques, such as covalent conjugation, physical adsorption, and electrostatic interaction.Citation13 One of the most common surface modifications of nanoparticles is the attachment of polyethylene glycol (PEG) to their surface. PEGylation can improve the stability and circulation time of nanoparticles in vivo by reducing their recognition by the immune system and preventing clearance by the reticuloendothelial system (RES).Citation25 Functionalization of the surface of nanotechnology-boosted biomaterials with ligands or peptides can also enhance their targeting and uptake by specific cells or tissues. For example, a prior study introduced an empty self-assembled hyaluronic acid (HA) nanoparticle as a promising therapeutic agent for OA therapy (). Specifically, this low-molecular-weight HA modified nanoparticles were demonstrated with long-term retention ability in knee joint, and the in vitro and in vivo results indicated that these functional nanoparticles can significantly enhance cartilage regeneration in OA models, providing a potential alternative for OA therapy.Citation26
Figure 2 Schematic illustration of HA-NPs for treatment of OA. Reprinted from Biomaterials, 275, Kang LJ, Yoon J, Rho JG et al. Self-assembled hyaluronic acid nanoparticles for osteoarthritis treatment. 120,967, Copyright 2021, with permission from Elsevier.Citation26
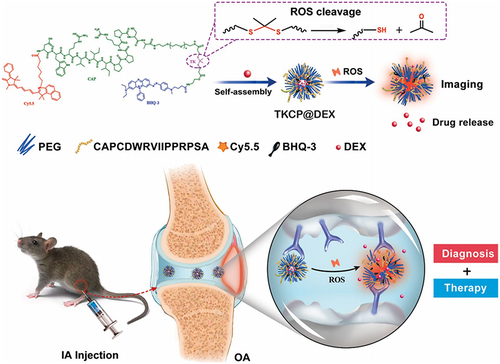
Targeted delivery of bioactive molecules to the site of injury is critical for the effective treatment of OA. Nanotechnology-boosted biomaterials can be designed to target specific cells, tissues, or organs using various mechanisms, such as passive targeting, active targeting, and stimuli-responsive targeting.Citation27 Passive targeting involves the accumulation of nanoparticles in the inflamed joints of OA due to the enhanced permeability and retention (EPR) effect. The EPR effect is a passive mechanism that allows nanoparticles to accumulate in the leaky blood vessels of inflamed tissues, resulting in enhanced uptake by target cells.Citation28 Accordingly, active targeting involves the attachment of ligands or peptides to the surface of nanoparticles that can recognize and bind to specific receptors on target cells.Citation29 Nanoparticles functionalized with antibodies or peptides that recognize receptors on the surface of chondrocytes have been shown to enhance their uptake and improve the efficacy of bioactive. In a previous study, a cartilage-targeting and ROS-responsive theranostic nanoprobe was developed to enable effective bioimaging and therapy for OA.Citation30 The fabrication of this nanoprobe involved modifying PEG micelles with ROS-sensitive thioketal linkers (TK) and integrating a cartilage-targeting peptide known as TKCP. Additionally, the nanoprobe was further engineered to encapsulate Dexamethasone (DEX), resulting in the formation of TKCP@DEX nanoparticles. The in vitro and in vivo results demonstrated the nanoprobe’s ability to facilitate sustained DEX release and leading to a notable reduction in cartilage damage within the OA-affected joints ().
Figure 3 Schematic illustration of the self-assembly of cartilage-targeting nanoparticles for OA treatment. Reproduced from Shen C, Gao M, Chen H, Zhan Y, Lan Q, Li Z et al. Reactive oxygen species (ROS)-responsive nanoprobe for bioimaging and targeting therapy of osteoarthritis. J Nanobiotechnology. 2021;19(1):395 under Creative Commons CC BY License.Citation30
Nanotechnology-boosted biomaterials represent a promising alternative to existing therapeutics, addressing critical challenges in drug delivery, bioavailability, targeted therapy, and regenerative medicine. Their unique properties and versatility open new avenues for improving treatment outcomes and enhancing therapies to individual patients. As the field of nanotechnology continues to advance, further research and translation into clinical applications hold great promise for transforming the landscape of medical treatments and patient care.
Fabrication and Modification of Nanotechnology-Boosted Biomaterials
The fabrication of these biomaterials is critical in determining their physical and chemical properties, and hence, their therapeutic efficacy. In this section, we will discuss the various fabrication methods of nanotechnology-boosted biomaterials for OA therapy.
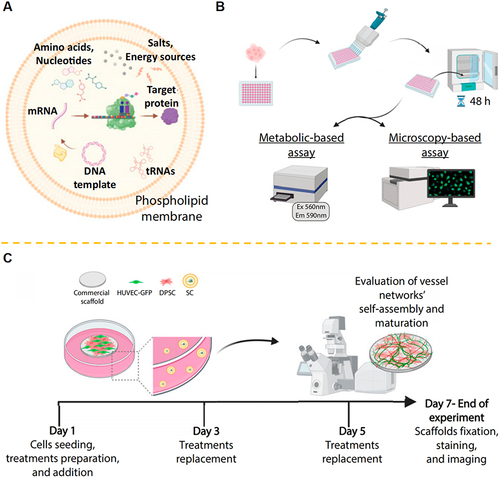
Generally, the bottom-up and top-down approaches are the two primary strategies for fabrication of nanotechnology-boosted biomaterials. The bottom-up approach involves the assembly of individual atoms or molecules to form a larger structure, while the top-down approach involves the breakdown of a larger structure into smaller components.Citation31,Citation32 In the bottom-up approach, various methods such as self-assembly, molecular epitaxy, and biomimetic synthesis can be used to fabricate nanotechnology-boosted biomaterials.Citation31 Self-assembly involves the spontaneous organization of molecules into ordered structures, such as micelles and vesicles. For instance, Hu et al presented self-assembly approach utilizing silica nanoparticles and DNA polymers to create hierarchically structured composite materials through clamped hybridization chain reactions. By self-assembling these nanocomposite materials, they fabricated thin layers within water-in-oil droplets generated microfluidically. This process enabled the production of mechanically stabilized hollow spheres with uniform size distributions at high throughput rates. Notably, these microcontainers have the potential for cell encapsulation, suggesting their utility in supramolecular bottom-up manufacturing and their application in the life sciences.Citation33 For molecular epitaxy approach, it involves the growth of thin films by sequentially depositing individual molecules on a substrate.Citation34 Accordingly, biomimetic synthesis involves the use of biological molecules, such as DNA and proteins, to assemble nanotechnology-boosted biomaterials.Citation35 In a recent study, Schroeder et al group designed synthetic cells that produce recombinant growth factors to support angiogenesis and tissue regeneration.Citation36 Interestingly, the in vivo results indicated that local injection of the synthetic cells in mice triggered the formation of new-borne blood vessels without recorded systemic immunogenicity. Therefore, these findings provide a potential therapeutic platform for mediating physiological processes by autonomously producing biological drugs inside the body ().
