?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Although magnesium is involved in a wide spectrum of vital functions in normal human physiology, the significance of hypomagnesemia and necessity for its treatment are under-recognized and underappreciated in clinical practice. In the current review, we first present an overview of the clinical significance of hypomagnesemia and normal magnesium metabolism, with a focus on renal magnesium handling. Subsequently, we review the literature for both congenital and acquired hypomagnesemic conditions that affect the various steps in normal magnesium metabolism. Finally, we present an approach to the routine evaluation and suggested management of hypomagnesemia.
Introduction
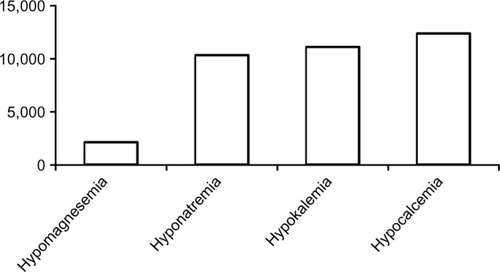
Despite being the second most abundant intracellular and fourth most abundant extracellular cation in the body, hypomagnesemia has received relatively poor attention in the medical literature compared with hyponatremia, hypokalemia, and hypocalcemia. As of November 14, 2013, there were 2,100 versus 10,298, 1,107, and 12,345 citations related to the respective electrolyte disorders, recorded on PubMed (). The low interest in hypomagnesemia may have stemmed from its relative lack of symptoms until plasma concentrations reach severely low levels, our poor understanding of magnesium metabolism until recent years, or both. Nonetheless, magnesium is involved in a wide spectrum of vital functions in human physiology. Magnesium is required for all enzymatic reactions requiring adenosine triphosphate (ATP), various reactions requiring kinases, neuromuscular excitability and cell permeability, regulation of ion channels and mitochondrial function, cellular proliferation and apoptosis, as well as immunity, among others.Citation1–Citation3
Clinical manifestations
Hypomagnesemia, while typically defined as having serum magnesium concentration below 0.66 mmol/L (1.6 mg/dL), with or without accompanying total body depletion, does not lead to clinically significant signs and symptoms until serum levels fall below 0.5 mmol/L (1.2 mg/dL).Citation3 Nonetheless, as magnesium is involved in an array of structural and physiological functions, adverse effects associated with hypomagnesemia may occur in almost every organ system, whether they are clinically acute and overt, or chronic and subtle.
Clinical manifestations of hypomagnesemia that promptly lead to medical attention involve neuromuscular hyperexcitability that may range from tremors, fasciculation, tetany, to convulsions, and neuropsychiatric disturbances including apathy, delirium, and even coma. Other potentially life-threatening complications may arise not solely from hypomagnesemia, but also from the associated hypocalcemia and/or hypokalemia, and include atrial and ventricular arrhythmias, torsades de pointe, enhanced sensitivity to digoxin toxicity, and sudden death. In contrast, long-term adverse complications where the association with hypomagnesemia is not often recognized include altered glucose homeostasis, hypertension, atherosclerosis, osteoporosis, asthma, migraines, and other end-organ damage. Presumed mechanisms involved in various hypomagnesemia-associated signs/symptoms are listed in .Citation2–Citation5 The mechanisms of many, if not most, clinical signs and symptoms of hypomagnesemia are likely multifactorial and beyond the scope of discussion in the current review.
Table 1 Magnesium functions and hypomagnesemia related clinical manifestations
Magnesium metabolism
Gastrointestinal absorption
While the Institute of Medicine recommends a daily magnesium intake of 310–420 mg/day in adults, with the end of normal range for women and higher range for men, it has been estimated that 300–350 mg of magnesium is consumed daily in a typical American diet.Citation6 Twenty percent to 80% of dietary magnesium is absorbed in the intestines, where absorption depends on both intake and body magnesium status, and occurs via both passive and active pathways.Citation6–Citation8 Passive Mg2+ absorption occurs paracellularly and predominantly in the small intestines, a process driven by a favorable electrochemical gradient and solvent drag with dietary intake. At low dietary magnesium intake, Mg2+ absorption relies heavily on active transcellular uptake via Mg2+-specific transporters in the large intestines.Citation7,Citation9 Two Mg2+-specific channels identified over recent years include the transient receptor potential melastatin (TRPM) 6 and TRPM7. TRPM7 is ubiquitously expressed among tissues, whereas TRPM6 is predominantly expressed along the full length of the intestine, the distal convoluted tubules (DCTs), the lungs, and the testis tissue. TRPM6 and TRPM7 can form heterodimers and may influence trafficking and activity of the TRPM6 Mg2+ channel. However, the extent and significance of the interactions between TRPM6 and 7 remain to be fully elucidated.Citation8 Nonetheless, it has been suggested that while TRPM6 plays an important role in epithelial Mg2+ transport, TRPM7 is involved in cellular Mg2+ homeostasis.Citation10 Loss-of-function mutations of TRPM6 have been reported in patients with familial hypomagnesemia with secondary hypocalcemia.Citation11
Magnesium exists as protein bound (20% to 30%), complexed with organic anions such as sulfates, phosphates, or bicarbonates and citrates (5% to 15%), or free ionized cations (55% to 70%), where the proportion of each form is dependent on plasma pH, ionic strength, and protein/organic anion contents. Intracellular magnesium concentration has been estimated to range from 5 t o 20 mmol/L (12–49 mg/dL), while extracellular magnesium concentration typically ranges from 0.70 to 1.05 mmol/L (1.7–2.6 mg/dL).Citation5
Cellular shift
Unlike potassium, regulation of Mg2+ cellular uptake or release occurs slowly and likely does not occur in all cell types. Bone is the largest reservoir of magnesium but its role and regulation in maintaining plasma levels remain poorly understood.Citation12
Kidney handling of magnesium
The kidneys are thought to play a key role in regulating and maintaining magnesium balance.
