 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
IHL-305 is a PEGylated liposomal formulation of irinotecan (CPT-11). The objective of this study was to evaluate the factors associated with interpatient variability in the pharmacokinetics and pharmacodynamics of IHL-305 in patients with advanced solid tumors. IHL-305 was administered intravenously once every 4 weeks as part of a Phase I study. Pharmacokinetic studies of the liposomal sum total CPT-11, released CPT-11, SN-38, SN-38G, 7-ethyl-10-[4-N-(5-aminopentanoic acid)-1-piperidino]-carbonyloxycamptothecin, and 7-ethyl-10-[4-amino-1-piperidino]-carbonyloxycamptothecin in plasma were performed. Noncompartmental and compartmental pharmacokinetic analyses were conducted using pharmacokinetic data for sum total CPT-11. The pharmacokinetic variability of IHL-305 is associated with linear and nonlinear clearance. Patients whose age and body composition (ratio of total body weight to ideal body weight [TBW/IBW]) were greater than the median age and TBW/IBW of the study had a 1.7-fold to 2.6-fold higher ratio of released CPT-11 area under the concentration versus time curve (AUC) to sum total CPT-11 AUC. Patients aged <60 years had a 1.3-fold higher ratio of percent decrease in monocytes at nadir to percent decrease in absolute neutrophil count at nadir as compared with patients aged ≥60 years. There was an inverse relationship between patient age and percent decrease in monocytes at nadir, ie, younger patients have a higher percent decrease in monocytes. Patients with a higher percent decrease in monocytes at nadir have a decreased plasma exposure of sum total CPT-11. The pharmacokinetics and pharmacodynamics of IHL-305 are consistent with those of other PEGylated liposomal carriers. Interpatient variability in the pharmacokinetics and pharmacodynamics of IHL-305 was associated with age, body composition, and monocytes.
Introduction
IHL-305 is a PEGylated liposomal formulation of irinotecan (CPT-11), a camptothecin analog that inhibits topoisomerase I and is approved for the treatment of metastatic colorectal cancer.Citation1–Citation4 The PEGylated liposomal formulation consists of phospholipids arranged in a bilayer with polyethylene glycol (PEG) covalently bound on the external surface. Encapsulation of CPT-11 allows for release of the active lactone form into the tumor over a protracted period of time, which is ideal for a cell cycle-specific drug.Citation1–Citation6 CPT-11 is a prodrug that requires metabolic transformation to the active metabolite, 7-ethyl-10-hydroxy-camptothecin (SN-38), which is approximately 100-fold to 1,000-fold more active than the parent drug. SN-38 is further conjugated to form an inactive glucuronide (SN-38G) by uridine diphosphate glucuronosyltransferases, primarily the UGT1A1 isoform. Other identified CPT-11 metabolites are 7-ethyl-10-[4-N-(5-aminopentanoic acid)-1-piperidino]-carbonyloxycamptothecin (APC) and 7-ethyl-10-[4-amino-1-piperidino]-carbonyloxycamptothecin (NPC).Citation3,Citation7
The pharmacokinetic disposition of carrier-mediated agents, such as liposomal agents, is dependent upon the carrier until the drug is released from the carrier.Citation1 Unlike small molecule anticancer agents, which are metabolized and cleared by the liver and kidney, the clearance of liposomes occurs via the mononuclear phagocyte system (MPS). The MPS is comprised of monocytes, macrophages, and dendritic cells located primarily within the liver and spleen.Citation8 The uptake of liposomes by the MPS may result in acute impairment or toxicity in the MPS, which in turn decreases clearance of PEGylated liposomal agents. Thus, there is a bidirectional interaction between PEGylated liposomal anticancer agents and the MPS. PEGylated liposomes are cleared at a slower rate through the MPS compared with non-PEGylated liposomes.Citation9 Once the drug is released from the carrier, the pharmacokinetic disposition of the drug will be the same as after administration of the noncarrier form of the drug.Citation4,Citation8 Thus, the pharmacokinetic properties of liposomal agents are unique, and there may be many factors attributed to their interpatient variability. Nanoparticle anticancer agents have higher variability in pharmacokinetic (eg, drug clearance, systemic exposure, distribution) disposition (20–100-fold), with potentially higher variability in pharmacodynamic (antitumor response and toxicity) responses as compared with small-molecule chemotherapy.Citation10,Citation11 The high interpatient variability in pharmacokinetics and pharmacodynamics threatens the clinical utility and activity of nanoparticle and liposomal agents. The factors that may explain the variability in the pharmacokinetics and pharmacodynamics of encapsulated and released forms of conventional and PEGylated liposomes remain unclear, but most likely include the MPS.Citation12–Citation18 Our group has evaluated factors affecting the pharmacokinetics and pharmacodynamics of liposomal anticancer agents in preclinical animal models and in patients.Citation10,Citation11 We were the first to report a reduced clearance of the liposomal encapsulated forms of PEGylated liposomal doxorubicin (Doxil®) and CKD-602 (S-CKD602) in patients aged ≥60 years.Citation18–Citation20 We have also reported that monocytes are more sensitive to S-CKD602 compared with neutrophils, and the increased sensitivity is related to the liposomal formulation and not CKD-602.Citation21,Citation22 These results suggest that monocytes engulf S-CKD602, which causes the release of CKD-602 from the liposome and toxicity to the monocytes, and that the effects are more prominent in patients aged <60 years.Citation20,Citation22,Citation23 We were also the first group to report that body composition alters the pharmacokinetics of PEGylated liposomal agents in mice and in patients.Citation20 In mice, there was greater exposure of drug in fat compared with muscle after administration of S-CKD602, whereas there was greater exposure of drug in muscle compared with fat after administration of non-liposomal CKD-602.Citation24 In addition, in patients, the exposure of encapsulated liposomal CKD-602 in plasma after administration of S-CKD602 was inversely related to the ratio of total body weight (TBW) to ideal body weight (IBW), suggesting that patients with a larger body composition have greater distribution of drug to fat which results in lower exposure in plasma. These results in patients are consistent with our prior studies in mice.