Figure 4 (A) Schematic illustration of the protein-producing synthetic cells. (B) The endothelial cells were cultured with synthetic cells prepared with/without inclusion of the TRX-bFGF DNA vector. (C) The examination of the formation of a three-dimensional vascular network in the different groups. Reprinted from Chen G, Levin R, Landau S et al. Implanted synthetic cells trigger tissue angiogenesis through de novo production of recombinant growth factors. Proc Natl Acad Sci U S A. 2022;119(38):e2207525119. Creative Commons.Citation36
In the top-down approach, various methods such as lithography, etching, and milling can be used for fabrication of nanotechnology-boosted biomaterials.Citation37 This approach can be applied toward the development of advanced therapies owing to the beneficial interactions enhanced through the retention of complex antigenic information.Citation38 Mechanistically, lithography involves the use of a mask to pattern a substrate, etching involves the removal of material from a substrate using a chemical or physical process, and milling involves the use of a milling tool to remove material from a substrate. In a prior study, Fang et al focused on the biological functionalization of polymeric nanoparticles with a layer of membrane coating derived from cancer cells. The core-shell nanostructures constructed by the top-down approach were demonstrated to carry the full array of cancer cell membrane antigens, and offer a promising platform with potential toward novel anti-cancer therapies.Citation38
In addition to the two main approaches, self-assembly is an another powerful technique for the fabrication of nanotechnology-boosted biomaterials, allowing for the spontaneous organization of molecules into ordered structures.Citation39 Self-assembly can be used to fabricate various types of nanotechnology-boosted biomaterials, such as nanoparticles, nanofibers, and nanotubes. Nanofabrication is also a feasible technique for the fabrication of nanotechnology-boosted biomaterials, involving the use of various methods such as electron beam lithography, focused ion beam milling, and atomic force microscopy.Citation40 Of note, nanofabrication can be used to create highly complex nanotechnology-boosted biomaterials with precise control over their size, shape, and composition.Citation41 For instance, Rogers et al group recently provided a series of hierarchical assembly concepts that leverage multiple layers of prestretched elastomeric substrates to produce 3D frameworks with complex, elaborate configurations.Citation42 One of the key features of this method is the control over strains used in the fabrication process, which provides reversible access to multiple different 3D layouts in a given structure. This means they can achieve different 3D geometries by controlling the applied strain during fabrication.Citation42
Moreover, surface functionalization and coating are critical steps in the fabrication of nanotechnology-boosted biomaterials, allowing for the modification of their surface properties and the attachment of bioactive molecules. Surface functionalization can be achieved through various methods such as chemical modification, plasma treatment, and physical adsorption. For example, Nie et al reported a novel functional modified-exosomes for anti-cancer therapy.Citation43 Specifically, the azide-modified macrophage-derived exosomes were conjugated with dibenzocyclooctyne-modified antibodies of CD47 and SIRPα, and the nano-bioconjugates can actively target tumors through the specific recognition between aCD47 and CD47 on the tumor cell surface.Citation43 In methodology, chemical modification involves the covalent attachment of functional groups to the surface of nanotechnology-boosted biomaterials, allowing for the precise control over the surface chemistry. Plasma treatment involves the use of a plasma to modify the surface chemistry of nanotechnology-boosted biomaterials, while physical adsorption involves the non-covalent attachment of molecules to the surface of nanotechnology-boosted biomaterials.Citation44 Furthermore, coating is another technique for the surface modification of nanotechnology-boosted biomaterials, allowing for the attachment of bioactive molecules and the formation of a biocompatible surface.Citation32 Electrospinning, layer-by-layer assembly, and physical vapor deposition are the main approaches for coating nanotechnology. Specifically, electrospinning is commonly use in electric field to fabricate nanofibers from a polymer solution, with the aim to achieve the precise control over the fiber diameter and orientation.Citation45 Accordingly, layer-by-layer assembly involves the sequential deposition of different layers of molecules onto the surface of nanotechnology-boosted biomaterials, allowing for the creation of complex surface architectures.Citation46 For physical vapor deposition, it involves the deposition of a thin film onto the surface of nanotechnology-boosted biomaterials, allowing for the precise control over the film thickness and composition.Citation47
Delivery Systems Based on Nanotechnology-Boosted Biomaterials
Nanotechnology-boosted biomaterials have provided precise and targeted delivery of therapeutic molecules to the site of injury, improving the therapeutic efficacy and reducing the risk of side effects.Citation44 In this section, we will discuss the various strategies for the delivery of bioactive agents and genes using nanotechnology-boosted biomaterials for OA treatment.
