Abstract
Polyethylene glycol (PEG) is a synthetic biocompatible polymer with many useful properties for developing therapeutics to treat spinal cord injury. Direct application of PEG as a fusogen to the injury site can repair cell membranes, mitigate oxidative stress, and promote axonal regeneration to restore motor function. PEG can be covalently or noncovalently conjugated to proteins, peptides, and nanoparticles to limit their clearance by the reticuloendothelial system, reduce their immunogenicity, and facilitate crossing the blood–brain barrier. Cross-linking PEG produces hydrogels that can act as delivery vehicles for bioactive molecules including growth factors and cells such as bone marrow stromal cells, which can modulate the inflammatory response and support neural tissue regeneration. PEG hydrogels can be cross-linked in vitro or delivered as an injectable formulation that can gel in situ at the site of injury. Chemical and mechanical properties of PEG hydrogels are tunable and must be optimized for creating the most favorable delivery environment. Peptides mimicking extracellular matrix protein such as laminin and n-cadherin can be incorporated into PEG hydrogels to promote neural differentiation and axonal extensions. Different hydrogel cross-linking densities and stiffness will also affect the differentiation process. PEG hydrogels with a gradient of peptide concentrations or Young’s modulus have been developed to systematically study these factors. This review will describe these and other recent advancements of PEG in the field of spinal cord injury in greater detail.
Introduction
Spinal cord injury (SCI) disrupts motor, sensory, and autonomic functions that can severely reduce the quality of life for patients. In the USA alone, there is an estimated 54 cases of SCI per million population with ~17,000 new cases each year. Most incidences of SCI occur because of traumatic incidents such as falls and accidents with men accounting for ~80% of new SCI cases in the USA.Citation1 Unfortunately, no clinical treatment to repair the damage of SCI, such as restoring motor function, exists. Furthermore, the cost of care for patients is significant and exerts a heavy burden on patients, families, and society at large as it can exceed >US$1 million over the first year.Citation2
After SCI, a glial scar forms around the injury area or lesion, which inhibits the growth of new axons and synapses.Citation3 Biomaterials offer a variety of strategies for treating SCI by bridging and filling the lesion, delivering cells to replace those that have been lost, or slowly releasing drugs that can mitigate the damage due to inflammation or make the environment more permissible for cell invasion. Reduced lesion formation has been reported with biomaterial use.Citation4,Citation5 Although a number of biomaterials are being pursued to treat SCI,Citation6 this review will highlight the use of polyethylene glycol (PEG), a synthetic material known for its capacity to immediately repair physical damage and reduce local glial scar formation,Citation7 and its applications in SCI.
PEG properties and characteristics
PEG is a relatively inexpensive, water-soluble, and linear polymer that is synthesized by the living anionic ring-opening polymerization of ethylene oxide with molecular weights ranging from 0.4 to 100 kDa. End-group modification with different reactive moieties makes it possible for PEG to participate in covalent bonding through a number of chemistries ().Citation8–Citation14 Attaching PEG through covalent or noncovalent interactions can prolong the circulation of proteins, peptides, and other molecules without compromising their bioactivity.Citation15,Citation16 In addition to PEGylation (bioconjugation), PEG can be cross-linked to form porous hydrogels, which can serve as biocompatible matrices that can closely mimic the extracellular matrix (ECM) found in tissues.Citation17 Typically, PEG hydrogels are formed by either photoinitiator- or redox-generated radicals, which initiate chain-growth and step-growth polymerization. The choice of the free radical initiator is important as it affects gelation time, mechanical strength, and the viability of embedded cells.Citation18 Photoinitiators, such as Irgacure 2959, have been frequently used for cross-linking PEG,Citation19–Citation21 but may be limited in their application in vivo as the light wavelength needed does not easily penetrate tissue. Wilems et al investigated the use of other free radical initiators such as ammonium persulfate (APS)/ tetramethylethylenediamine (TEMED), VA044 (a thermal initiator), and a Fenton chemistry reaction (driven by glucose/ glucose oxidase oxidation/reduction of Fe).Citation8 APS/TEMED was determined to be the most viable replacement for Irgacure 2959 of the tested initiators in that study.
Figure 1 Reactive moieties that have been used as functional end groups on PEG.
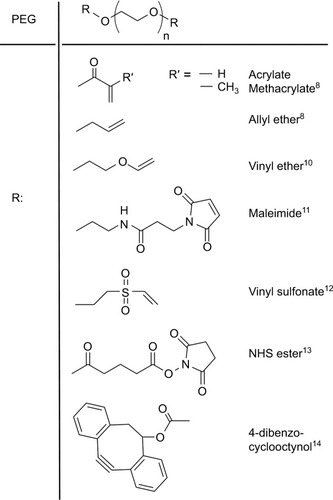
Beyond changes due to radical initiator choice, polymer concentration, chain length, and chain configuration (linear, multiarm, grafted, and so on) are known to result in diverse network structures and affect physical and rheologic properties of PEG hydrogels.Citation22,Citation23 This makes the hydrogels’ mechanical and material properties (such as Young’s and shear modulus, mesh size, and so on) easily tailorable.Citation24 Copolymers, additives, additional processing (such as electrospinning), and other means can be used to fine-tune matrix properties further, making it applicable to not only SCI but also for other tissue repair.Citation25,Citation26
Systematic optimization of PEG systems
Although various mechanical and physical properties of PEG systems must be optimized to maximize the survival and function of encapsulated cells and promote interaction with the host environment, let us first consider the optimization of the general biochemical environment. The injury environment contains a host of nonpermissive molecules (e.g., chondroitin sulfate proteoglycan or CSPG, Nogo-A, and myelin-associated glycoproteins), which can inhibit axonal growth, and also permissive factors (e.g., laminin and tenascin) that promote axon growth and hence are targets for SCI therapeutics. CSPG is particularly important as it is a key component of the glial scar that forms after SCI. PEG does not prevent the formation of the glial scar but can reduce it.Citation27 Glycosaminoglycan (GAG) chains on CSPGs can serve as guidance cues during development but will block axonal growth after SCI.Citation3 Traditionally, it has been thought that enzymatic digestion of the chondroitin sulfate GAG chains with enzymes such as chondroitinase ABC (chABC) may promote regeneration.Citation28 However, the instability of chABC has limited its use despite efforts to stabilize it.Citation29,Citation30 Another strategy for limiting the role of CSPG could be to neutralize specific CSPG sugar epitopes that are responsible for the inhibitory effects of CSPG.Citation31 Comparatively, there has been much more effort to engineer and optimize systems for delivering growth-permissive substrates.