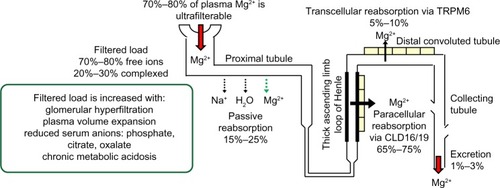
Approximately 70% to 80% of plasma magnesium in ionized or complexed forms is ultrafilterable in the kidneys. Once filtered, 15% to 25% is reabsorbed passively with sodium and water in the proximal tubules.Citation13 Sixty-five percent to 75% of the filtered magnesium load is reabsorbed paracellularly in the thick ascending limb of the loop of Henle (TAL), a process facilitated by the tight junction protein claudin-16, also known as paracellin-1.Citation14,Citation15 Mutation of claudin-16 is associated with severe hypomagnesemia with hypercalciuria and nephrolithiasis.Citation16 More recently, mutations encoding the tight junction protein claudin-19 have also been reported to cause the inherited human renal disorder, familial hypomagnesemia with hypercalciuria and nephrocalcinosis.Citation17 Although further studies are required, current evidence from both in vitro and in vivo studies suggests that claudin-16 and claudin-19 cogenerate a cation channel at the TAL tight junction necessary for normal Mg2+ reabsorption at this nephron segment.Citation18
Five percent to 10% of the filtered magnesium, or 70% to 80% of magnesium delivered from the TAL, is reabsorbed at the DCT subsegment 1, the final nephron segment where Mg2+ can be reabsorbed, via an active and regulated transcellular pathway through the apical TRPM6 ().Citation19,Citation20 Although the percentage of filtered magnesium reabsorbed in the DCT is lower than that at more proximal segments, regulated reabsorption at this segment is essential to magnesium balance because it determines the final urinary Mg2+ loss.Citation21
DCT magnesium reabsorption
Apical factors
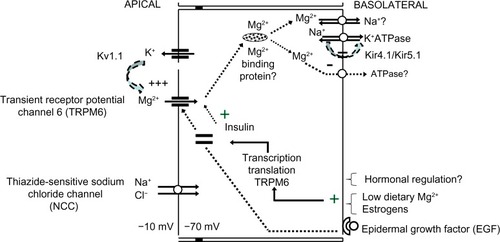
In Gitelman syndrome, where there is a homozygous or compound heterozygous mutation or deletion in the SLC12A3 gene encoding the apical thiazide-sensitive NaCl cotransporter (NCC), significant hypomagnesemia occurs in association with hypokalemic metabolic alkalosis and hypocalciuria.Citation22 Although mechanistically unclear, Nijenhuis et al demonstrated reduced TRPM6 expression in mice chronically treated with thiazide, a Gitelman-equivalent condition.Citation23 Additionally, it has been suggested that the compensatory hyperaldosteronism associated with reduced NCC sodium reabsorption in Gitelman may lead to reduced TRPM6 activity, particularly in concert with low dietary magnesium intake.Citation24 It may also be speculated that reduced NCC sodium reabsorption could lead to suboptimal basolateral (3)Na+-(2)K+-ATPase activity, hence reduced generation of the favorable potential difference (ie, more negative intracellular voltage) to facilitate apical Mg2+ reabsorption (). Whatever the mechanism(s) is/are, normal NCC function is likely necessary for DCT Mg2+ reabsorption.
The apical voltage-gated potassium channel Kv1.1 has also been implicated to play a role in normal DCT Mg2+ reabsorption. A mutation involving substitution of asparagine for aspartic acid leading to nonfunctional Kv1.1 channels in the DCT has been reported in a family with isolated autosomal-dominant hypomagnesemia paired with signs and symptoms of neuromuscular dysfunction including recurrent muscle cramps and weakness, tremors, tetany, cerebellar atrophy, and myokymia.Citation25 It was suggested that the loss of Kv1.1 function reduces apical K+ intraluminal secretion, hence the associated favorable intraluminal positive voltage to facilitate Mg2+ reabsorption via TRPM6.