Delivery of Bioactive Agents
Bioactive agents such as growth factors, cytokines, and anti-inflammatory agents play a critical role in promoting tissue regeneration and reducing inflammation in OA. However, the systemic administration of these agents can lead to off-target effects and reduced efficacy. Nanotechnology-boosted biomaterials can be used to deliver these agents locally to the site of injury, improving their therapeutic efficacy and reducing the risk of side effects.Citation48
Nanoparticles are one of the most widely used nanotechnology-boosted biomaterials for the delivery of bioactive agents.Citation49 These particles can be engineered to have a specific size, shape, and surface chemistry, allowing for the targeted delivery of therapeutic molecules to the site of injury.Citation50 Various types of nanoparticles such as liposomes, polymeric nanoparticles, and dendrimers have been used for the delivery of bioactive agents for OA treatment.
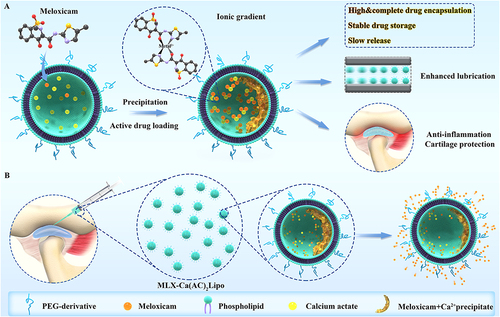
Liposomes are spherical nanoparticles composed of a phospholipid bilayer, allowing for the encapsulation of both hydrophilic and hydrophobic therapeutic molecules. These nanoparticles can be modified to improve their stability and targeting ability, such as the addition of PEG to reduce their clearance by the immune system.Citation51 For a recent example, Zhong et al reported an actively-loaded liposomal composites using meglumine to enhance aqueous solubility and divalent metal (Ca2+) solution to improve encapsulation efficiency. The in vitro and in vivo examination indicated that this liposomal biomaterial was capable of significantly ameliorating chondrocytes apoptosis and extracellular matrix degeneration via restoring the inflammatory microenvironment of OA joint (). Thus, these findings provided a promising nanomedicine for OA therapy by intra-articular injection.Citation52
Figure 5 Schematic illustration of fabrication and main advantages of articular injection of these liposomal nanoparticles in OA therapy. (A) Diagrammatic representation depicting the configuration of meloxicam-loaded liposomes through active means (MLX-Ca(AC)2Lipo), along with its primary merits when juxtaposed with the passive loading technique. These advantages encompass augmented and exhaustive drug encapsulation, enduring drug retention stability, and controlled drug liberation. (B) Schematic visualization delineating the process of administering MLX-Ca(AC)2Lipo through localized intra-articular injection to address osteoarthritic conditions of rats. The mode of meloxicam emancipation from the system is concurrently elucidated.
Furthermore, polymeric nanoparticles are an another type of nanoparticle that can be used for the delivery of bioactive agents. Polymeric nanoparticles are solid particles that can incorporate hydrophilic and hydrophobic drugs, such as small molecules, proteins, and nucleic acids. They are extensively used in the field of nanomedicine due to their structural versatility, facile synthesis, and higher stability when compared to other nanoparticle types.Citation53 These particles are composed of biocompatible and biodegradable polymers such as poly (lactic-co-glycolic acid) (PLGA) and can be engineered to have a specific size and surface chemistry.Citation54 For instance, the Qin et al group developed a novel cartilage targeting therapy based on the polymeric nanoparticles.Citation3 In this study, the in vitro and in vivo results indicated that polymeric micellar nanoparticles conjugated with a potent epidermal growth factor receptor (EGFR) ligand, were stable and nontoxic and had long joint retention, high cartilage uptake, and penetration capabilities. More important, in the OA mice model, intra-articular injection of these functional nanoparticles effectively attenuated cartilage degeneration, subchondral bone plate sclerosis, and joint pain. All these findings suggest the feasibility and safety of using nanotechnology to target EGFR signaling for OA therapy.Citation3
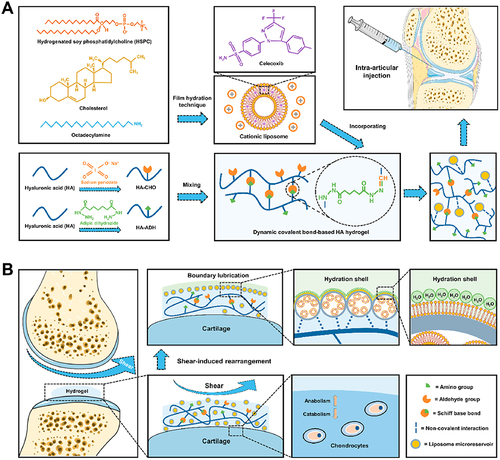
In addition to nanoparticles, other types of nanotechnology-boosted biomaterials such as hydrogels and scaffolds can also be used for the delivery of bioactive agents.Citation55–57 Hydrogels are three-dimensional networks of cross-linked polymers that can absorb large amounts of water, allowing for the encapsulation and release of therapeutic molecules. These biomaterials can be engineered to have a specific pore size and degradation rate, allowing for the controlled release of therapeutic molecules.Citation58 In a recent study, Huang et al group reported a novel drug-delivery hydrogel by incorporating celecoxib-loaded liposomes within dynamic covalent bond-based HA. Functionally, the in vitro and in vivo examinations suggested that this hydrogel acted as a stable drug delivery system for sustained release of celecoxib, and was able to protect anabolic-catabolic balance, improve cartilage wear, and attenuate OA progression ().Citation59 Therefore, this study shed beneficial sights on the therapeutic application of hydrogel-based drug delivery system in cartilage degenerative diseases like OA.