Based on our previous preclinical and clinical studies of PEGylated liposomal agents, we hypothesized that age, body composition, and monocyte changes are fundamental patient-related factors that alter the pharmacokinetics and pharmacodynamics of all liposomal, nanoparticle, and conjugated drugs.Citation10,Citation11,Citation18 However, these factors have not been evaluated for other nanoparticle or liposomal agents in mice or patients.
The clinical and standard pharmacokinetic results of the Phase I study of IHL-305 have been published previously.Citation25 IHL-305 was associated with higher interpatient variability in the pharmacokinetic disposition of sum total (encapsulated + released) and released CPT-11 compared with nonliposomal CPT-11.Citation25 However, the factors associated with the high pharmacokinetic variability of IHL-305 have not been evaluated. Thus, based on our hypothesis described above, the objective of this study was to evaluate the factors (ie, age, body composition, monocytes) associated with interpatient variability in the pharmacokinetics and pharmacodynamics of IHL-305 in patients with advanced solid tumors.
Patients and methods
Patients
Written informed consent, approved by the institutional review board of the Sarah Cannon Research Institute and Vanderbilt University Medical Center, was obtained from all patients prior to study entry. Patients aged 18 years or older with a histologically confirmed malignant solid tumor for which no known regimen or protocol treatments of higher efficacy were available were eligible for this study. Pertinent eligibility criteria included an Eastern Cooperative Oncology Group performance status of 0 to 2 and normal bone marrow, hepatic, and renal function as defined by the following: absolute neutrophil count (ANC) ≥1,500 cells/μL, platelets ≥100,000 cells/μL, total bilirubin within normal institutional limits, aspartate aminotransferase/alanine aminotransferase ratio ≤2.5× institutional upper limit of normal (ULN) or ≤5.0× ULN if liver metastases were present, and plasma creatinine ≤1.5× institutional ULN or creatinine clearance ≥60 mL/min/1.73 m2 for patients with creatinine levels above institutional normal. Patients were excluded from the study for any of the following: prior treatment with CPT-11; chemotherapy or radiotherapy within 4 weeks (6 weeks for nitrosoureas or mitomycin C); known brain metastases; significant cardiac disease including heart failure; a history of myocardial infarction; a history of serious ventricular arrhythmias. All other eligibility criteria have been previously reported.Citation25
Dosage and administration
IHL-305 is a formulation of CPT-11 encapsulated in long-circulating PEGylated liposomes. In IHL-305, the PEGylated liposome bilayer is composed of cholesterol and hydrogenated soybean phosphatidylcholine, and the surface of the liposomes is modified with PEG. The mean particle diameter is approximately 100 nm and the drug to lipid mass ratio is 1:4 (0.25 mg CPT-11 per mg of lipid). The PEGylated liposomal formulation was generated by Terumo Corporation (Tokyo, Japan). IHL-305 was supplied by Yakult Honsha Corporation (Tokyo, Japan) in sterile 10 mL light-resistant, single-use glass vials as a translucent white to pale yellow liquid with a nominal total CPT-11 concentration of 5 mg/mL. IHL-305 was diluted 25-fold in 5% dextrose or normal saline prior to administration. Prior to administration of the study drug, patients were premedicated with ondansetron (or an other 5-HT3 inhibitor should circumstances require) and dexamethasone, according to each institution’s standard of care.
IHL-305 was administered as a 60-minute intravenous infusion every 4 weeks. Doses administered (expressed in mg of CPT-11) were 3.5, 7, 10.5, 14, 28, 33.5, 37, 50, 67, 80, 88, 120, 160, and 210 mg/m2. This Phase I study followed a standard dose escalation design with patients enrolled in cohorts of three, with the possibility of extending the cohort up to six patients depending on the number of dose-limiting toxicities.Citation26 No intrapatient dose escalation was permitted. The maximum tolerated dose was defined based on standard criteria.