Peptides are short sequences of amino acids designed to mimic the biologic function of various growth-promoting molecules such as ECM proteins and neurotrophic growth factors,Citation32,Citation33 but are easier to embed because of their smaller size that allows for better dispersion through the three-dimensional (3D) material and are more easily tethered with the right regio- and chemoselectivity. Serotonergic innervation,Citation34 locomotor recovery,Citation35,Citation36 and neural progenitor cell survival and integrationCitation37,Citation38 have been improved by application of peptides. Peptides can be incorporated into PEG for delivery to the SCI lesion ().Citation20,Citation39 In addition, a variety of peptide-conjugated PEG hydrogels have been created and used for studying neural differentiation in vitro. RGD–tenascin-conjugated PEG hydrogels promoted the survival and differentiation of neural stem cells.Citation40 An amino acid sequence based on IKVAV, a peptide with similar biologic effect as laminin-1,Citation41,Citation42 was incorporated at 10 µM concentrations into a PEG tetra-acrylate hydrogel to support growth and differentiation of human neural stem cells.Citation43 IKVAV also influences growth cone movement.Citation44
Figure 2 (A) Schematic for tethering peptides to PEG dimethacrylate gels. (B) Diagram of a syringe pump system used for embedding a linear gradient of peptide within a hydrogel. For two-dimensional cultures, only two polymer (with/without peptide) syringes are needed to generate the hydrogel. For three-dimensional cultures, the cell syringe is added and pumped along with the other two syringes. There is no disruption to the peptide gradient with the third syringe. (C) Effect of different HAVDI peptide concentrations on Tuj1 expression and neurite extension from human neural stem cells. *P-value <0.05.
Abbreviation: PEG, polyethylene glycol.
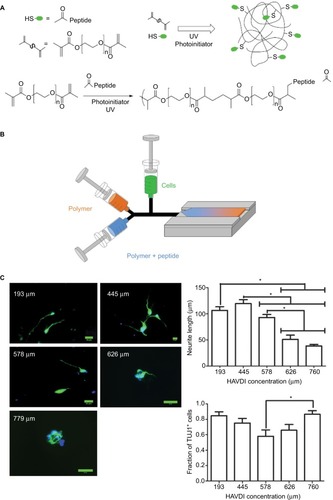
ECM proteins that peptides, like IKVAV, mimic play critical roles in supporting axonal regeneration into the injury area.Citation45 Without a simple and systematic method, the optimization process can be tedious and expensive for parameters, such as peptide concentration, that affect the success of regenerative medicine approaches for SCI. One can use a continuous-gradient approach to see how changes in the matrix properties will affect the cell behavior.Citation46 Using a system of syringe pumps (), Lim et al were able to quickly fabricate a linear gradient of an N-cadherin peptide, HAVDI, embedded within a PEG hydrogel and apply it to studying the survival and neuronal differentiation of mouse embryonic stem cells (mESCs).Citation47 They found that there was a biphasic response in the lengths of the neurite extension and Tuj1 expression. After differentiating for 6 days, cells exhibited significantly higher Tuj1 expression at both 292 and 467 µM compared to other concentrations. This type of cellular response is often not identified with traditional optimization strategies. Increasing the amount of HAVDI further led to greater caspase activation and cellular apoptosis. Using this construct, Lim also explored the effects of HAVDI on the differentiation of neural stem cells derived from human-induced pluripotent stem cells. No biphasic response was seen in terms of neurite extensions with the human cells (). The HAVDI concentration with the highest Tuj1 mRNA expression was 577 µM,Citation48 which indicates a potential shift in optimal peptide concentration between the species. These two studies illustrate the usefulness of a gradient system to identify the optimal peptide concentration for neural differentiation.
Aside from molecular gradients, the dimensionality (2D vs 3D) will most likely affect experimental outcomes because cells will behave differently depending on whether they are embedded within a matrix or simply plated on a 2D surface.Citation46 Using a system of three syringe pumps, Yang et al fabricated a PEG hydrogel with a linear gradient of IKVAVCitation49 and once again studied the neural differentiation of mESCs cultured both on top of (2D) or encapsulated within (3D) the gels. The difference in dimensionality led to drastically different peptide concentration requirements for successful neuronal differentiation. In 2D, the optimal amount of IKVAV for mESC’s neuronal differentiation and survival was 570 µM with lower IKVAV concentrations resulting in decreased cell attachment. Whereas in 3D, 60 µM of IKVAV showed greater neuronal marker expression compared to that of higher concentrations (). It is clear from the results of this study that dimensionality is an important consideration when interpreting biologic data and translating the results of in vitro studies to in vivo experiments. The 2D environment may not recapitulate all of the signaling found in a 3D system; gradient hydrogels provide a facile way for studying the response of cells to changing extrinsic factors in an environment that may better emulate that which is found in vivo.