Figure 6 (A) The assembly of CLX@Lipo@HA-gels was accomplished through the integration of CLX-loaded HSPC liposomes within HA hydrogels linked by Schiff base bonds. (B) The resulting CLX@Lipo@HA-gels exhibit the capacity to unveil internal liposomal microreservoirs at external interfaces through shear-induced structural reconfigurations. This phenomenon engenders the creation of boundary layers, concurrently facilitating the dispensation of CLX to ameliorate the homeostatic equilibrium of the extracellular matrix by modulating anabolic and catabolic processes. Reprinted from Lei Y, Wang X, Liao J, Shen J, Li Y, Cai Z et al. Shear-responsive boundary-lubricated hydrogels attenuate osteoarthritis. Bioact Mater. 2022;16:472–484. Creative Commons.Citation59
Delivery of Genes and Non-Coding RNAs
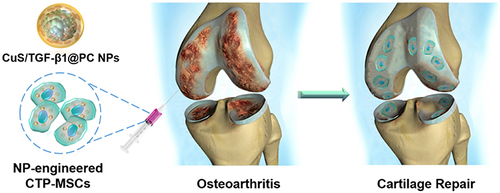
Gene therapy is an emerging approach for the treatment of OA, providing a mechanism for the delivery of therapeutic genes to the site of injury. However, the delivery of genes to the target tissue is a significant challenge due to the complexity of the extracellular matrix and the cellular barriers present in the joint. Nanotechnology-boosted biomaterials can be used to deliver genes and non-coding RNAs locally to the joint, improving their therapeutic efficacy and reducing the risk of off-target effects.Citation60 Various types of nanotechnology-boosted biomaterials such as nanoparticles, liposomes, and viral vectors have been used for the delivery of genes and non-coding RNAs for OA treatment.Citation61 Nanoparticles can be used to deliver plasmid DNA encoding therapeutic genes, allowing for the sustained expression of the therapeutic protein in the target tissue.Citation62 For example, Ding et al group recently introduced a novel nanoparticle encapsulated with plasmid DNA (pDNA) encoding transforming growth factor-beta 1 (TGF-β1) to engineer mesenchymal stem cells (MSCs) for promotion of cartilage regeneration in OA ().Citation63 Noticeably, they found that the nanoparticles were able to enhance cell proliferation and present a higher pDNA transfection efficiency compared with lipofectamine 3000. Furthermore, the in vitro and in vivo results all showed that the nanoparticles-engineered MSCs exhibited a better therapeutic efficacy in promotion of cartilage repair compared with pure MSCs.Citation63 Thus, these findings provides a potential strategy for overcoming the limitation of current stem cell therapy in OA therapy through designing and fabricating nano-engineered stem cells.
Figure 7 Schematic illustrations of the fabrication of nanoparticle-based gene therapy in OA treatment. Reprinted from Cai Y, Wu C, Ou Q, Zeng M, Xue S, Chen J et al. Enhanced osteoarthritis therapy by nanoengineered mesenchymal stem cells using biomimetic CuS nanoparticles loaded with plasmid DNA encoding TGF-beta1. Bioact Mater. 2023;19:444–457. Creative Commons.Citation63
Viral vectors are another type of nanotechnology-boosted biomaterial that can be used for the delivery of genes and non-coding RNAs. These vectors are derived from viruses and can be engineered to be non-pathogenic and target specific cell types. Adeno-associated virus (AAV) vectors have shown promise for gene therapy in OA, as they can efficiently transduce chondrocytes and have a low immunogenicity.Citation64 However, the use of viral vectors for gene therapy can raise safety concerns, such as the potential for integration into the host genome. In addition to genes, non-coding RNAs such as microRNAs (miRNAs) and small interfering RNAs (siRNAs) have also emerged as potential therapeutic targets for OA.Citation65 These non-coding RNAs regulate gene expression and can modulate pathways involved in OA pathology, such as inflammation and matrix degradation.Citation66 Nanoparticles, liposomes, and other nanotechnology-boosted biomaterials can be used to deliver these non-coding RNAs to the target tissue, allowing for the modulation of gene expression and the improvement of OA pathology.Citation67 Overall, nanotechnology-boosted biomaterials provide a promising platform for the delivery of bioactive agents and genes for the treatment of OA. These biomaterials can improve the targeted delivery of therapeutic molecules, enhancing their therapeutic efficacy and reducing the risk of off-target effects.
Green Nanomaterials for Cell and Mitochondria Targeting
The utilization of green nanomaterials for cell and mitochondria targeting represents an innovative and environmentally friendly approach in nanomedicine.Citation68 Green nanomaterials are derived from sustainable and biocompatible sources, minimizing potential adverse effects on both human health and the environment.Citation69 Utilizing their unique physicochemical properties, green nanomaterials have shown great potential for precise and efficient targeting of specific cells and mitochondria, offering promising opportunities for advanced therapeutic interventions.
Green nanomaterials are typically derived from natural sources such as plant extracts, proteins, polysaccharides, and lipids. These materials are renewable, cost-effective, and biocompatible, reducing concerns about toxicity and long-term environmental impact.Citation69 Furthermore, the surface of green nanomaterials can be easily functionalized to improve their selectivity and affinity for specific cells or organelles. Ligands, antibodies, or peptides that recognize receptors or biomarkers overexpressed on the target cells’ surface can be conjugated to the nanomaterials.Citation70–72 This active targeting allows the green nanomaterials to bind preferentially to the desired cells, increasing their accumulation and uptake while minimizing interactions with non-targeted cells. Mitochondria play a pivotal role in cellular energy production and are involved in various diseases, including neurodegenerative disorders, cancer, and metabolic diseases.Citation69,Citation72 Targeting mitochondria is a promising therapeutic approach. Importantly, green nanomaterials can be engineered to deliver therapeutic agents directly to the mitochondria. To achieve this, positively charged nanomaterials can be designed to interact with the negatively charged mitochondrial membrane, facilitating their uptake into these organelles. Peptide-based mitochondrial targeting sequences, such as mitochondria-penetrating peptides (MPPs), can also be incorporated into the green nanomaterials to improve their mitochondrial delivery efficiency.Citation70,Citation72
The utilization of green nanomaterials for cell and mitochondria targeting presents a promising avenue for developing precision medicine and therapeutic strategies. Their sustainable nature, combined with the ability to actively and selectively target specific cells and organelles, makes them an attractive alternative to conventional targeting approaches. Continued research in this field holds great potential for developing safe and effective therapies for a wide range of diseases, while contributing to sustainable and eco-friendly nanomedicine practices.