Blood counts
ANC and monocyte counts were obtained at least once per week on cycle 1 of the IHL-305 study. Additional counts were obtained as clinically required. The percent decrease in ANC and monocytes at nadir was calculated using the standard formula [(pre-value-nadir)/pre-value] ×100.
Sample collection, processing, and analytical studies
Plasma samples for pharmacokinetic assessment were obtained from all patients. On cycle 1, blood (5 mL) was collected in tubes containing sodium heparin at prior to administration, at the end of the infusion (approximately 1 hour), and at 1.5, 2, 3, 5, 9, 13, and 25 hours after the start of the infusion for patients treated at <67 mg/m2 and the first three patients treated at 67 mg/m2. Additional samples at 49, 73, 97, 169 (day 7), 192 (day 8), and 216 (day 9) hours after the start of the infusion were also collected for patients treated at >67 mg/m2 and the last three patients treated at 67 mg/m2.
The blood samples were centrifuged at 3,000× g for 15 minutes at 4°C to collect the plasma fraction. Plasma samples were processed to measure sum total (encapsulated + released) CPT-11 and released CPT-11, SN-38, SN-38G, APC, and NPC, as previously described.Citation27 The sum total CPT-11, released CPT-11, SN-38, SN-38G, APC, and NPC concentrations were measured using high-performance liquid chromatography.Citation28 The total (lactone + hydroxy acid) form of camptothecin was measured for sum total CPT-11, released CPT-11, SN-38, SN-38G, APC, and NPC samples. The lower limit of quantitation of the total form sum total CPT-11, released CPT-11, SN-38, SN-38G, APC, and NPC were 100, 2, 2, 2, 2, and 2 ng/mL, respectively.
Compartmental pharmacokinetic analysis
Compartmental pharmacokinetic analysis of sum total CPT-11 after administration of IHL-305 was performed using WinNonlin (version 5.0.1; Pharsight Corporation, Mountain View, CA, USA).Citation29 Different pharmacokinetic model structures were considered to characterize the disposition of IHL-305 in plasma. In the development of the model, one-compartment and two-compartment models with linear and nonlinear (Michaelis-Menten) clearance were evaluated to describe the plasma disposition of IHL-305. The final model structure used for the pharmacokinetic analysis produced identifiable parameters in all patients except one.
Pharmacokinetic model parameters for sum total CPT-11 after administration of IHL-305 included the volume of the central compartment (Vc) and intercompartment rate constants, (k12, k21).Citation29 The elimination rate constant from the central compartment (k10) was used to represent linear clearance. For nonlinear clearance, the maximum rate (velocity, Vmax) and a Michaelis constant (Km) were estimated using the standard Michaelis-Menten equation described below, where X1 represents the amount remaining and t is the time after administration of the study drug.
Using standard equations, clearance and elimination half-life were calculated using parameter estimates from the models. The area under the IHL-305 plasma concentration versus time curve from 0 to infinity (AUC0–∞) was calculated using the log trapezoidal method by simulating the concentration versus time data from each patient using patient-specific parameters.Citation29 The AUC was also normalized by dose (AUC/dose).
Evaluation of the goodness of fit and the estimated parameters was based on the Akaike information criterion, the precision of the parameter estimates, the random distribution of weighted residuals between measured and predicted concentrations with respect to time, and the absence of a significant correlation between independent model parameters (<0.95).Citation29
Evaluation of factors
The patient’s age, TBW/IBW ratio, and percent decrease in monocytes at nadir were evaluated as potential factors associated with the pharmacokinetic variability of IHL-305. The TBW/IBW ratio was calculated using standard equations and used as a measure of body composition. These same factors were evaluated as potential factors associated with the pharmacodynamic variability of IHL-305.
Statistical analysis
The relationship between TBW/IBW and AUC/dose was analyzed using multiple linear regression controlling for age. The relationship between clearance and the percent decrease in monocytes was analyzed using a simple linear regression. The relationship between dose-normalized sum total CPT-11 AUC and the percent decrease in monocytes was analyzed using simple linear regression. The relationship between the percent decrease in monocytes and age was analyzed using multiple linear regression controlling for dose. The percent decrease in monocytes and ANC at nadir within a patient were compared using the Wilcoxon signed ranked test. The percent decrease in monocytes and ANC at nadir in patients aged <60 and ≥60 years were compared using the two-sample t-test. All statistical analyses were performed using SAS version 9.2 software (SAS Institute, Cary, NC, USA).Citation30
Results
Patient characteristics
The characteristics of the 42 patients enrolled in this study from December 14, 2006 to December 15, 2008 have been described previously.Citation25 Pharmacokinetic studies of IHL-305 were performed in 39 of these patients with a mean (median, range) age of 59.3 years (60 years, 41–75 years), and the majority being female (n=26, 66%).