Basolateral factors
Renal magnesium handling at the DCT occurs via the apical TRPM6, whose shuttling from intracellular vesicles toward apical membranes requires binding of epidermal growth factor (EGF) to its basolateral receptor. Mutations of the EGF gene have been shown to be the causative defect in recessive isolated renal hypomagnesemia, where apical TRPM6 expression is reduced.Citation26,Citation27
It is speculated that following apical reabsorption, Mg2+ binds to Mg2+-binding proteins for transport to the basolateral sides, where eventual reabsorption into the interstitium occurs via transporters such as Na+/Mg2+ exchangers and/or Mg2+-ATPase. While basolateral transport of Mg2+ has not been elucidated, mutations involving the γ-subunit of the basolateral Na+-K+-ATPase or the regulatory protein of Na+-K+-ATPase, the hepatocyte nuclear factor 1 homeobox B (HNF1B), are associated with renal Mg2+ wasting.Citation28–Citation30 Mutation in the FXYD2 gene encoding the γ-subunit of Na+-K+-ATPase is thought to affect normal routing, hence activity of the transporter. Suboptimal Na+-K+-ATPase activity theoretically leads to depolarization of the DCT due to the 3Na+-to-2K+ exchange ratio (ie, reduced intracellular negative voltage that normally favors reabsorption of the divalent cation Mg2+) thus reduced Mg2+ reabsorption via TRPM6. If basolateral Mg2+ reabsorption significantly relies on Na+/Mg2+ exchangers, it may be also be deduced that reduced Na+-K+-ATPase activity, hence reduced basolateral Na+ recycling, would also lead to reduced basolateral Mg2+ reabsorption. Heterozygous mutations of the HNF1B gene, either whole-gene deletion or point mutations, are linked to a dominant renal cysts and diabetes syndrome, where up to 50% of patients also present with renal magnesium wasting with hypocalciuria.Citation31 HNF1B is thought to play a regulatory role in the transcription of the FXYD2 gene, specifically at the promoter responsible for the transcription the γa-subunit of Na+-K+-ATPase.Citation30
The basolateral, heteromeric, inwardly rectifying Kir4.1/Kir5.1 K+ channel has also been found to affect Mg2+ reabsorption, presumably via its K+ recycling function necessary to maintain optimal Na+-K+-ATPase activity. Mutations involving the KCNJ10 gene encoding Kir4.1 have been reported to cause a clinical constellation involving hypomagnesemia with associated SEizures, Sensorineural deafness, Ataxia, Mental retardation, and Electrolyte imbalance Epilepsy, Ataxia, Sensorineural deafness, and renal Tubulopathy, known as (SeSAME/EAST) syndrome.Citation32,Citation33 It is possible that the associated suboptimal Na+-K+-ATPase function leads to reduced sodium reabsorption at the DCT thiazide-sensitive sodium chloride channel, a defect observed with Gitelman syndrome. Accordingly, with the exception of hypocalcemia, a Gitelman-like clinical syndrome including sodium wasting, hypokalemia, hypomagnesemia, and metabolic alkalosis may be observed with Kir4.1 loss-of-function mutation. Additionally, it has been reported that the Kir4.1/Kir5.1 K+ channel is extremely sensitive to inhibition by intracellular pH, a characteristic thought to be conferred by the intact Kir5.1 subunit.Citation34 Interestingly, activation or gain-of-function mutation of the calcium-sensing receptor (CaSR) has been shown to reduce basolateral Kir4.1 expression, presumably via altered caveolin-mediated trafficking of the channel, with resultant reduction in Mg2+ reabsorption and salt-wasting at the DCT.Citation35
Finally, although mechanistically unclear, both deletion and missense mutations involving cyclin M2, a protein localized to the basolateral side in both the TAL and the DCT, have been identified in two unrelated families with unexplained dominant hypomagnesemia.Citation36
Hormonal regulation
Regulatory hormones reported to influence magnesium homeostasis via TRPM6 and TRPM7 protein expression and activity include angiotensin II, aldosterone, bradykinin, thrombin, estrogen, and insulin. Parathyroid hormone and 1,25 vitamin D, however, do not appear to have any effect on TRPM6 expression.Citation8,Citation37–Citation41 Of interest, the inability of insulin activation of TRPM6 in two single nucleotide TRPM6 polymorphisms, Ile1393Val and Lys1584Glu, has been linked to hypomagnesemia and presumed hypomagnesemia-induced glucose intolerance in women on low dietary magnesium intake.Citation37 Low dietary magnesium intake and estrogens have been shown to upregulate renal TRPM6 expression and reduce urinary magnesium excretion.Citation38
Aldosterone has been reported to induce renal Mg2+ wasting, an effect that may be ameliorated by aldosterone antagonists.Citation39,Citation40 Additionally, the use of aldosterone antagonists has also been shown to maintain plasma magnesium levels in patients receiving routine therapy for congestive heart failure, presumably via enhancing cellular Mg2+ efflux, thus implicating a role for aldosterone in cellular Mg2+ shift.Citation40 Sontia et al have suggested that aldosterone may influence urinary Mg2+ excretion through redistribution of Mg2+ (Mg2+ efflux) in muscle, bone, and the gastrointestinal tract via stimulation of the Na+/Mg2+ exchanger and downregulation of renal TRPM7.Citation41
Congenital conditions associated with hypomagnesemia are summarized in .
Table 2 Congenital causes of hypomagnesemia
Etiologies of acquired hypomagnesemia
Similar to congenital conditions, acquired etiologies of hypomagnesemia may be categorized based on the presumed defects in the various steps of magnesium metabolism and are summarized in .
Table 3 Acquired causes of hypomagnesemia
Gastrointestinal causes
Common conditions include severely low dietary Mg2+ intake; prolonged nasogastric suction; and overall malabsorptive states including diarrhea, steatorrhea, celiac disease, regional enteritis, and short-gut syndrome. Gastrointestinal magnesium loss due to malabsorptive states may be due to rapid intestinal transit, Mg2+ binding to undigested free fatty acids, and/or reduction in both passive and active absorption.Citation42–Citation44 Of interest, diarrhea has been attributed as an important cause of hypomagnesemia in patients with chronic alcoholism. In a study involving 127 patients with chronic alcoholism, 20 out of 38 cases of hypomagnesemia were thought to be due to alcohol withdrawal syndrome and diarrhea. The remaining patients with hypomagnesemia had evidence of hypermagnesiuria, thought to be associated with hypophosphatemia and/or metabolic acidosis.Citation45
Cellular shift/tissue sequestration