Challenges and Opportunities
Previous studies have extensively discussed the noteworthy nanotechnology-boosted biomaterials utilized in OA therapy.Citation73,Citation74 For instance, the study conducted by the Vahedi et al group performed a thorough examination of diverse optimization strategies applied to nanofibrous scaffolds, aiming to augment cartilage tissue engineering.Citation73 Specifically, their review analyzed the impact of nanoscaffold architecture on overall performance, and also provided a prospective outlook to promote progress in this promising domain.Citation73 However, despite these efforts, further investigations are warranted to comprehensively compile the most recent advances of nanotechnology-boosted biomaterials in OA therapy. Therefore, in this comprehensive review, we present an extensive survey of the current status and future prospects concerning nanotechnology-boosted biomaterials for OA therapy. Although nanotechnology-boosted biomaterials have exhibited huge potential for improved targeting, sustained release, and enhanced therapeutic efficacy for OA,Citation75 the development and translation of these biomaterials face several challenges and limitations, which need to be addressed to achieve successful clinical translation.
First of all, the development of nanotechnology-boosted biomaterials for OA treatment requires overcoming various technical challenges and limitations, including:
Synthesis and characterization of nanoparticles: The production of nanoparticles with the desired size, shape, and surface properties for targeted OA therapy can be challenging. The characterization of nanoparticles is also crucial for their successful use in vivo, as the properties of nanoparticles can change significantly in biological environments.
Biocompatibility and toxicity: The safety and biocompatibility of nanoparticles are essential factors for clinical translation. It is crucial to assess the potential toxicity and immunogenicity of nanoparticles, as well as their interactions with biological systems, such as cells, tissues, and organs.
Control of nanoparticle release: The controlled release of nanoparticles is crucial for achieving sustained therapeutic effects. However, achieving this control can be challenging due to the complex interactions between nanoparticles and the surrounding biological environment.
Targeting and penetration: Nanoparticles should be designed to target specific tissues and penetrate the extracellular matrix to reach the desired site of action. However, the heterogeneity of the OA tissue and the presence of various barriers can hinder effective targeting and penetration.
The safety and regulatory issues associated with nanotechnology-boosted biomaterials are also significant challenges that need to be addressed for successful clinical translation. The regulatory approval process for nanotechnology-boosted products is rigorous, and it requires extensive safety and efficacy data. The safety concerns associated with nanoparticles, such as their potential toxicity, immunogenicity, and long-term effects, need to be addressed in preclinical studies before clinical trials can be conducted. The development of effective strategies to evaluate the safety and efficacy of nanoparticles is crucial for their successful clinical translation.
Hence, to overcome the challenges and limitations of nanotechnology-boosted biomaterials for OA treatment, several future directions and recommendations can be considered:
Advanced characterization techniques: The development of advanced characterization techniques that can provide more accurate and detailed information on the properties of nanoparticles are essential. Techniques such as high-resolution microscopy and spectroscopy can provide valuable insights into the interaction of nanoparticles with biological systems.
In vitro and in vivo toxicity assessment: Toxicity assessment of nanoparticles should be conducted using relevant models to determine their safety and biocompatibility. These studies should consider the long-term effects of nanoparticles and their potential to cause immunogenicity and other adverse effects.
Development of targeted delivery systems: Targeted delivery systems should be developed to improve the targeting and penetration of nanoparticles in OA tissue. These delivery systems can include various targeting ligands and surface modifications to enhance the binding and internalization of nanoparticles into OA cells.
Integration with other therapies: nanotechnology-boosted biomaterials can be integrated with other OA therapies, such as cell and gene therapy, to enhance their therapeutic effects. Combination therapies can provide a synergistic effect that can improve the overall efficacy of OA treatment.
In conclusion, nanotechnology-boosted biomaterials have shown great potential for OA treatment, offering beneficial advantages such as improved drug delivery, enhanced tissue regeneration, and reduced inflammation. However, there are still technical challenges that need to be addressed, including the limited availability of suitable biomaterials, the complexity of the design and synthesis of nanoparticles, and the difficulty in achieving effective targeting of the affected joint. Additionally, safety and regulatory issues need to be thoroughly considered and addressed to ensure the clinical translation of these technologies.
The field of nanotechnology-boosted biomaterials for OA therapy holds significant potential for advancing treatment options and improving patient outcomes. Several future research directions can further enhance the application of nanotechnology in OA therapy, including advanced drug delivery systems, personalized nanomedicine, biomimetic nanomaterials, combination therapies, and clinical trials and translational research. By focusing on these future research directions, the field of nanotechnology-boosted biomaterials for OA therapy can make significant strides towards revolutionizing treatment approaches, promoting cartilage regeneration, and improving the quality of life for OA patients.
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgments
This review was funded by Science and technology plan of Xi’an (grant numbers 22YXYJ0021 and 22YXYJ0024). We express our gratitude to Medpeer software (https://image.medpeer.cn/) for its valuable assistance in providing the image source used in preparation.