Linear and nonlinear pharmacokinetic disposition of IHL-305
The variability in the pharmacokinetic disposition of sum total CPT-11 was related to linear and nonlinear (saturable) clearance of IHL-305 in patients, which was dose-dependent. At doses from 3.5 to 50 mg/m2, the IHL-305 sum total CPT-11 plasma concentration versus time profiles were best described using a model with linear clearance in all patients (n=14). At doses from 67 to 210 mg/m2, the IHL-305 sum total CPT-11 plasma concentration versus time profiles were best described using a model with linear (n=16) and nonlinear clearance (n=8). The dose of IHL-305 was significantly higher in patients with nonlinear clearance than in patients with linear clearance (P=0.01). The dose-normalized sum total CPT-11 AUC in patients with linear clearance and patients with nonlinear clearance are presented in .
Table 1 Compartmental pharmacokinetic parameters of sum total CPT-11 after IHL-305 in patients with linear and nonlinear disposition
Relationship between age, body composition, and pharmacokinetic disposition of IHL-305
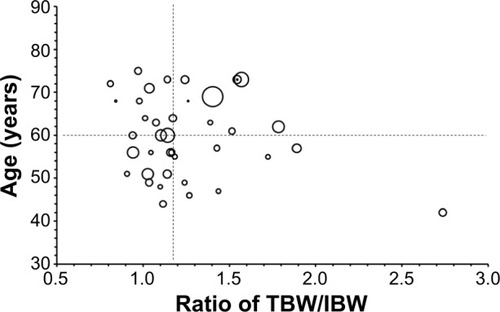
Based on our previous studies reporting both age and TBW/IBW ratio affecting the pharmacokinetic disposition of S-CKD602, we evaluated the relationship between these two factors and the pharmacokinetic disposition of IHL-305. The relationship between TBW/IBW and dose-normalized CPT-11 AUC (AUC/dose) in all patients is presented in . Controlling for age, there was an inverse relationship between TBW/IBW ratio and AUC/dose (R2=0.12, P=0.41), whereby low TBW/IBW was associated with high AUC/dose in patients aged <60 years. The effect of age and TBW/IBW together on the ratio of released CPT-11 AUC to sum total CPT-11 AUC in all patients was evaluated using a bubble chart and is presented in . Patients whose age and TBW/IBW were greater than the median of the study had a 1.7-fold to 2.6-fold higher ratio of released CPT-11 AUC to sum total CPT-11 AUC.
Figure 1 Relationship between the ratio of TBW/IBW and dose-normalized IHL-305 sum total AUC (AUC/dose). AUC/dose in patients aged <60 and ≥60 years are represented by the solid triangles and open triangles, respectively.
Notes: The best-fit line of the data is represented by the curved solid line (R2=0.12). After controlling for age, there was an inverse relationship between TBW/IBW and AUC/dose, with a low TBW/IBW being associated with high AUC/dose in patients aged <60 years; CPT-11 is irinotecan; IHL-305 is a PEGylated liposomal formulation of irinotecan.
Abbreviations: AUC, area under the concentration versus time curve; TBW/IBW, total body weight to ideal body weight.
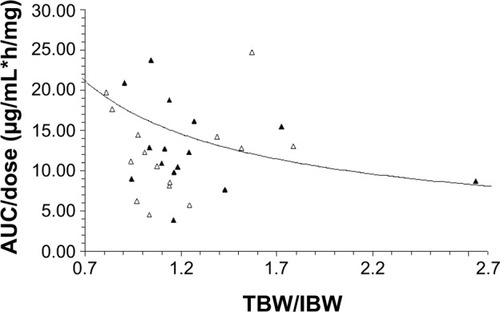
Figure 2 Relationship between two factors, age, and the ratio of TBW/IBW, and ratio of released CPT-11 AUC to sum total CPT-11 AUC.
Notes: Patients are divided into four groups according to the median value of age and TBW/IBW. The size of each circle correlates with ratio of released CPT-11 AUC to sum total CPT-11 AUC in a patient at the specific age and ratio of true body weight to ideal body weight. The mean ± standard deviation values for the ratio of released CPT-11 AUC to sum total CPT-11 AUC were 0.0042±0.0028, 0.0038±0.0038, 0.0066±0.0084, and 0.0025±0.0013 in patients aged <60 years and TBW/IBW <1.16, patients aged ≥60 years and TBW/IBW <1.16, patients aged ≥60 years and TBW/IBW ≥1.16, and patients aged <60 years and TBW/IBW ≥1.16, respectively; CPT-11 is irinotecan; IHL-305 is a PEGylated liposomal formulation of irinotecan.
Abbreviations: AUC, area under the concentration versus time curve; TBW/IBW, total body weight to ideal body weight.