Acute pancreatitis is known to be associated with hypocalcemia and hypomagnesemia, both presumably via saponification in necrotic fat. The accompanying hypocalcemia-induced hyperparathyroidism and resultant hypophosphatemia may also lead to concurrent renal Mg2+ loss.Citation43 Post-parathyroidectomized patients are thought to develop hypomagnesemia predominantly due to increased bone formation and mineralization, and in some cases, concurrent hypermagnesiuria.Citation46 Conditions associated with increased anabolic states and cellular magnesium uptake leading to reduced plasma magnesium concentration include refeeding syndrome, excessive parenteral alimentation, advanced pregnancy, and lactation.Citation47–Citation49 Hypomagnesemia associated with massive blood transfusions is not uncommon and presumed to be due to citrate toxicity, hemodilution, and/or associated comorbidities.Citation50 Cardiopulmonary bypass surgeries without magnesium supplementation may be associated with hypomagnesemia, thought to be due to increased cellular uptake with the high catecholamine state and chelation by free fatty acids and/or citrate.Citation51 Hypomagnesemia associated with foscarnet has been proposed to occur via magnesium incorporation into bone matrix following complex formation with foscarnet.Citation52
Renal wasting
Hyperfiltration
Hyperfiltration associated with various conditions (including diabetes mellitus; post-obstructive, osmotic, or acute tubular necrosis diuresis; post-kidney transplantation; or excessive volume expansion) may lead to enhanced filterable magnesium load, thus overwhelming the kidneys’ Mg2+ reabsorption capacity, and resultant hypermagnesiuria. Conditions favoring magnesium in its ionized form such as chronic metabolic acidosis or states with low organic anions may also contribute to a high filterable magnesium load and renal Mg2+ wasting.Citation53
Reduced proximal tubular reabsorption
As Mg2+ is reabsorbed passively at the proximal tubules, severe injury to this nephron segment in the presence of normal glomerular filtration can lead to hypermagnesiuria. Acquired Fanconi’s syndrome and tubular toxicities induced by drugs such as cisplatin, aminoglycosides, and pentamidine, have all been reported to be associated with hypomagnesemia. Of note, pentamidine may also be associated with acute pancreatitis, which could be contributory to hypomagnesemia.Citation54 Dietary salt loading has been shown in rats to increase distal tubular Mg2+ delivery, presumably via reduced proximal passive reabsorption, and upregulation of TRPM6 in the DCT, but overall increases urinary Mg2+ excretion.Citation55
Reduced TAL reabsorption
Acquired causes of hypomagnesemia that target the TAL include loop diuretics, hypokalemia, hypercalcemia, and aminoglycosides. Effective reabsorption of Mg2+ at this nephron segment relies on luminal K+ recycling, which requires the functional Na+-K+-2Cl− cotransporter (NKCC2) for cellular K+ uptake, the renal outer medullary K+ channel (ROMK) for K+ recycling back into the lumen, and the CaSR for regulation of the ROMK. Potassium recycling into the lumen via the ROMK creates a more positively charged lumen, which facilitates divalent cation (Ca2+, Mg2+) reabsorption paracellularly. This process is facilitated by the tight junction proteins claudin-16 and claudin-19. Any transporter defect leading to suboptimal K+ recycling or claudin-16/claudin-19 function may induce Ca2+ and Mg2+ wasting. While loop diuretics specifically block NKCC2, hypokalemia may cause both suboptimal NKCC2 functioning as well as K+ recycling. Activation of the CaSR with Ca2+ can inhibit ROMK, and hence cause ineffective K+ tubular recycling. Reduction in K+ recycling, hence favorable intraluminal positive voltage necessary to facilitate Ca2+ and Mg2+ paracellular reabsorption, leads to wasting of both divalent cations. Aminoglycosides have been suggested to directly inhibit tubular Mg2+ reabsorption via binding to and activation of the CaSR. Of interest, this class of antibiotics has been suggested to mimic type 5 Bartter syndrome where there is a gain-of-function mutation of the CaSR.Citation56–Citation58
Reduced DCT reabsorption
Drugs known to be associated with hypomagnesemia and likely exert their magnesiuric effect at the DCTs include calcineurin inhibitors and rapamycin. Both cyclosporine and tacrolimus have been shown to reduce TRPM6 expression in the DCT in animal studies.Citation59–Citation61 Additionally, cyclosporine has been shown to induce reduced messenger (m)RNA expression of NCC.Citation59 Whether the latter effect affects TRPM6 expression or plays a contributory role in magnesiuria is not known. Clinically, tacrolimus has been observed to induce more significant magnesiuria compared with cyclosporine.Citation62
While rapamycin (sirolimus) has been reported to induce hypomagnesemia, the mechanism(s) whereby rapamycin causes magnesiuria is (are) not well defined. In an in vitro study using NRK-52E cells derived from normal rat renal tubules, Ikari et al revealed that sirolimus reduces mRNA expression TRPM6 at the DCT via inhibition of EGF-induced increase in TRPM6 expression, presumably by reducing the stability of TRPM6 mRNA.Citation63 In contrast, in a study using male Wistar rats treated with rapamycin, da Silva et al reported increased TRPM6 expression.Citation64 The magnesiuric effect of rapamycin in the latter study was thought to be due to the primary downregulation of NKCC2 protein expression leading to magnesiuria, followed by a secondary compensatory response with increased DCT TRPM6 protein expression. A direct rapamycin stimulatory effect on TRPM6 expression, however, could not be ruled out. Interestingly, rosiglitazone was shown to ameliorate the rapamycin-associated electrolyte disturbances including hypokalemia and downregulation of NKCC2.Citation64
Although both calcineurin and mammalian target of rapamycin (mTOR) inhibitors may induce renal Mg2+ wasting, mTOR inhibitors may have lower magnesiuric effect compared with calcineurin inhibitors. In a retrospective review involving 138 renal transplant patients who were converted from calcineurin inhibitors to mTOR inhibitors over a 6-month period, magnesium levels significantly improved in association with reduced fractional excretion of Mg2+.65 In addition to drugs, acid-base status has also been shown to determine renal expression of TRPM6; whereas chronic metabolic acidosis reduces, chronic metabolic alkalosis enhances TRPM6 expression.Citation66
To our knowledge, acquired causes of hypomagnesemia associated with inhibition of basolateral Kir4.1/5.1 have not been reported. Although there is evidence of direct inhibition of basolateral Kir4.1/5.1 and Kir4.1 channels in the cortical collecting duct by dopamine and antibody production against the potassium channel Kir4.1 in patients with multiple sclerosis, hypomagnesemia is not associated with these conditions.Citation67,Citation68 Similarly, there are no known reported cases of acquired hypomagnesemia due to direct inhibition of Kv1.1.