References
- Glyn-Jones S, Palmer AJ, Agricola R, et al. Osteoarthritis. Lancet. 2015;386(9991):376–387. doi:10.1016/S0140-6736(14)60802-3
- Martel-Pelletier J, Barr AJ, Cicuttini FM, et al. Osteoarthritis. Nat Rev Dis Primers. 2016;2:16072. doi:10.1038/nrdp.2016.72
- Wei Y, Luo L, Gui T, et al. Targeting cartilage EGFR pathway for osteoarthritis treatment. Sci Transl Med. 2021;13(576). doi:10.1126/scitranslmed.abb3946
- Zhao Y, Song S, Wang D, et al. Nanozyme-reinforced hydrogel as a H(2)O(2)-driven oxygenerator for enhancing prosthetic interface osseointegration in rheumatoid arthritis therapy. Nat Commun. 2022;13(1):6758. doi:10.1038/s41467-022-34481-5
- Zhen G, Wen C, Jia X, et al. Inhibition of TGF-beta signaling in mesenchymal stem cells of subchondral bone attenuates osteoarthritis. Nat Med. 2013;19(6):704–712. doi:10.1038/nm.3143
- Jiang Y. Osteoarthritis year in review 2021: biology. Osteoarthritis Cartilage. 2022;30(2):207–215. doi:10.1016/j.joca.2021.11.009
- Zhang X, Chen X, Zhao Y. Nanozymes: versatile Platforms for Cancer Diagnosis and Therapy. Nanomicro Lett. 2022;14(1):95. doi:10.1007/s40820-022-00828-2
- Zhang X, Chen X, Song J, Zhang J, Ren X, Zhao Y. Size-Transformable nanostructures: from design to biomedical applications. Adv Mater. 2020;32(48):e2003752. doi:10.1002/adma.202003752
- Li M, Luo Z, Zhao Y. Hybrid nanoparticles as drug carriers for controlled chemotherapy of cancer. Chem Rec. 2016;16(4):1833–1851. doi:10.1002/tcr.201600029
- Liang S, Yao J, Liu D, Rao L, Chen X, Wang Z. Harnessing Nanomaterials for Cancer Sonodynamic Immunotherapy. Adv Mater. 2023;35. doi:10.1002/adma.202211130:e2211130
- Yang K, Yang Z, Yu G, Nie Z, Wang R, Chen X. Polyprodrug nanomedicines: an emerging paradigm for cancer therapy. Adv Mater. 2022;34(6):e2107434. doi:10.1002/adma.202107434
- Kunz-Schughart LA, Dubrovska A, Peitzsch C, et al. Nanoparticles for radiooncology: mission, vision, challenges. Biomaterials. 2017;120:155–184. doi:10.1016/j.biomaterials.2016.12.010
- Nethi SK, Das S, Patra CR, Mukherjee S. Recent advances in inorganic nanomaterials for wound-healing applications. Biomater Sci. 2019;7(7):2652–2674. doi:10.1039/C9BM00423H
- Piktel E, Niemirowicz K, Watek M, Wollny T, Deptula P, Bucki R. Recent insights in nanotechnology-boosted drugs and formulations designed for effective anti-cancer therapy. J Nanobiotechnology. 2016;14(1):39. doi:10.1186/s12951-016-0193-x
- Qian W-M, Vahid MH, Sun Y-L, et al. Investigation on the effect of functionalization of single-walled carbon nanotubes on the mechanical properties of epoxy glass composites: experimental and molecular dynamics simulation. J Materials Res Technol. 2021;12:1931–1945. doi:10.1016/j.jmrt.2021.03.104
- Maghsoudlou MA, Nassireslami E, Saber-Samandari S, Khandan A. Bone regeneration using bio-nanocomposite tissue reinforced with bioactive nanoparticles for femoral defect applications in medicine. Avicenna J Med Biotechnol. 2020;12(2):68–76.
- Abdellahi M, Karamian E, Najafinezhad A, Ranjabar F, Chami A, Khandan A. Diopside-magnetite; A novel nanocomposite for hyperthermia applications. J Mech Behav Biomed Mater. 2018;77:534–538. doi:10.1016/j.jmbbm.2017.10.015
- Cheng H, Chawla A, Yang Y, et al. Development of nanomaterials for bone-targeted drug delivery. Drug Discov Today. 2017;22(9):1336–1350. doi:10.1016/j.drudis.2017.04.021
- Nga NK, Thuy Chau NT, Viet PH. Facile synthesis of hydroxyapatite nanoparticles mimicking biological apatite from eggshells for bone-tissue engineering. Colloids Surf B Biointerfaces. 2018;172:769–778. doi:10.1016/j.colsurfb.2018.09.039
- Chintapula U, Chikate T, Sahoo D, et al. Immunomodulation in age-related disorders and nanotechnology interventions. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2023;15(1):e1840. doi:10.1002/wnan.1840
- Tang F, Li L, Chen D. Mesoporous silica nanoparticles: synthesis, biocompatibility and drug delivery. Adv Mater. 2012;24(12):1504–1534. doi:10.1002/adma.201104763
- Maturavongsadit P, Luckanagul JA, Metavarayuth K, et al. Promotion of In vitro chondrogenesis of mesenchymal stem cells using in situ hyaluronic hydrogel functionalized with rod-like viral nanoparticles. Biomacromolecules. 2016;17(6):1930–1938. doi:10.1021/acs.biomac.5b01577
- Huang W, Ling S, Li C, Omenetto FG, Kaplan DL. Silkworm silk-based materials and devices generated using bio-nanotechnology. Chem Soc Rev. 2018;47(17):6486–6504. doi:10.1039/C8CS00187A
- Tang B, Xia W, Cai W, Liu J. Luminescent gold nanoparticles with controllable hydrophobic interactions. Nano Lett. 2022;22(20):8109–8114. doi:10.1021/acs.nanolett.2c02486
- Jebari-Benslaiman S, Uribe KB, Benito-Vicente A, et al. Boosting Cholesterol Efflux from Foam Cells by Sequential Administration of rHDL to Deliver MicroRNA and to Remove Cholesterol in a Triple-Cell 2D Atherosclerosis Model. Small. 2022;18(13):e2105915. doi:10.1002/smll.202105915
- Kang LJ, Yoon J, Rho JG, et al. Self-assembled hyaluronic acid nanoparticles for osteoarthritis treatment. Biomaterials. 2021;275:120967. doi:10.1016/j.biomaterials.2021.120967
- Wang Y, Zhang P, Wei Y, et al. Cell-Membrane-Display Nanotechnology. Adv Healthc Mater. 2021;10(1):e2001014. doi:10.1002/adhm.202001014
- Bertrand N, Wu J, Xu X, Kamaly N, Farokhzad OC. Cancer nanotechnology: the impact of passive and active targeting in the era of modern cancer biology. Adv Drug Deliv Rev. 2014;66:2–25. doi:10.1016/j.addr.2013.11.009
- Wang Y, Zhang K, Li T, et al. Macrophage membrane functionalized biomimetic nanoparticles for targeted anti-atherosclerosis applications. Theranostics. 2021;11(1):164–180. doi:10.7150/thno.47841