Relationship between percent decrease in monocytes and pharmacokinetic disposition of IHL-305
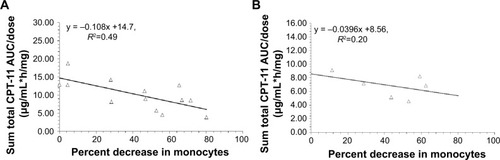
Based on our prior studies, the percent decrease in monocytes at nadir on cycle 1 was used as a surrogate measure of monocyte function. The relationship between the percent decrease in monocytes and dose normalized CPT-11 AUC in patients with linear clearance and nonlinear clearance are presented in , respectively. For patients with linear clearance, there was a statistically significant linear relationship between percent decrease in monocytes and AUC/dose (P=0.008, R2=0.49), where high percent decrease in monocytes was associated with low AUC/dose. However, the relationship between the percent decrease in monocytes and dose-normalized CPT-11 AUC in patients with nonlinear clearance was not significant (P=0.37, R2=0.20) which may be due to saturation of the interaction between IHL-305 and monocytes.
Figure 3 Relationship between percent decrease in monocytes and dose normalized CPT-11 AUC (AUC/dose).
Notes: (A, B) Represent the relationship between percent decrease in monocytes and AUC/dose in patients with linear clearance and nonlinear clearance, respectively. The relationship between AUC/dose and percent decrease in monocytes was best described by a linear relationship in patients with linear clearance (P=0.008, y = −0.108x +14.7, R2=0.49); CPT-11 is irinotecan; IHL-305 is a PEGylated liposomal formulation of irinotecan.
Abbreviation: AUC, area under the concentration versus time curve.
Neutropenia and monocytopenia associated with IHL-305
To evaluate the differential effects of IHL-305 on neutrophils and monocytes, we compared the percent decrease in ANC and monocytes at nadir in the blood of patients administered IHL-305 on cycle 1. The day of nadir (mean ± standard deviation) for ANC and monocytes after administration of IHL-305 was 18.7±7.4 days and 11.2±6.1 days, respectively (P=0.0006). The extent of neutropenia and monocytopenia following administration of IHL-305 is summarized in . After administration of IHL-305, the percent decrease in ANC and monocytes at nadir were 29%±20% and 42%±24%, respectively (P=0.19) in all patients. The ratio of percent decrease in monocytes to percent decrease in ANC at their nadir within a patient was 1.4±1.0.
Table 2 Summary of ANC and monocyte decrease at nadir after administration of IHL-305
To evaluate age-related effects on the relationship between neutropenia and monocytopenia after administration of IHL-305, we compared the percent decrease in ANC and monocytes in the blood of patients aged <60 and ≥60 years. Categorizing patients as aged <60 or ≥60 years was based on our previous studies reporting a reduced clearance of PEGylated liposomal anticancer agents in patients aged ≥60 years compared with patients aged <60 years.Citation20 The age (mean ± standard deviation) of patients in groups aged <60 and ≥60 years was 51.4±4.8 years and 67.3±5.2 years, respectively (P<0.001). The extent of neutropenia and monocytopenia following administration of IHL-305 in patients aged <60 and ≥60 years is summarized in . The percent decrease in ANC and monocytes in patients aged <60 years was 30%±23% and 45%±30%, respectively (P=0.46). The ratio of percent decrease in monocytes to percent decrease in ANC within a patient aged <60 years was 1.7±1.4. The percent decrease in ANC and monocytes in patients aged ≥60 years was 28%±19% and 40%±20%, respectively (P=0.30). The ratio of percent decrease in monocytes to percent decrease in ANC within a patient aged ≥60 years was 1.2±0.7.
Relationship between age and pharmacodynamics of IHL-305
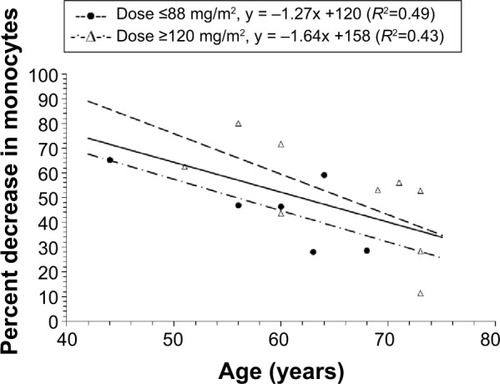
The relationship between age and percent decrease in monocytes at nadir in patients treated at a dose ≥50 mg/m2 is presented in . Patients treated at a dose <50 mg/m2 were not included because the majority of these patients were not evaluated for monocyte counts. There was an inverse linear relationship between the percent decrease in monocytes and age in all patients (R2=0.32, P=0.029), in patients with a dose ≤88 mg/m2 (R2=0.49, P=0.121), and in patients with a dose ≥120 mg/m2 (R2=0.43, P=0.056), where in all cases younger patients had a higher percent decrease in monocytes compared with younger patients. In addition, the percent decrease in monocytes was lower in patients with a dose ≤88 mg/m2 than in those with a dose ≥120 mg/m2.
Figure 4 Relationship between percent decrease in monocytes and age in all patients with a dose ≥50 mg/m2.