Figure 3 (A) Effect of IKVAV concentration on axon length of mouse embryonic stem cells (Tuj1/DAPI staining) changes depending on whether the cells are cultured in a 2D or a 3D setting. A much lower concentration of IKVAV is necessary for inducing differentiation in 3D culture than that in 2D culture. (B) Effect of different Young’s modulus on axon length (Tuj1/DAPI staining) of human-induced pluripotent stem cells. Second row is simply higher-magnification images of the axons. Scale bar indicates 20 µm.
Abbreviations: 3D, three-dimensional; DAPI, 4′,6-diamidino-2-phenylindole.
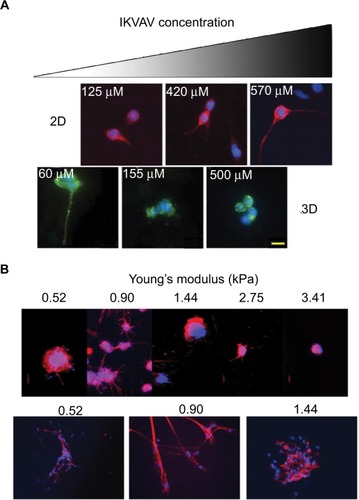
Mechanical properties such as stiffness and cross-linking density are other important parameters that can be conveniently studied with gradient hydrogels. Mosley et al demonstrated the fabrication of a PEG hydrogel with a continuous linear gradient of Young’s moduli and examined how varying the stiffness of a hydrogel can affect neuronal differentiation and neurite extensions of human neural stem cells.Citation50 They determined that a Young’s modulus of 907 Pa allowed for the longest axonal extensions () and MAP2 expression, which closely abide with the Young’s modulus of the brain and spinal cord.Citation51 Hydrogels with a lower moduli (502 Pa) showed decreased levels of Tuj1 despite other matrix properties such as swelling ratio and mesh size being the same. At higher Young’s moduli (2–3 kPa), hydrogels demonstrated decreased Tuj1 and MAP2, but higher GFAP expression. Neurons are not the only cell types affected by matrix stiffness. A study compared the effects of storage modulus and cross-linking density on oligodendrocyte progenitor differentiation. Compared to long-chain-length PEG, hydrogels derived from shorter-chain-length PEGs possessed a higher cross-linking density that resulted in a stiffer material and led to higher expressions of PDGFRα.Citation52 These studies reinforce the importance of modulating mechanical properties to fit the differentiating neuralCitation53,Citation54 or glial cellsCitation55,Citation56 for treating SCI.
PEG in preclinical models of SCI
It is long known that the mechanical force of the primary injury leads to an influx of Ca2+ into neurons.Citation57 This accumulation of Ca2+ generates free oxidative radicals that have disastrous effects on axons leading to apoptosis and proteases activation.Citation58 PEG is a surfactant because of its hydrophilic nature. These properties promote fusion of cell membranes and membrane fluidity.Citation59 During the acute phase of SCI, PEG may inhibit nerve fiber degeneration and create a favorable microenvironment for the regeneration of nerve filaments.Citation7 The combined actions of sealing cell membranes with PEG and applying an electric field were able to block inward flux of Ca2+ ions and lead to enhanced functional recovery.Citation60
PEG can also stimulate angiogenesis, isolate or reduce local glial scar invasion, promote and guide axonal regeneration, and restore synaptic connections with target tissue, hence stimulating injury repair.Citation61 PEG hydrogels have high water content and porosity, which make them behave like aqueous solutions at a microscopic scale while being macroscopically solid. This facilitates the uptake and diffusion of molecules and cells. Molecules smaller than hydrogel pores can be released through diffusion, while larger molecules can be released through degradation and swelling.Citation62 PEG has a wide range of bioengineering applications ranging from a membrane patch to 3D scaffolds and drug delivery vehicles.Citation9–Citation13,Citation49,Citation63 In this section, we will discuss specific applications of PEG in SCI in greater depth ().
Table 1 A summary of technologies and methods in which PEG has been used to treat different phases of SCI
PEG as a fusogen
The loss of membrane integrity seriously harms cells because of disruptions in the balance of ions and subsequent release of reactive oxygen species from the mitochondria and threatens to deleteriously affect spinal cord functionality as the damage spread beyond the initial injury site. In SCI, PEG has been shown to act as a fusogen with the ability to repair compromised neuronal membranes.Citation59,Citation64 While the exact mechanism of fusing the cell membrane is unclear, it has been hypothesized that either PEG dehydrates the cell membrane and allows lipid elements to resolve into each other or PEG reduces the surface tension and improves the membrane’s fluidity so that sealing may occur.Citation65
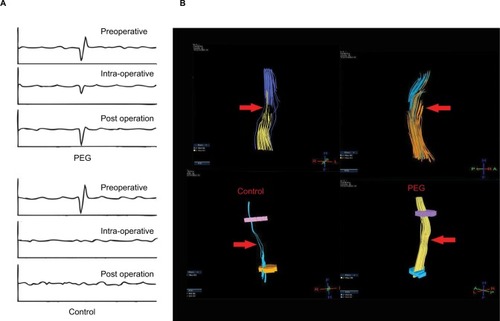
Ren et al performed laminectomy at T-10 in rats and immediately applied either saline or PEG-600 directly to the point of transection in an attempt to refuse the thoracic spinal cord.Citation66 The blood–brain barrier (BBB) score of the PEG-treated group steadily improved over a period of 28 days, whereas the saline-treated group did not. Recordings of somatosensory evoked potentials (SSEP) showed recovery of the waveform in PEG-treated animals, whereas the SSEP form did not recover in control animals (). Direct tensor imaging (DTI) showed that this recovery may be partially due to the tissue continuity found in PEG group (). In a recent but still ongoing study, Liu et al applied PEG as a fusogen in an acute complete transection of dog’s spinal cord at T-10.Citation67 Using a modified BBB score for assessing dog locomotion, the PEG-treated group had a median score of 8 while the negative control group had a score of 3 (scale is 0–15 with 0 being no hindlimb movement). DTI showed some fiber reconstitution in PEG-treated group along with increased SSEP waveforms compared to no treatment. As of the writing of this review, the study is still incomplete, but it is important to note that this extremely acute injury model is a clean transection, which is quite different from blunt trauma and generates a comparatively less substantial glial scar. Other work has shown that formation of the astrocyte scar may beneficially participate in the regeneration process until the subacute phase and then it becomes prohibitive.Citation68 Ren notes that PEG does not prevent the formation of the scar and hypothesizes that axon regrowth through the PEG matrix occurs before the subacute phase and helps to bridge the ends of the severed cords, thus contributing to the restoration of function.Citation66 Similarly, Kim conducted cervical laminectomy at C5 in a rat SCI model and then immediately applied PEG-600 or saline. Measurements of motor evoked potential (MEP) found that PEG-treated animals showed an increase in the measurement of MEP’s amplitude (mean of 0.081 vs 0.156 mV) at 1 hour after injury.Citation69 While it is not yet clear how well these results would translate to a clinical setting, they are encouraging and are suggestive of the benefits of early and direct application of PEG to the severed spinal cord in order to mitigate neural damage and promote the regenerative process.