- Shen C, Gao M, Chen H, et al. Reactive oxygen species (ROS)-responsive nanoprobe for bioimaging and targeting therapy of osteoarthritis. J Nanobiotechnology. 2021;19(1):395. doi:10.1186/s12951-021-01136-4
- Andreo J, Ettlinger R, Zaremba O, et al. Reticular nanoscience: bottom-up assembly nanotechnology. J Am Chem Soc. 2022;144(17):7531–7550. doi:10.1021/jacs.1c11507
- Fang RH, Kroll AV, Gao W, Zhang L. Cell membrane coating nanotechnology. Adv Mater. 2018;30(23):e1706759. doi:10.1002/adma.201706759
- Hu Y, Grosche M, Sheshachala S, et al. Bottom-Up Assembly of DNA-Silica Nanocomposites into Micrometer-Sized Hollow Spheres. Angew Chem Int Ed Engl. 2019;58(48):17269–17272. doi:10.1002/anie.201910606
- Wang Z, Blaszczyk A, Fuhr O, Heissler S, Woll C, Mayor M. Molecular weaving via surface-templated epitaxy of crystalline coordination networks. Nat Commun. 2017;8:14442. doi:10.1038/ncomms14442
- Gopfrich K, Platzman I, Spatz JP. Mastering complexity: towards bottom-up construction of multifunctional eukaryotic synthetic cells. Trends Biotechnol. 2018;36(9):938–951. doi:10.1016/j.tibtech.2018.03.008
- Chen G, Levin R, Landau S, et al. Implanted synthetic cells trigger tissue angiogenesis through de novo production of recombinant growth factors. Proc Natl Acad Sci U S A. 2022;119(38):e2207525119. doi:10.1073/pnas.2207525119
- Stanev TK, Liu P, Zeng H, et al. Direct patterning of optoelectronic nanostructures using encapsulated layered transition metal dichalcogenides. ACS Appl Mater Interfaces. 2022;14(20):23775–23784. doi:10.1021/acsami.2c03652
- Fang RH, Hu CM, Luk BT, et al. Cancer cell membrane-coated nanoparticles for anticancer vaccination and drug delivery. Nano Lett. 2014;14(4):2181–2188. doi:10.1021/nl500618u
- Bishop KJM. Self-assembly across scales. Nat Mater. 2022;21(5):501–502. doi:10.1038/s41563-022-01235-z
- Wang Y, Mirkin CA, Park SJ. Nanofabrication beyond electronics. ACS Nano. 2009;3(5):1049–1056. doi:10.1021/nn900448g
- Zhang G, Surwade SP, Zhou F, Liu H. DNA nanostructure meets nanofabrication. Chem Soc Rev. 2013;42(7):2488–2496. doi:10.1039/C2CS35302D
- Zhao H, Cheng X, Wu C, et al. Mechanically Guided Hierarchical Assembly of 3D Mesostructures. Adv Mater. 2022;34(12):e2109416. doi:10.1002/adma.202109416
- Nie W, Wu G, Zhang J, et al. Responsive exosome nano-bioconjugates for synergistic cancer therapy. Angew Chem Int Ed Engl. 2020;59(5):2018–2022. doi:10.1002/anie.201912524
- Hu Q, Li H, Wang L, Gu H, Fan C. DNA nanotechnology-enabled drug delivery systems. Chem Rev. 2019;119(10):6459–6506. doi:10.1021/acs.chemrev.7b00663
- Farokhi M, Mottaghitalab F, Reis RL, Ramakrishna S, Kundu SC. Functionalized silk fibroin nanofibers as drug carriers: advantages and challenges. J Control Release. 2020;321:324–347. doi:10.1016/j.jconrel.2020.02.022
- Correa S, Boehnke N, Barberio AE, et al. Tuning nanoparticle interactions with ovarian cancer through layer-by-layer modification of surface chemistry. ACS Nano. 2020;14(2):2224–2237. doi:10.1021/acsnano.9b09213
- Zhao Z, Hou T, Wu N, et al. Polycrystalline few-layer graphene as a durable anticorrosion film for copper. Nano Lett. 2021;21(2):1161–1168. doi:10.1021/acs.nanolett.0c04724
- Patra JK, Das G, Fraceto LF, et al. Nano based drug delivery systems: recent developments and future prospects. J Nanobiotechnology. 2018;16(1):71. doi:10.1186/s12951-018-0392-8
- Garbayo E, Pascual-Gil S, Rodriguez-Nogales C, Saludas L, Estella-Hermoso De Mendoza A, Blanco-Prieto MJ. Nanomedicine and drug delivery systems in cancer and regenerative medicine. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2020;12(5):e1637. doi:10.1002/wnan.1637
- Onugwu AL, Nwagwu CS, Onugwu OS, et al. Nanotechnology based drug delivery systems for the treatment of anterior segment eye diseases. J Control Release. 2023;354:465–488. doi:10.1016/j.jconrel.2023.01.018
- Wang W, Lu KJ, Yu CH, Huang QL, Du YZ. Nano-drug delivery systems in wound treatment and skin regeneration. J Nanobiotechnology. 2019;17(1):82. doi:10.1186/s12951-019-0514-y
- Zhong Y, Zhou Y, Ding R, et al. Intra-articular treatment of temporomandibular joint osteoarthritis by injecting actively-loaded meloxicam liposomes with dual-functions of anti-inflammation and lubrication. Mater Today Bio. 2023;19:100573. doi:10.1016/j.mtbio.2023.100573
- Pontes AP, Welting TJM, Rip J, Creemers LB. Polymeric nanoparticles for drug delivery in osteoarthritis. Pharmaceutics. 2022;14(12):2639. doi:10.3390/pharmaceutics14122639
- Bruno MC, Cristiano MC, Celia C, et al. Injectable Drug Delivery Systems for Osteoarthritis and Rheumatoid Arthritis. ACS Nano. 2022;16(12):19665–19690. doi:10.1021/acsnano.2c06393
- Lin X, Tsao CT, Kyomoto M, Zhang M. Injectable Natural Polymer Hydrogels for Treatment of Knee Osteoarthritis. Adv Healthc Mater. 2022;11(9):e2101479. doi:10.1002/adhm.202101479
- Soleimani M, Asgharzadeh Salmasi A, Asghari S, et al. Optimization and fabrication of alginate scaffold for alveolar bone regeneration with sufficient drug release. Int Nano Lett. 2021;11(3):295–305. doi:10.1007/s40089-021-00342-0
- Angili SN, Morovvati MR, Kardan-Halvaei M, et al. Fabrication and finite element simulation of antibacterial 3D printed Poly L-lactic acid scaffolds coated with alginate/magnesium oxide for bone tissue regeneration. Int J Biol Macromol. 2023;224:1152–1165. doi:10.1016/j.ijbiomac.2022.10.200
- Zhao T, Wei Z, Zhu W, Weng X. Recent Developments and Current Applications of Hydrogels in Osteoarthritis. Bioengineering. 2022;9(4):567.