Notes: For patients with a dose ≥50 mg/m2 and ≤88 mg/m2, individual values are represented by the solid circles. For patients with a dose ≥120 mg/m2, individual values are represented by the open triangles. There was a linear relationship between the percent decrease in monocytes and age in all patients (R2=0.32), patients with a dose ≤88 mg/m2 (R2=0.49), and in patients with a dose ≥120 mg/m2 (R2=0.43); CPT-11 is irinotecan; IHL-305 is a PEGylated liposomal formulation of irinotecan.
Discussion
Major advances in the use of liposomes, conjugates, and nanoparticles as vehicles to deliver drugs have occurred in the past 10 years.Citation4,Citation9,Citation31 Doxil and the albumin-stabilized nanoparticle formulation of paclitaxel (Abraxane®) are now approved by the US Food and Drug Administration.Citation32–Citation34 In addition, there are more than 300 liposomal and nanoparticle formulations of anticancer agents currently in development.Citation4 This is the first study to identify age, body composition, and monocyte counts as factors associated with pharmacokinetic variability of a PEGylated liposomal CPT-11 formulation. These results are consistent with our prior studies of Doxil and S-CKD602.Citation19,Citation20
The percent decrease in monocytes was significantly correlated with clearance of sum total CPT-11, where patients with a higher percent decrease in monocytes at nadir have an increased clearance of sum total CPT-11. The relationship between changes in monocytes and the pharmacokinetic disposition of IHL-305 suggest that the monocytes engulf liposomal anticancer agents via their phagocytic function as part of the MPS, which causes the release of drug from the liposome and subsequent cytotoxicity to monocytes.Citation9,Citation34 There are two potential explanations for the relationship between changes in monocytes and the pharmacokinetic disposition of IHL-305. The first theory is that the monocytes engulf liposomal anticancer agents via their phagocytic function as part of the MPS, which causes release of drug from the liposome and subsequent cytotoxicity to monocytes. The second theory is that the reduction in monocytes after administration of liposomal agents may also be a result of movement of monocytes out of the bloodstream and into other MPS organs, such as the liver and spleen. Additionally, monocytes were more sensitive to IHL-305 as compared with neutrophils in our study. This is consistent with our previous study, that the increased sensitivity is related to the liposomal formulation and not to the encapsulated drug.Citation35 The overall difference in monocyte and neutrophil sensitivity to IHL-305 is less than that reported for S-CKD602. This may be due to CPT-11 being less potent than CKD-602 or due to the different liposomal formulations used in each product. In our study, the decrease in monocytes is reversible, monocytopenia resolved in 2 weeks for most patients, and was not a dose-limiting toxicity in our study. However, the long-term effects of liposomal and other nanoparticles on the function of the MPS and other parts of the immune system are unknown and need to be evaluated.
The nonlinear clearance of IHL-305 was associated with high doses of IHL-305 (≥67 mg/m2 CPT-11 or 268 mg/m2 lipid). We previously reported that nonlinear clearance of S-CKD602 was associated with high doses of S-CKD602 (>1.7 mg/m2 CKD-602 or 15.2 mg/m2 lipid).Citation20 The nonlinear clearance of sum total CPT-11 after administration of IHL-305 and other nanoparticle agents may be related to saturation of the clearance capacity of the MPS. The difference in the lipid dose of IHL-305 and S-CKD602 resulting in saturable clearance of each agent suggests that the lipid dose is not the predominant factor associated with saturating the MPS, and that other constituents (eg, number of liposomes administered) and the patient’s MPS function and capacity may be more important issues. Age and body composition were not associated with the pharmacokinetic variability of IHL-305 in patients with nonlinear clearance, which is consistent with our prior studies.Citation20
Patients who were younger than 60 years and had a lean body composition had an increased plasma exposure of IHL-305. The relationship between body composition and plasma exposure of IHL-305 in patients is consistent with our prior studies of S-CKD602 which showed that patients with a lean body composition had a higher plasma exposure of S-CKD602.Citation20 Our previous studies in mice also showed that the distribution of S-CKD602 in fat relative to muscle is greater compared with nonliposomal CKD-602.Citation24 In addition, overweight mice were reported to express more macrophages in fat.Citation36 The lower exposure of liposomal agents in patients with a greater TBW/IBW ratio may be a result of greater distribution of IHL-305 to adipose tissue and greater uptake by macrophages in adipose tissue. Thus, adipose tissue could be considered an MPS-related organ, similar to the liver and spleen. In addition, studies suggest that obesity induces an inflammatory state, so patients with a greater TBW/IBW ratio may have heightened MPS function, which would result in faster clearance of liposomal agents.Citation37 The influence of age on the pharmacodynamics of PEGylated liposomal agents has been reported by our group. There was an inverse relationship between patient age and percent decrease in monocytes at nadir, with younger patients having a higher percent decrease in monocytes. This is consistent with our study of S-CKD602, indicating that an age-related decrease in the function of monocytes may account for the reduced uptake and clearance of PEGylated liposomes and cytotoxicity to monocytes.Citation22
We evaluated factors affecting SN-38 AUC but did not see any relationship. The lack of a relationship between SN-38 pharmacokinetics and factors associated with the MPS and pharmacology of liposomal agents is not unexpected given that the factors affecting liposomal agents (MPS) and SN-38 (phase I and II hepatic enzymes) are different. In addition, we evaluated the relationship between CPT-11 AUC (not dose-normalized) and percent decrease in monocytes. There was no significant relationship between CPT-11 AUC and percent decrease in monocytes in patients with linear clearance or in patients with nonlinear clearance.