As previously discussed, thiazide-, and to some extent, cyclosporine-induced hypomagnesemia may occur via downregulation of TRPM6 expression. Moreover, Loffing et al have shown that thiazide administration over three days in rats can provoke apoptosis of distal tubule cells and associated focal peritubular inflammation.Citation69 The direct effect of thiazides on the terminal nephron segment for magnesium reabsorption may explain the severe hypomagnesemia associated with Gitelman and not necessarily Bartter syndrome. As observed in animal studies where high-salt treatment was associated with increased distal delivery of Mg2+ and upregulation of TRPM6, the increased distal delivery of Mg2+ in patients with various types of Bartter syndromes may similarly upregulate DCT TRPM6, and hence reduce net Mg2+ wasting compared with Gitelman syndrome.Citation55
Factors known to be associated with hypomagnesemia that could act via inhibition of the DCT basolateral Na+-K+-ATPase include hypophosphatemia, calcineurin inhibitors, and ethanol.Citation70,Citation71 As phosphate is required for all ATP-requiring cellular activities, it is conceivable that hypophosphatemia can lead to suboptimal Na+-K+-ATPase activity and, if proven present and significant in the DCT, basolateral Mg2+-ATPase.
While mutations involving EGF affect apical TRPM6 expression hence magnesium reabsorption, the use of anti-EGF receptor antibodies, particularly cetuximab and panitumumab, has accordingly been reported to be associated with hypomagnesemia. Other commonly used drugs associated with hypomagnesemia that have been shown to reduce EGF expression include cisplatin and cyclosporine.Citation59,Citation72,Citation73
Clinical conditions and drugs that could potentially be equivalent to CaSR gain-of-function mutations to induce hypomagnesemia include hypercalcemia and aminoglycosides. Both may stimulate the CaSR and in turn reduce ROMK activity hence reduce the favorable potential difference necessary for optimal paracellular Mg2+ reabsorption at the TAL. Moreover, CaSR activation at the DCT is also thought to induce renal Mg2+ wasting via inhibition of TRPM6 expression. Nonetheless, common conditions associated with hypercalcemia including primary hyperparathyroidism and granulomatous disease, however, have not been reported to be associated with significant hypomagnesemia. In contrast, aminoglycosides including amikacin and gentamicin are well known to induce hypomagnesemia, hypothesized to occur via binding to and activation of the CaSR.Citation74,Citation75 Whether the CaSR agonist cinacalcet can induce hypomagnesemia is not known. Incidentally, our research into this question led to the review of a case series with reported pre- and post-cinacalcet treatment serum magnesium levels in four patients with familial hypocalciuric hypercalcemia. Notably, all four patients’ serum magnesium levels were lower at follow-up than at baseline. The serum magnesium levels before and at follow-up were 1.01±0.07 mmol/L and 0.95±0.01 mmol/L, respectively, P=0.11. Whether this observation is fortuitous, further investigation is warranted.Citation76
Diabetes mellitus is well known to be associated with hypomagnesemia, likely via a plethora of mechanisms.Citation53 Most recently, given reports of reduced insulin activation of TRPM6 in patients with TRPM6 polymorphisms, it may be speculated that the lack of insulin in the diabetic state may reduce TRPM6 activity and could thus contribute to hypomagnesemia.Citation77
Other drugs associated with hypomagnesemia include amphotericin and pentamidine. The former is thought to self-insert into renal tubular membranes and act as an ionophore for urinary magnesium leak, whereas the latter is thought to induce hypomagnesemia via nonspecific, yet to be determined kidney injury.Citation54,Citation78
Finally, hypomagnesemia has been reported in severe burn patients. The involved mechanisms are likely multifactorial and may involve the need for aminoglycoside administration, or associated hypokalemia, among others.Citation79
Diagnosis of hypomagnesemia
The clinical evaluation for the underlying cause of hypomagnesemia requires a thorough investigation for the presence of diabetes mellitus, alcoholism, gastrointestinal conditions involving poor absorption and/or poor nutritional intake, or a family history of hypomagnesemia without or without other electrolyte abnormalities, and a complete list of medications used. The suspected underlying etiology may be confirmed with urinary studies based on its mechanism via renal wasting or extrarenal cause. Patients with hypomagnesemia due to renal Mg2+ wasting have been suggested to present with a fractional excretion of Mg2+ greater than 4%, whereas those with extrarenal causes present with a much lower percentage, typically 2% or less. The fractional excretion of Mg2+ is defined as:
where the 0.7 factor indicates that in most circumstances, only 70% of serum magnesium is filterable.Citation80
Management of hypomagnesemia: authors’ opinion
Magnesium metabolism and kidney handling of magnesium have been significantly elucidated over the last decade. A correlation between clinical conditions and specific mechanisms leading to hypomagnesemia will no doubt lead to better prevention and mechanism-specific therapy. Although mild-to-moderate hypomagnesemia is typically asymptomatic, long-term deficiencies have been reported to be associated with a spectrum of adverse micro- and macrovascular outcomes including increased risk of diabetes mellitus, various diabetic complications, arrhythmias particularly in association with congestive heart failure, hypertension, and more rapid progression of kidney disease, among others. Therefore, if safely tolerated, patients should receive therapy to correct the hypomagnesemic state.Citation2–Citation5,Citation81,Citation82 Currently, however, the management of hypomagnesemia still relies on relatively nonspecific management including avoidance or discontinuation of responsible agents if possible, corrections of underlying metabolic derangements, and/or magnesium supplementation.