- Lei Y, Wang X, Liao J, et al. Shear-responsive boundary-lubricated hydrogels attenuate osteoarthritis. Bioact Mater. 2022;16:472–484. doi:10.1016/j.bioactmat.2022.02.016
- Jiang Y, Fan M, Yang Z, et al. Recent advances in nanotechnology approaches for non-viral gene therapy. Biomater Sci. 2022;10(24):6862–6892. doi:10.1039/D2BM01001A
- Xu X, Liu C, Wang Y, et al. nanotechnology-boosted delivery of CRISPR/Cas9 for cancer treatment. Adv Drug Deliv Rev. 2021;176:113891. doi:10.1016/j.addr.2021.113891
- Hald Albertsen C, Kulkarni JA, Witzigmann D, Lind M, Petersson K, Simonsen JB. The role of lipid components in lipid nanoparticles for vaccines and gene therapy. Adv Drug Deliv Rev. 2022;188:114416. doi:10.1016/j.addr.2022.114416
- Cai Y, Wu C, Ou Q, et al. Enhanced osteoarthritis therapy by nanoengineered mesenchymal stem cells using biomimetic CuS nanoparticles loaded with plasmid DNA encoding TGF-beta1. Bioact Mater. 2023;19:444–457. doi:10.1016/j.bioactmat.2022.04.021
- Ji Q, Xu X, Kang L, et al. Hematopoietic PBX-interacting protein mediates cartilage degeneration during the pathogenesis of osteoarthritis. Nat Commun. 2019;10(1):313. doi:10.1038/s41467-018-08277-5
- Shen S, Yang Y, Shen P, et al. circPDE4B prevents articular cartilage degeneration and promotes repair by acting as a scaffold for RIC8A and MID1. Ann Rheum Dis. 2021;80(9):1209–1219. doi:10.1136/annrheumdis-2021-219969
- Ji ML, Jiang H, Wu F, et al. Precise targeting of miR-141/200c cluster in chondrocytes attenuates osteoarthritis development. Ann Rheum Dis. 2021;80(3):356–366. doi:10.1136/annrheumdis-2020-218469
- Zhao Y, Deng X, Tan S, et al. Co-Polymer Carrier with Dual Advantages of Cartilage-Penetrating and Targeting Improves Delivery and Efficacy of MicroRNA Treatment of Osteoarthritis. Adv Healthc Mater. 2023;12(6):e2202143. doi:10.1002/adhm.202202143
- Bae Y, Jung MK, Song SJ, et al. Functional nanosome for enhanced mitochondria-targeted gene delivery and expression. Mitochondrion. 2017;37:27–40. doi:10.1016/j.mito.2017.06.005
- Cheng Y, Ji Y, Tong J. Triple stimuli-responsive supramolecular nanoassembly with mitochondrial targetability for chemophotothermal therapy. J Control Release. 2020;327:35–49. doi:10.1016/j.jconrel.2020.08.006
- Wani AK, Akhtar N, Mir TUG, et al. Targeting Apoptotic Pathway of Cancer Cells with Phytochemicals and Plant-Based Nanomaterials. Biomolecules. 2023;13(2):194. doi:10.3390/biom13020194
- Xiong Y, Lin Z, Bu P, et al. A Whole-course-repair system based on neurogenesis-angiogenesis crosstalk and macrophage reprogramming promotes diabetic wound healing. Adv Mater. 2023;35(19):e2212300. doi:10.1002/adma.202212300
- Zeng WN, Yu QP, Wang D, et al. Mitochondria-targeting graphene oxide nanocomposites for fluorescence imaging-guided synergistic phototherapy of drug-resistant osteosarcoma. J Nanobiotechnology. 2021;19(1):79. doi:10.1186/s12951-021-00831-6
- Ahmadian E, Eftekhari A, Janas D, Vahedi P. Nanofiber scaffolds based on extracellular matrix for articular cartilage engineering: a perspective. Nanotheranostics. 2023;7(1):61–69. doi:10.7150/ntno.78611
- Vahedi P, Moghaddamshahabi R, Webster TJ, et al. The use of infrapatellar fat pad-derived mesenchymal stem cells in articular cartilage regeneration: a review. Int J Mol Sci. 2021;22(17):9215. doi:10.3390/ijms22179215
- Brown S, Kumar S, Sharma B. Intra-articular targeting of nanomaterials for the treatment of osteoarthritis. Acta Biomater. 2019;93:239–257. doi:10.1016/j.actbio.2019.03.010