IHL-305 exhibits all of the pharmacologic, antitumor, and cytotoxic advantages of a long-acting, liposomal anticancer agent.Citation4,Citation25,Citation38,Citation39 The high interpatient variability in the pharmacokinetics and pharmacodynamics of sum total IHL-305 was associated with age, body composition, saturable clearance, and monocyte function. Our data also suggest that IHL-305 undergoes nonlinear or saturable clearance at higher doses.Citation25 The clinical significance of these differences and the factors associated with them need to be evaluated for IHL-305 and other liposomal and nanoparticle anticancer agents. Ultimately, the best predictor of the pharmacokinetic and pharmacodynamic variability of IHL-305 and other liposomal and nanoparticle agents may be a phenotypic probe that measures the clearance capacity of liposomes in individual patients.Citation40 This phenotypic probe can then be used to individualize the dosages of liposomal and nanoparticle agents for each patient to achieve a target exposure and thus reduce the pharmacodynamic variability of these agents.Citation40 As there are more than 300 nanoparticle anticancer agents currently in development, as well as numerous other nanoparticles in development for other diseases, the results of our study may have a wide and long-term impact on the development of these agents.Citation10,Citation11,Citation18
Acknowledgments
This work was supported by a grant from Yakult Honsha Co Ltd to the participating institutions.
Disclosure
The authors report no conflicts of interest in this work.
References
- ZamboniWCConcept and clinical evaluation of carrier-mediated anticancer agentsOncologist200813324826018378535
- InnocentiFKroetzDLSchuetzEComprehensive pharmacogenetic analysis of irinotecan neutropenia and pharmacokineticsJ Clin Oncol200927162604261419349540
- SlatterJGSchaafLJSamsJPPharmacokinetics, metabolism, and excretion of irinotecan (CPT-11) following I.V. infusion of [(14)C]CPT-11 in cancer patientsDrug Metab Dispos200028442343310725311
- ZamboniWCLiposomal, nanoparticle, and conjugated formulations of anticancer agentsClin Cancer Res200511238230823416322279
- ZamboniWCStewartCFThompsonJRelationship between topotecan systemic exposure and tumor response in human neuroblastoma xenograftsJ Natl Cancer Inst19989075055119539245
- StewartCFZamboniWCCromWRTopoisomerase I interactive drugs in children with cancerInvest New Drugs199614137478880392
- XieRMathijssenRHSparreboomAVerweijJKarlssonMOClinical pharmacokinetics of irinotecan and its metabolites in relation with diarrheaClin Pharmacol Ther200272326527512235447
- AllenTMHansenCPharmacokinetics of stealth versus conventional liposomes: effect of doseBiochim Biophys Acta1991106821331411911826
- PapahadjopoulosDAllenTMGabizonASterically stabilized liposomes: improvements in pharmacokinetics and antitumor therapeutic efficacyProc Natl Acad Sci U S A1991882411460114641763060
- SongGWuHYoshinoKZamboniWCFactors affecting the pharmacokinetics and pharmacodynamics of liposomal drugsJ Liposome Res201222317719222332871
- PrabhakarUMaedaHJainRKChallenges and key considerations of the enhanced permeability and retention effect for nanomedicine drug delivery in oncologyCancer Res20137382412241723423979
- Ahmad KhanbeigiRKumarASadoukiFThe delivered dose: applying particokinetics to in vitro investigations of nanoparticle internalization by macrophagesJ Control Release2012162225926622824784
- ShahNBVercellottiGMWhiteJGFeganAWagnerCRBischofJCBlood-nanoparticle interactions and in vivo biodistribution: impact of surface PEG and ligand propertiesMol Pharm2012982146215522668197
- SkoczenSLPotterTMDobrovolskaiaMAIn vitro analysis of nanoparticle uptake by macrophages using chemiluminescenceMethods Mol Biol201169725526121116975
- MoghimiSMParhamifarLAhmadvandDParticulate systems for targeting of macrophages: basic and therapeutic conceptsJ Innate Immun201245–650952822722900
- AndersenAJWibroePPMoghimiSMPerspectives on carbon nanotube-mediated adverse immune effectsAdv Drug Deliv Rev201264151700170522634159
- CristRMGrossmanJHPatriAKCommon pitfalls in nanotechnology: lessons learned from NCI’s Nanotechnology Characterization LaboratoryIntegr Biol (Camb)201251667322772974