Figure 4 (A) Waveform of somatosensory evoked potentials before and after (15 days) spinal cord transection for PEG-treated and negative control groups. PEG treatment elicited some recovery in the shape of the waveform compared to no treatment at all. (B) Diffused tensor imaging of spinal cord transection with/without PEG treatment. PEG treatment group led to greater continuity of nerve fibers compared to no treatment.
Abbreviation: PEG, polyethylene glycol.
Injectable PEG
Various PEG formulations have been developed to create injectable platforms that offer the ability to deliver cells or other drugs directly to the site of SCI.Citation70,Citation71 One of these has highlighted the potential of using PEG beyond the early phases of SCI. Estrada et al compared the application of PEG-600, matrigel, or alginate to rats in a chronic model (5 weeks) of SCI. After either a hemisection or complete transection, the lesion scar was resectioned and treated with the polymers. Matrigel did not elicit any axonal in-growth into the biopolymers, whereas there was no difference between the axonal extensions of the alginate- or resection-only group. PEG-600 group showed the highest axon density within the lesion area and correspondingly had the greatest vascularization (in terms of number of blood vessels and their size) within the graft. Aside from axons, they also identified the presence of Schwann cells, astrocytes, and endothelial cells within the PEG-600 graft with limited number of B- and T-cells and ED-1-positive inflammatory cells. Even though there was some heterogeneity in the BBB score of animals receiving PEG-600, overall, they still performed better than the transection-only group. This work highlights the idea that even in a chronic injury setting, PEG can act as a supportive matrix and promote the infiltration of glial cells such as astrocytes and Schwann cells as well as angiogenesis to promote axonal regrowth and some functional recovery.Citation70
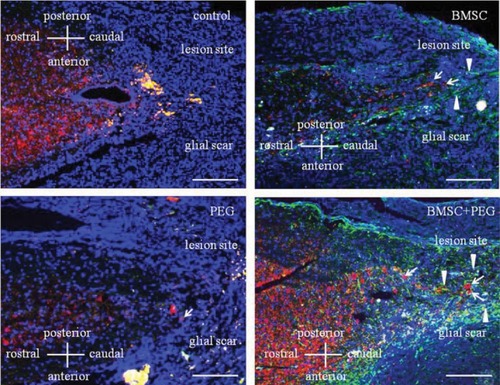
Oda et al studied the effects of delivering combinations of PEG and bone marrow-derived mesenchymal stem cells (MSC) in a mouse model of SCI. Female ICR mice underwent T-10 dorsal laminectomy and were then injected with PEG, MSC, or PEG+MSC. Only MSC alone or PEG+MSC showed significant migration of neuronal cells toward the glial scar area (). Interestingly, all of the groups showed higher but similar motor function scores compared to the negative control, so PEG and MSC did not have any synergistic effect on functional recovery.Citation72
Figure 5 Image of glial fibrillary acidic protein (green), microtubule-associated protein 2 (red), and 4′,6-diamidino-2-phenylindole staining of thoracic cord in mice 4 weeks after injury with transplantation of MSC with/without PEG. Compared to all other groups, mice treated with both PEG and MSC showed many neuronal cells migrating toward the glial scar.
Abbreviations: MSC, mesenchymal stem cells; PEG, polyethylene glycol; BMSC, bone marrow derived mesenchymal stem cell.
Huang et al investigated PEG infused with magnesium sulfate (AC105) and its impact on reducing excitotoxic glutamate exposure in a rat SCI model.Citation73 As Mg2+ homeostasis is important for a variety of cell functions such as membrane stabilization and energy metabolism, restoring this homeostasis may be beneficial for SCI. After a T9/10 laminectomy, the extracellular Mg concentration decreased significantly. Single injections of AC105 showed some recovery in the levels of Mg, whereas either delivery of saline or MgSO4 did not. Multiple deliveries of AC105 raised extracellular Mg concentration even more. Though not statistically significant, extracellular glutamate levels increased by 20% after SCI; this elevation was abolished by treatment with AC105 but not MgSO4. It is yet unclear whether a potential PEG–Mg complex could explain AC105’s superior performance over direct Mg delivery but, nevertheless, as glutamate–N-methyl-D-aspartate excitotoxicity is a major driver of secondary SCI damage, injectable PEG with Mg or other ions may help to mitigate its damage.