Minimization of renal Mg2+ wasting
Routine corrections of the hyperfiltrative state, chronic metabolic acidosis, and low organic anion state (eg, hypophosphatemia and hypoproteinemia/hypoalbuminemia) should be done whenever applicable to minimize filtration of large amounts of free ionized Mg2+. Consultation with a dietitian may be necessary to increase dietary magnesium intake as well as protein supplementation in malnourished patients. Reduction of glomerular hyperfiltration may be achieved with the addition of any renin–angiotensin inhibitors. The use of an aldosterone antagonist may be considered if safely tolerated. Diarrheal states should be evaluated by a gastrointestinal specialist and promptly treated.
Dietary intake of foods containing high levels of magnesium should be encouraged. In general, grains, and beans are good sources of magnesium. Selected food sources of magnesium may be found at ods.od.nih.gov/factsheets/Magnesium-HealthProfessional/#h3.Citation83 A few food sources containing at least 10% of the daily value of recommended total daily dietary magnesium (400 mg for adults and children aged 4 and older) as developed by the US Food and Drug Administration are listed in . If all measures fail to achieve “normal” range or serum magnesium concentration >0.66 mmol/L (1.6 mg/dL), magnesium supplement should be added. Various magnesium formulations are available on the market and are listed in . In the authors’ experience, Mg Plus Protein (Miller Pharmacal Group, Inc., Carol Stream, IL, USA) appears to be best tolerated by patients in terms of magnesium supplement-induced diarrhea.
Table 4 Selected food sources of magnesium
Table 5 Common oral magnesium formulations
In conclusion, recent research has contributed a great deal to our understanding of various congenital and acquired hypomagnesemic states. Although no specific targeted therapy is currently available, routine corrections of underlying etiologies and electrolyte and metabolic derangements, increase in dietary magnesium intake, and/or magnesium supplementation are recommended, as chronic hypomagnesemic state has been reported to be associated with various micro- and macrovascular complications.
Disclosure
The authors report no conflicts of interest in this work.
References
- SandersGTHuijenHJSandersRMagnesium in disease: a review with special emphasis on the serum ionized magnesiumClin Chem Lab Med19993711–121011103310726809
- SarisNEMervaalaEKarppanenHKhawajaJALewenstamAMagnesium: an update on physiological, clinical and analytical aspectsClin Chim Acta20002941–212610727669
- SwaminathanRMagnesium metabolism and its disordersClin Biochem Rev2003242476618568054
- FlinkEBMagnesium deficiency: etiology and clinical spectrumActa Med Scand Suppl19816471251377020347
- Jahnen-DechentWKettelerMMagnesium basicsClin Kidney J20125Suppl 1i3i14
- SchwartzRSpencerHWelshJJMagnesium absorption in human subjects from leafy vegetables, intrinsically labeled with stable 26 MgAm J Clin Nutr19843945715766711467
- FineKDSanta AnaCAPorterJLFordtranJSIntestinal absorption of magnesium from food and supplementsJ Clin Invest19918823964021864954
- QuammeGARecent developments in intestinal magnesium absorptionCur Opin Gastroenterol2008242230235
- SchweigelMMartensHMagnesium transport in the gastrointestinal tractFront Biosci20005D666D67710922297
- SchlingmannKPGudermannTA critical role of TRPM channel-kinase for human magnesium transportJ Physiol2005566Pt 230130815845589
- SchlingmannKPWeberSPetersMHypomagnesemia with secondary hypocalcemia is caused by mutations in TRPM6, a new member of the TRPM gene familyNat Genet200231216617012032568
- [no authors listed]Plasma and bone magnesiumNutrition Reviews196018721421613858038
- QuammeGADirksJHThe physiology of renal magnesium handlingRen Physiol1986952572693544106
- QuammeGAControl of magnesium transport in the thick ascending limbAm J Physiol19892562 Pt 2F197F2102644845
- BlanchardAJeunemaitreXCoudolPParacellin-1 is critical for magnesium and calcium reabsorption in the human thick ascending limb of HenleKidney Int20015962206221511380823
- WeberSSchneiderLPetersMNovel paracellin-1 mutations in 25 families with familial hypomagnesemia with hypercalciuria and nephrocalcinosisJ Am Soc Nephrol20011291872188111518780
- KonradMSchallerASeelowDMutations in the tight-junction gene claudin 19 (CLDN19) are associated with renal magnesium wasting, renal failure, and severe ocular involvementAm J Hum Genet200679594995717033971
- HouJGoodenoughDAClaudin-16 and claudin-19 function in the thick ascending limbCurr Opin Nephrol Hypertens201019548348820616717
- VoetsTNiliusBHoefsSTRPM6 forms the Mg2+ influx channel involved in intestinal and renal Mg2+ absorptionJ Biol Chem20042791192514576148
- KonradMSchlingmannKPGudermanTInsights into the molecular nature of magnesium homeostasisAm J Physiol Renal Physiol20042864F599F60515001450
- ReilyRFEllisonDHMammalian distal tubule: physiology, pathophysiology, and molecular anatomyPhysiol Rev200080127731310617770
- LemminkHHKnoersNVKárolyiLNovel mutations in the thiazide-sensitive NaCl cotransporter gene in patients with Gitelman syndrome with predominant localization to the C-terminal domainKidney Int19985437207309734597