- CaronWPSongGKumarPRawalSZamboniWCInterpatient pharmacokinetic and pharmacodynamic variability of carrier-mediated anticancer agentsClin Pharmacol Ther201291580281222472987
- SidoneBJEdwardsRPZamboniBAStrychorSMarucaLJZamboniWCEvaluation of body surface area (BSA) based dosing, age, and body composition as factors affecting the pharmacokinetic (PK) variability of STEALTH liposomal doxorubicin (Doxil)Paper presented at the 2007 AACR-NCI-EORTC International Conference on Molecular Targets and Cancer Therapeutics: Discovery, Biology, and Clinical ApplicationsOctober 22–26, 2007San Francisco, CA, USA
- ZamboniWCStrychorSMarucaLPharmacokinetic study of PEGylated liposomal CKD-602 (S-CKD602) in patients with advanced malignanciesClin Pharmacol Ther200986551952619675541
- WuHRamanathanRKZamboniBAMechanism-based model characterizing bidirectional interaction between PEGylated liposomal CKD-602 (S-CKD602) and monocytes in cancer patientsInt J Nanomedicine201275555556423112576
- ZamboniWCMarucaLJStrychorSBidirectional pharmacodynamic interaction between pegylated liposomal CKD-602 (S-CKD602) and monocytes in patients with refractory solid tumorsJ Liposome Res201121215816520626314
- De MartinisMModestiMGinaldiLPhenotypic and functional changes of circulating monocytes and polymorphonuclear leucocytes from elderly personsImmunol Cell Biol200482441542015283852
- ZamboniWCStrychorSJosephEPlasma, tumor, and tissue disposition of STEALTH liposomal CKD-602 (S-CKD602) and nonliposomal CKD-602 in mice bearing A375 human melanoma xenograftsClin Cancer Res200713237217722318056203
- InfanteJRKeedyVLJonesSFPhase I and pharmacokinetic study of IHL-305 (PEGylated liposomal irinotecan) in patients with advanced solid tumorsCancer Chemother Pharmacol201270569970522941375
- ZamboniWCTondaMENew designs of clinical trialsHighlights in Oncology Practice200018127
- InfanteJRKeedyVLJonesSFPhase I and pharmacokinetic study of IHL-305 (PEGylated liposomal irinotecan) in patients with advanced solid tumorsCancer Chemother Pharmacol201270569970522941375
- KuritaAKanedaNHigh-performance liquid chromatographic method for the simultaneous determination of the camptothecin derivative irinotecan hydrochloride, CPT-11, and its metabolites SN-38 and SN-38 glucuronide in rat plasma with a fully automated on-line solid-phase extraction system, PROSPEKTJ Chromatogr B Biomed Sci Appl1999724233534410219676
- GabrielssonJLWeinerDLPharmacokinetic and Pharmacodynamic Data Analysis: Concepts and Applications3rd edLondon, UKTaylor and Francis2000
- RosnerGFundamentals of Biostatistics5th edPacific Grove, CA, USADuxbury2000
- MaedaHWuJSawaTMatsumuraYHoriKTumor vascular permeability and the EPR effect in macromolecular therapeutics: a reviewJ Control Release2000651–227128410699287
- MarkmanMGordonANMcGuireWPMuggiaFMLiposomal anthracycline treatment for ovarian cancerSemin Oncol2004316 Suppl 139110515717739
- KrownSENorthfeltDWOsobaDStewartJSUse of liposomal anthracyclines in Kaposi’s sarcomaSemin Oncol2004316 Suppl 13365215717737
- RoyVLaPlantBRGrossGGBaneCLPalmieriFMPhase II trial of weekly nab (nanoparticle albumin-bound)-paclitaxel (nab-paclitaxel) (Abraxane) in combination with gemcitabine in patients with metastatic breast cancer (N0531)Ann Oncol200920344945319087987
- DeLoiaJAZamboniWCJonesJMStrychorSKelleyJLGallionHHExpression and activity of taxane-metabolizing enzymes in ovarian tumorsGynecol Oncol2008108235536018063021
- WeisbergSPMcCannDDesaiMRosenbaumMLeibelRLFerranteAWJrObesity is associated with macrophage accumulation in adipose tissueJ Clin Invest2003112121796180814679176
- WellenKEHotamisligilGSObesity-induced inflammatory changes in adipose tissueJ Clin Invest2003112121785178814679172
- SparreboomAZamboniWCCancer Chemotherapy and Biotherapy: Principles and Practice4th edPhiladelphia, PA, USALippincott Williams & Wilkins2005
- StewartCFZamboniWCCromWRTopoisomerase I interactive drugs in children with cancerInvest New Drugs19961437478880392
- ZamboniWCEdwardsRPMountzJMThe development of liposomal and nanoparticle anticancer agents: methods to evaluate the encapsulated and released drug in plasma and tumor and phenotypic probes for pharmacokinetic (PK) and pharmacodynamic (PD) dispositionPaper presented at the NSTI Nanotechnology ConferenceMay 7–11, 2007Boston, MA, USA