Injectable PEG can also be delivered as a polymer that can cross-link and gel in situ; this offers the advantage of a matrix that can conform geometrically to the defect without requiring a pregelled patient-specific hydrogel or causing additional excision of healthy tissue. After injection, functionalized polymeric scaffolds can support spinal tissue regeneration by promoting revascularizationCitation74 and encouraging axonal infiltration into the matrix.Citation75 Comolli et al had developed a poly(N-isopropylacrylamide)-co-poly(ethylene glycol) injectable platform for SCI, which allowed for the slow release of growth factors such as brain-derived neurotrophic factor (BDNF) and neurotrophin-3 as well as enabling the survival of MSC.Citation76 Using this platform as an inspiration, Cai et al developed a shear thinning hydrogel designed to protect the cells from the injection process and slowing down the degradation time after the gel has formed at the injury site.Citation77 The ex vivo network is formed between an eight-arm peptide–PEG copolymer that assembles with a recombinant protein and then in situ cross-linking occurs because of the thermal phase transition of a PNIPAM chain conjugated to the PEG copolymer. By increasing the density of the PNIPAM network, it is possible to modulate and slow the degradation rate of the gel, which can then improve cell retention and lower the initial number of cells that must be implanted.
PEG and nanoparticles (NPs)
PEG can also act as a surface ligand for improving the delivery of NPs in order to treat SCI. The small size of NP offers the advantage of easily crossing cell membranes for targeted drug delivery.Citation78 A variety of NP systems have been developed for neural drug delivery applications such as transporting methylprednisolone,Citation79 growth factors such as bFGF-2,Citation80 and serotonin receptor agonists.Citation81 For systemic delivery of NP, commonly encountered obstacles that can prevent NP from reaching their target are clearance by the reticuloendothelial system and/or being blocked by the BBB. Covalent/noncovalent attachment of PEG (PEGylation) to NP allows them to have reduced immunogenicityCitation82,Citation83 and better penetrate the BBB.Citation84
Despite its reputation of being a “stealth” coating material, there have been reports of anti-PEG antibodies present in human and animal models.Citation85–Citation87 PEG hydrogels have also been known to stimulate foreign body response.Citation88,Citation89 Interestingly, anti-PEG antibodies (ab) have been detected in patients treated with and without PEGylated drugs, perhaps due to the widespread use of PEG in household products including toothpaste and shampoo.Citation90 Consequently, the α-PEG ab may lead to the accelerated blood clearance of PEGylated therapeutics in the liver and effectively decrease their efficacy.Citation91,Citation92
Previously, it was found that PEGylated liposomes stimulated immunoglobulin M (IgM) ab production in marginal zone (MZ) B cells without any dependence on T-cells.Citation93 Subsequently, the anti-PEG IgM ab binding to PEGylated liposomes and their recognition by complement receptors on the surface of the MZ B-cell can lead to transport of the liposomes to spleen follicles.Citation94 The exact binding epitope is uncertain but B-cell activation may occur because of identical repeating epitopes.Citation95 It may also be that PEG acts as a hapten and only elicits an immune response when conjugated to another molecule.Citation96
Papastefanaki et al investigated the use of PEG-functionalized 40 nm AuNP for treating SCI.Citation97 In vitro, the AuNP offered protection against H2O2-induced toxicity of mouse cerebellar neurons. Using a mouse model of compression SCI, they showed that animals treated with AuNP had higher BBB scores as well as other hindlimb improvements such as foot stepping angles and ladder climbing. The PEG-AuNP also decreased the microglia presence at the injury site, which suggests the NP’s capacity to attenuate the immune response. Animals with AuNP also showed higher number of motor neuron survival compared to PBS controls along with greater remyelination of existing axonal fibers.
Papa et al studied the effects of surface charge and PEGylation on the uptake of poly methyl(methacrylate) NP by microglia and used this system to deliver anti-inflammatory drugs.Citation98 Their results showed that positive surface charges increased uptake, whereas PEGylation slowed but did not block uptake of the NP by activated microglia. After uptake, drugs were released through a Fickian diffusion mechanism. In accordance with this result, Jenkins et al studied the effects of PEGylation on magnetic NP and their uptake by major cells within the central nervous system such as microglia, oligodendrocyte precursor cells, astrocyte, and neurons. They found that PEGylation reduces uptake by all of these cells, which then increased the extracellular availability of the NPs.Citation99 Therefore, depending on the application, PEGylation must be carefully tuned and modified or otherwise it may produce an undesired result by limiting the amount of NP being delivered to the target.
PEGylation is not the only application of PEG in nano-medicine. Nanomicelles have been derived from triblock monomethyl PEG-poly(l-lactide)-poly(trimethylene carbonate) copolymers. These nanomicelles were loaded with zonisamide that slowly released over a period of 2 weeks. These nanomicelles protected CTX TNA2 cells against H2O2- induced toxicity and when cultured with cortical neurons, increased the axon lengths. When used in an in vivo rat SCI model, animals treated with the nanomicelles showed improved BBB scores.Citation100 PEG-DSPE micelles formulated with apamin, a small 18-amino-acid peptide known to penetrate the BBB, was used to target micelles to the spinal cord.Citation101 Delivery of the micelles that carried curcumin, known to possess antioxidant properties, significantly improved the BBB score of the animals.