- NijenhuisTVallonVvan der KempAWLoffingJHoenderopJGBindelsRJEnhanced passive Ca2+ reabsorption and reduced Mg2+ channel abundance explains thiazide-induced hypocalciuria and hypomagnesemiaJ Clin Invest200511561651165815902302
- YogiACalleraGEO’ConnorSEDysregulation of renal transient receptor potential melastatin 6/7 but not paracellin-1 in aldosterone-induced hypertension and kidney damage in a model of hereditary hypomagnesemiaJ Hypertens20112971400141021602712
- GlaudemansBvan der WijstJScolaRHA missense mutation in the Kv1.1 voltage-gated potassium channel-encoding gene KCNA1 is linked to human autosomal dominant hypomagnesemiaJ Clin Invest2009119493694219307729
- GroenestegeWMThébaultSvan der WijstJImpaired basolateral sorting of pro-EGF causes isolated recessive renal hypomagnesemiaJ Clin Invest200711782260226717671655
- ThebaultSAlexanderRTTiel GroenestegeWMHoenderopJGBindelsRJEGF increases TRPM6 activity and surface expressionJ Am Soc Nephrol2009201788519073827
- MeijICKoenderinkJBvan BokhovenHDominant isolated renal magnesium loss is caused by misrouting of the Na(+), (K+)-ATPase gamma-subunitNat Genet200026326526611062458
- ArystarkhovaESweadnerKJSplice variants of the gamma subunit (FXYD2) and their significance in regulation of the Na, K-ATPase in kidneyJ Bioenerg Biomembr200537638138616691469
- FerrèSVeenstraGJBouwmeesterRHoenderopJGBindelsRJHNF-1B specifically regulates the transcription of the gamma a-subunit of the Na+/K+-ATPaseBiochem Biophys Res Commun2011404128429021130072
- Kolatsi-JoannouMBinghamCEllardSHepatocyte nuclear factor-1 beta: a new kindred with renal cysts and diabetes and gene expression in normal human developmentJ Am Soc Nephrol200112102175218011562418
- SchollUIChoiMLiuTSeizures, sensorineural deafness, ataxia, mental retardation, and electrolyte imbalance (SeSAME syndrome) caused by mutations in KCNJ10Proc Natl Acad Sci U S A2009106145842584719289823
- BockenhauerDFeatherSStanescuHCEpilepsy, ataxia, sensorineural deafness, tubulopathy, and KCNJ 10 mutationsN Engl J Med2009360191960197019420365
- PaulaisMBloch-FaureMPicardNRenal phenotype in mice lacking the Kir5.1 (Kcnj16) K+ channel subunit contrasts with that observed in SeSame/EAST syndromeProc Natl Acad Sci U S A201110825103611036621633011
- ChaSKHuangCDingYQiXHuangCLMillerRTCalcium-sensing receptor decreases cell surface expression of the inwardly rectifying K+ channel Kir4.1J Biol Chem201128631828183521084311
- StuiverMLainezSWillCCNNM2, encoding a basolateral protein required for renal Mg2+ handling, is mutated in dominant hypomagnesemiaAm J Hum Genet201188333334321397062
- SongYHsuYHNiuTMansonJEBuringJELiuSCommon genetic variants of the ion channel transient receptor potential membrane melastatin 6 and 7 (TRPM6 and TRPM7), magnesium intake, and risk of type 2 diabetes in womenBMC Med Genet200910419149903
- GroenestegeWMHoenderopJGvan den HeuvelLKnoersNBindelsRJThe epithelial Mg2+ channel transient receptor potential melastatin 6 is regulated by dietary Mg2+ content and estrogensJ Am Soc Nephrol20061741035104316524949
- BarrCSLangCCHansonJArnottMKennedyNStruthersADEffects of adding spironolactone to an angiotensin-converting enzyme inhibitor in chronic congestive heart failure secondary to coronary artery diseaseAm J Cardiol19957617125912657503007
- GaoXPengLAdhikariCMLinJZuoZSpironolactone reduced arrhythmia and maintained magnesium homeostasis in patients with congestive heart failureJ Cardiac Fail2007133170177
- SontiaBMontezanoACParaviciniTTabetFTouyzRMDownregulation of renal TRMP7 and increased inflammation and fibrosis in aldosterone-infused mice: effects of magnesiumHypertension200851491592118268139
- MacIntyreIRobinsonCJMagnesium and the gut: experimental and clinical observationsAnn N Y Acad Sci196916228658735259576
- HershTSiddiquiDAMagnesium and the pancreasAm J of Clin Nutr19732633623664570229
- AgusZSHypomagnesemiaJ Am Soc Nephrol19991071616162210405219
- ElisafMMerkouropoulosMTsianosEVSiamopoulosKCPathogenetic mechanisms of hypomagnesemia in alcoholic patientsJ Trace Elem Med Biol1995942102148808192
- TambyahPARauffALeeKOPersistent hypomagnesaemia following parathyroid surgery, hypermagnesuria as a possible causeAnn Acad Med Singapore19901945365392221815
- WeiselbergECGonzalezMFisherMEating disorders in the twenty-first centuryMinerva Ginecol201163653154522036757
- FrazierTGMuchaMERushIHTrullEJCarlsonSAO’ConnorJAHypomagnesemia: higher risk using total parenteral nutrition in the treatment of patients with malignanciesJ Surg Oncol198013135386766197
- FlinkEBMagnesium deficiency. Etiology and clinical spectrumActa Med Scand Suppl19816471251377020347
- HoKMLeonardARisk factors and outcome associated with hypomagnesemia in massive transfusionTransfusion201151227027620735766
- AglioLSStanfordGGMaddiRBoydJL3rdNussbaumSChernowBHypomagnesemia is common following cardiac surgeryJ Cardiothorac Vasc Anesth1991532012081863738
- GearhartMOSorgTBFoscarnet-induced severe hypomagnesemia and other electrolyte disordersAnn Pharmacother19932732852898384030
- PhamPCTPhamPMTPhamSVMillerJMPhamPTTHypomagnesemia in patients with type 2 diabetesClin J Am Soc Nephrol20072236637317699436