PEG as drug and cell delivery vehicles
In addition to PEGylation, various PEG-based structures such as micelles and hydrogels have been extensively used as delivery vehicles. The payloads can be either proteins such as growth factors, bioactive peptides, or cells.Citation102 Various modifications to confer degradability to PEG hydrogels and incorporation of growth factors/immunomodulatory drugs have been developed for PEG combinatorial tissue engineering strategies.Citation103,Citation104 Cholesterol coupled to PEG to create a cholesterol–ortho-nitrobenzyl–PEG complex, which was then used to fuse two distinct liposomal membranes in situ.Citation105 This system may be used for intracellular release of liposome-encapsulated drugs. Lampe et al developed that PLGA microspheres loaded with BDNF and glial cell line– derived neurotrophic factor (GDNF) can be embedded within degradable PEG hydrogels for a slow release of their cargo. This controlled delivery of neurotrophic factors reduced the microglial response.Citation106 Recent work with a layered agarose gel functionalized with PEG, poly(acrylic acid), and BDNF has shown that this construct is capable of controlled release of BDNF over 10 days. In addition to providing chemical signaling, this gel also possesses linear channels to provide physical cues to guide axonal growth.Citation107
However, low-molecular-weight PEG may be deleterious to the release of encapsulated proteins. An injectable hybrid NP/hydrogel system was used to study the release of PDGF-AA from PLGA NPs that were embedded in a PEG-400 hydrogel.Citation108 The addition of PEG-400 lowered the release of detectable PDGF-AA; over half of the encapsulated protein remain unaccounted for after 21 days. As PEG did not affect the release of encapsulated bovine serum albumin, it could have altered the protein stability of PDGF-AA rather than the NP. It is unclear by what mechanism PEG affected the PDGF-AA as the authors did not find any effects of PEG on the release of BDNF. Other studies have noted widely varying results on how PEG-400 affects other neurotrophic factors.Citation109,Citation110
Additionally, the stability and availability of encapsulated protein in PEG hydrogels may be affected by the cross-linking reaction. Hammer et al studied the modifications to lysozyme after encapsulation in various PEG gels fabricated from PEG derivatives including PEG5k-maleimide, -acryl-amide, -thiol, and -furan.Citation111 Depending on both the pH and type of PEG derivative, multiple PEG chains were attached to lysozyme, which also affected the proportion of free and bound proteins within the gels and may potentially affect the protein’s function. The susceptibility of each protein may be affected by the nucleophilicity of the amino acids that may react with α,β-unsaturated carbonyl compounds in Michael-type addition reactions. Also, the distribution of the amino acids should be considered as those on the protein surface are more easily available to reaction than those found within the protein core. Precipitation with PEG or Zn2+,Citation112 addition of transition metal chelators,Citation113 or separating hydrophobic polymerization sites from hydrophilic protein areas have been presented as strategies for preserving protein function.Citation114 Therefore, continued study of the mechanism underlying this phenomenon is highly warranted.
Cell delivery is a particularly attractive candidate for treating SCI because not only can the cells replace those cells that were lost, they can also secrete a variety of cytokines and growth factors to stimulate native tissue regeneration. These cells may increase cell–cell interaction, adhesion, survival and proliferation, and differentiation.Citation115–Citation117 Common cell types for inclusion into PEG-based scaffolds include neural progenitors derived from embryonic or induced pluripotent stem cells or MSC. Neural progenitor cells can differentiate into neurons for restoring connectivityCitation118 or glial cells such as Schwann cells to secrete growth factors such as GDNF for promoting axonal regeneration and remyelination.Citation119 Human MSC were delivered in a hydrogel PEG/carbomer/agarose with RGD peptide and ECM matrix deposits secreted by MSC. These MSC delivered paracrine factors. The cells survived and proliferated in vivo inside these gels for 9 days. During that time, they modulated the immune response as evidenced by the increased M2 macrophage presence.Citation120 A rat model with acute SCI was treated with scaffolds of PEG fumarate and Schwann cells to successfully trigger axonal regeneration.Citation121 They observed that animals with implanted scaffolds, with or without cells, had similar levels of collagen scarring and cyst formation, and accumulation of CSPG after 8 weeks. However, when animals with scaffold were compared to animals with injury only, these factors were significantly reduced.
Conclusion
Despite having a simple structure, PEG has a flexibility in processing, which has made it useful in a number of applications for SCI. As we deepen our understanding of cell–material interface and the SCI lesion biology, we expect to see further utilization of PEG in treatment development. Combination treatments that use PEG as both a fusogen and a structural component like a hydrogel matrix will likely be developed. Adoption of high-throughput methods to systematically optimize PEG-based and other experimental treatments will further expedite the transition of PEG from experimental to clinical treatments for SCI. This transition toward greater use of PEG in the clinic is already occurring in other tissues.
Acknowledgments
The author would like to acknowledge financial support from the following sources: Mission Connect, a TIRR program (014-120), The Staman Ogilvie Fund, William Stamps Farish Fund, Bentsen Stroke Center, and Vivian L. Smith Department of Neurosurgery.
Disclosure
The authors report no conflicts of interest in this work.