- ShahGMAlvaradoPKirschenbaumMASymptomatic hypocalcemia and hypomagnesemia with renal magnesium wasting associated with pentamidine therapy in a patient with AIDSAm J Med19908933803822393042
- LeeCTLienYHHLaiLWNgHYChiouTTChenHCVariations of dietary salt and fluid modulate calcium and magnesium transport in the renal distal tubuleNephron Physiol20121223–4192723774784
- HolmesAMHeslingCMWilsonTMDrug-induced secondary hyperaldosteronism in patients with pulmonary tuberculosisQ J Med1970391542993155449594
- ChouCLChenYHChauTLinSHAcquired Bartter-like syndrome associated with gentamicin administrationAm J Med Sci2005329314414915767821
- ZietseRZoutendijkRHoornEJFluid, electrolyte and acid-base disorders associated with antibiotic therapyNat Rev Nephrol20095419320219322184
- LedeganckKJBouletGAHorvathCAEffects of renal distal tubule transporters TRPM6 and NCC in a rat model of cyclosporine nephrotoxicity and effect of EGF treatmentAm J Physiol Renal Physiol20113013F486F49321653632
- NijenhuisTHoenderopJGBindelsRJDownregulation of Ca(2+) and Mg(2+) transport proteins in the kidney explains tacrolimus (FK506)-induced hypercalciuria and hypomagnesemiaJ Am Soc Nephrol200415354955714978156
- IkariAOkudeCSawadaHTakahashiTSugataniJMiwaMDown-regulation of TRPM6-mediated magnesium influx by cyclosporin ANaunyn Schmiedebergs Arch Pharmacol20083774–633334318026717
- AisaYMoriTNakazatoTEffects of immunosuppressive agents on magnesium metabolism early after allogeneic hematopoietic stem cell transplantationTransplantation20058081046105016278584
- IkariASanadaASawadaHOkudeCTonegawaCSugataniJDecrease in transient receptor potential melastatin 6 mRNA stability caused by rapamycin in renal tubular eptiherlial cellsBiochim Biophys Acta2011180861502150821073857
- da SilvaCAde BragançaACShimizuMHRosiglitazone prevents sirolimus-induced hypomagnesemia, hypokalemia, and downregulation of NKCC2 protein expressionAm J Physiol Renal Physiol20092974F916F92219656910
- Sánchez-FructuosoAISantín CanteroJMPérez FloresIValero San CecilioRCalvo RomeroNVilalta CasasRChanges in magnesium and potassium homeostasis after conversion from a calcineurin inhibitor regimen to an mTOR inhibitor-based regimenTransplant Proc20104283047304920970606
- NijenhuisTRenkemaKYHoenderopJGBindelsRJAcid-base status determines the renal expression of Ca2+ and Mg2+ transport proteinsJ Am Soc Nephrol200617361762616421227
- ZaikaOLMamenkoMPalyginOBoukelmouneNStaruschenkoAPochynyukODirect inhibition of basolateral Kir4.1/5.1 and Kir4.1 channels in the cortical collecting duct by dopamineAm J Physiol Renal Physiol20133059F1277F128723986512
- SrivastavaRAslamMKalluriSRPotassium channel KIR4.1 as an immune target in multiple sclerosisN Engl J Med2012367211512322784115
- LoffingJLoffing-CueniDHegylIThiazide treatment of rats provokes apoptosis in distal tubule cellsKidney Int1996504118011908887276
- Younes-IbrahimMBarneseMBurthPCastro-FariaMVInhibition of purified human kidney Na+-K+-ATPase by cyclosporine A: a possible mechanism for drug human nephrotoxicityAnn N Y Acad Sci200398663363512763906
- RodrigoRThielemannLEffects of chronic and acute ethanol exposure on renal (Na+K)-ATPase in the ratGen Pharmacol19972957197239347316
- do Pazo-OubiñaFEstefanell-TejeroARiu-ViladomsGAnglada-MartínezHMolas-FerrerGCreus-BaróNMagnesium monitoring practice in monoclonal anti-epidermal growth factor receptor antibodies therapyJ Clin Pharma Ther2013382101103
- LedeganckKJBouletGABogersJJVerpootenGADe WinterBYThe TRPM6/EGF pathway is downregulated in a rat model of cisplatin nephrotoxicityPLoS One201382e5701623457647
- ChrispalABooruguHPrabhakarATMosesVAmikacin-induced type 5 Bartter-like syndrome with severe hypocalcemiaJ Postgrad Med200955320821019884751
- ChouCLChenYHChauTLinSHAcquired Bartter-like syndrome associated with gentamicin administrationAm J Med Sci2005329314414915767821
- RasmussenAQJørgensenNRSchwarzPClinical and biochemical outcomes of cinacalcet treatment of familial hypocalciuric hypercalcemia: a case seriesJ Med Case Rep20115156422142470
- NairAVHocherBVerkaartSLoss of insulin-induced activation of TRPM6 magnesium channels results in impaired glucose tolerance during pregnancyProc Natl Acad Sci U S A201210928113241132922733750
- FinkelsteinAHolzRAqueous pores created in thin lipid membranes by the polyene antibiotics nystatin and amphotericin BMembranes197323774084585230
- CunninghamJJAnbarRDCrawfordJDHypomagnesemia: a multifactorial complication of treatment of patients with severe burn traumaJPEN J Parenter Enteral Nutr19871143643673112426
- ElisafMPanteliKTheodorouJSiamopoulosKCFractional excretion of magnesium in normal subjects and in patients with hypomagnesemiaMagnes Res19971043153209513927
- PhamPCPhamPMPhamPALower serum magnesium levels are associated with more rapid decline of renal function in patients with diabetes mellitus type 2Clin Nephrol200563642943615960144
- Van LaeckeSVan BiesenWVanholderRHypomagnesaemia, the kidney and the vesselsNephrol Dial Transplant201227114003401022610987
- US Department of Health and Human Services. National Institutes of HealthMagnesium fact sheet for health professionals Available from ods.od.nih.gov/factsheets/Magnesium-HealthProfessional/#h3Accessed March 27, 2014