References
- CenterNSFacts and Figures at A GlanceBirmingham, ALUniversity of Alabama at Birmingham2016
- FrenchDDCampbellRRSabharwalSNelsonALPalaciosPAGavin-DreschnackDHealth care costs for patients with chronic spinal cord injury in the Veterans Health AdministrationJ Spinal Cord Med200730547748118092564
- SilverJMillerJHRegeneration beyond the glial scarNat Rev Neurosci20045214615614735117
- BibleEDell’AcquaFSolankyBNon-invasive imaging of transplanted human neural stem cells and ECM scaffold remodeling in the stroke-damaged rat brain by (19)F- and diffusion-MRIBiomaterials201233102858287122244696
- RitfeldGJRauckBMNovosatTLThe effect of a polyurethane-based reverse thermal gel on bone marrow stromal cell transplant survival and spinal cord repairBiomaterials20143561924193124331711
- ZiembaAMGilbertRJBiomaterials for local, controlled drug delivery to the injured spinal cordFront Pharmacol2017824528539887
- LuoJBorgensRShiRPolyethylene glycol improves function and reduces oxidative stress in synaptosomal preparations following spinal cord injuryJ Neurotrauma2004218994100715318999
- WilemsTSLuXKurosuYEKhanZLimHJSmith CallahanLAEffects of free radical initiators on polyethylene glycol dimethacrylate hydrogel properties and biocompatibilityJ Biomed Mater Res A2017105113059306828744952
- PeraleGRossiFSundstromEHydrogels in spinal cord injury repair strategiesACS Chem Neurosci20112733634522816020
- BoomerJAQuallsMMInerowiczHDCytoplasmic delivery of liposomal contents mediated by an acid-labile cholesterol-vinyl ether-PEG conjugateBioconjug Chem2009201475919072698
- KangCETatorCHShoichetMSPoly(ethylene glycol) modification enhances penetration of fibroblast growth factor 2 to injured spinal cord tissue from an intrathecal delivery systemJ Control Release20101441253120114065
- ZustiakSPLeachJBHydrolytically degradable poly(ethylene glycol) hydrogel scaffolds with tunable degradation and mechanical propertiesBiomacromolecules20101151348135720355705
- SoderquistRGMilliganEDSloaneEMPEGylation of brain-derived neurotrophic factor for preserved biological activity and enhanced spinal cord distributionJ Biomed Mater Res A200991371972919048635
- ZhengJSmith CallahanLAHaoJStrain-promoted cross-linking of PEG-based hydrogels via copper-free cycloadditionACS Macro Lett2012181071107323205321
- TurecekPLBossardMJSchoetensFIvensIAPEGylation of biopharmaceuticals: a review of chemistry and nonclinical safety information of approved drugsJ Pharm Sci2016105246047526869412
- RobertsMJBentleyMDHarrisJMChemistry for peptide and protein PEGylationAdv Drug Deliv Rev201264116127
- GeckilHXuFZhangXMoonSDemirciUEngineering hydrogels as extracellular matrix mimicsNanomedicine (Lond)20105346948420394538
- LinCCAnsethKSPEG hydrogels for the controlled release of bio-molecules in regenerative medicinePharm Res200926363164319089601
- MahoneyMJAnsethKSThree-dimensional growth and function of neural tissue in degradable polyethylene glycol hydrogelsBiomaterials200627102265227416318872
- Smith CallahanLAPolicastroGMBernardSLChildersEPBoettcherRBeckerMLInfluence of discrete and continuous culture conditions on human mesenchymal stem cell lineage choice in RGD concentration gradient hydrogelsBiomacromolecules20131493047305423844746
- WilliamsCGMalikANKimTKMansonPNElisseeffJHVariable cytocompatibility of six cell lines with photoinitiators used for polymerizing hydrogels and cell encapsulationBiomaterials200526111211121815475050
- LinSSangajNRazafiarisonTZhangCVargheseSInfluence of physical properties of biomaterials on cellular behaviorPharm Res20112861422143021331474
- KimJKongYPNiedzielskiSMSinghRKPutnamAJShikanovACharacterization of the crosslinking kinetics of multi-arm poly(ethylene glycol) hydrogels formed via Michael-type additionSoft matter20161272076208526750719
- TanHMarraKGInjectable, biodegradable hydrogels for tissue engineering applicationsMaterials20103317461767
- SongARaneAAChristmanKLAntibacterial and cell-adhesive polypeptide and poly(ethylene glycol) hydrogel as a potential scaffold for wound healingActa Biomaterialia201281415022023748
- HutanuDFrishbergMDGuoLDarieCCRecent applications of polyethylene glycols (PEGs) and PEG derivativesMod Chem Appl2014221000132
- SpencerKCSyJCRamadiKBGraybielAMLangerRCimaMJCharacterization of mechanically matched hydrogel coatings to improve the biocompatibility of neural implantsSci Rep2017711952
- BradburyEJCarterLMManipulating the glial scar: chondroitinase ABC as a therapy for spinal cord injuryBrain Res Bull201184430631620620201
- Nazari-RobatiMKhajehKAminianMMollaniaNGolestaniAEnhancement of thermal stability of chondroitinase ABC I by site-directed mutagenesis: an insight from Ramachandran plotBiochim Biophys Acta20131834247948623159774
- ShahaboddinMEKhajehKMalekiMGolestaniAImprovement of activity and stability of Chondroitinase ABC I by introducing an aromatic cluster at the surface of proteinEnzyme Microb Technol2017105384428756859
- BrownJMXiaJZhuangBA sulfated carbohydrate epitope inhibits axon regeneration after injuryProc Natl Acad Sci USA2012109134768477322411830
- StephenDSThe biology of neurotrophins, signalling pathways, and functional peptide mimetics of neurotrophins and their receptorsCNS Neurol Disord Drug Targets200871466218289031
- O’LearyPDHughesRADesign of potent peptide mimetics of brain-derived neurotrophic factorJ Biol Chem200327828257382574412730196
- LangBTCreggJMDePaulMAModulation of the proteoglycan receptor PTPsigma promotes recovery after spinal cord injuryNature2015518753940440825470046
- MaoYTonkinRSNguyenTSystemic administration of connexin43 mimetic peptide improves functional recovery after traumatic spinal cord injury in adult ratsJ Neurotrauma201734370771927629792
- OhtakeYParkDAbdul-MuneerPMThe effect of systemic PTEN antagonist peptides on axon growth and functional recovery after spinal cord injuryBiomaterials201435164610462624630093
- IwasakiMWilcoxJTNishimuraYSynergistic effects of self-assembling peptide and neural stem/progenitor cells to promote tissue repair and forelimb functional recovery in cervical spinal cord injuryBiomaterials20143592617262924406216
- ZweckbergerKAhujaCSLiuYWangJFehlingsMGSelf-assembling peptides optimize the post-traumatic milieu and synergistically enhance the effects of neural stem cell therapy after cervical spinal cord injuryActa Biomater201642778927296842
- ZustiakSPDurbalRLeachJBInfluence of cell-adhesive peptide ligands on poly(ethylene glycol) hydrogel physical, mechanical and transport propertiesActa Biomater2010693404341420385260
- NaghdiPTiraihiTGanjiFDarabiSTaheriTKazemiHSurvival, proliferation and differentiation enhancement of neural stem cells cultured in three-dimensional polyethylene glycol-RGD hydrogel with tenascinJ Tissue Eng Regen Med201610319920825312025
- TashiroKSephelGCWeeksBA synthetic peptide containing the IKVAV sequence from the A chain of laminin mediates cell attachment, migration, and neurite outgrowthJ Biol Chem19892642716174161822777785
- SilvaGACzeislerCNieceKLSelective differentiation of neural progenitor cells by high-epitope density nanofibersScience200430356621352135514739465
- LiuLi XJoseyXBShort laminin peptide for improved neural stem cell growthStem Cells Transl Med20143566267024692587
- AdamsDNKaoEYHypoliteCLDistefanoMDHuWSLetourneauPCGrowth cones turn and migrate up an immobilized gradient of the laminin IKVAV peptideJ Neurobiol200562113414715452851
- FawcettJWThe extracellular matrix in plasticity and regeneration after CNS injury and neurodegenerative diseaseProg Brain Res201521821322625890139
- Smith CallahanLAThe concentration game: differential effects of bioactive signaling in 2D and 3D cultureNeural Regen Res2016111666826981082
- LimHJMosleyMCKurosuYSmith CallahanLAConcentration dependent survival and neural differentiation of murine embryonic stem cells cultured on polyethylene glycol dimethacrylate hydrogels possessing a continuous concentration gradient of n-cadherin derived peptide His-Ala-Val-Asp-LleActa Biomater20175615316027915022
- LimHJKhanZWilemsTSHuman induced pluripotent stem cell derived neural stem cell survival and neural differentiation on polyethylene glycol dimethacrylate hydrogels containing a continuous concentration gradient of N-Cadherin derived peptide His-Ala-Val-Asp-IleACS Biomater Sci Eng201735776781
- YangYHKhanZMaCLimHJSmith CallahanLAOptimization of adhesive conditions for neural differentiation of murine embryonic stem cells using hydrogels functionalized with continuous Ile-Lys-Val-Ala-Val concentration gradientsActa Biomater201521556225931018
- MosleyMCLimHJChenJNeurite extension and neuronal differentiation of human induced pluripotent stem cell derived neural stem cells on polyethylene glycol hydrogels containing a continuous Young’s Modulus gradientJ Biomed Mater Res A2017105382483327798956
- OzawaHMatsumotoTOhashiTSatoMKokubunSComparison of spinal cord gray matter and white matter softness: measurement by pipette aspiration methodJ Neurosurg2001952 Suppl22122411599840
- RussellLNLampeKJOligodendrocyte precursor cell viability, proliferation, and morphology is dependent on mesh size and storage modulus in 3D Poly(ethylene glycol)-based hydrogelsACS Biomater Sci Eng201731234593468
- YanYLiYSongLZengCPluripotent stem cell expansion and neural differentiation in 3-D scaffolds of tunable Poisson’s ratioActa Biomater20174919220327845272
- RammenseeSKangMSGeorgiouKKumarSSchafferDVDynamics of mechanosensitive neural stem cell differentiationStem Cells201735249750627573749
- LourencoTPaes de FariaJBippesCAModulation of oligodendrocyte differentiation and maturation by combined biochemical and mechanical cuesSci Rep201662156326879561
- RussellLNLampeKJEngineering biomaterials to influence oligodendroglial growth, maturation, and myelin productionCells Tissues Organs20162021–28510127701172
- HappelRDSmithKPNaren BanikLJames PowersMHoganELDouglas BalentineJCa2+-accumulation in experimental spinal cord traumaBrain Research198121124764797237138
- HuffTBShiYSunWWuWShiRChengJXReal-time CARS imaging reveals a calpain-dependent pathway for paranodal myelin retraction during high-frequency stimulationPLoS One201163e1717621390223
- NehrtAHamannKOuyangHShiRPolyethylene glycol enhances axolemmal resealing following transection in cultured cells and in ex vivo spinal cordJ Neurotrauma201027115116119691421
- WangAZhangGXiaochenWZhangCTaoSHuoXCombination of applied electric field and polyethylene glycol effectively enhance functional recovery in acute spinal cord injury of ratsPaper presented at: 2016 Asia-Pacific International Symposium on Electromagnetic Compatibility (APEMC)17–21 May 20162016
- Carballo-MolinaOAVelascoIHydrogels as scaffolds and delivery systems to enhance axonal regeneration after injuriesFront Cell Neurosci2015913
- CensiRDi MartinoPVermondenTHenninkWEHydrogels for protein delivery in tissue engineeringJ Control Release2012161268069222421425
- TrimailleTPerticiVGigmesDRecent advances in synthetic polymer based hydrogels for spinal cord repairComptes Rendus Chimie2016191157166
- LuoJBorgensRShiRPolyethylene glycol immediately repairs neuronal membranes and inhibits free radical production after acute spinal cord injuryJ Neurochem200283247148012423257
- ShiRPolyethylene glycol repairs membrane damage and enhances functional recovery: a tissue engineering approach to spinal cord injuryNeurosci Bull201329446046623893430
- RenSLiuZ-HWuQPolyethylene glycol-induced motor recovery after total spinal transection in ratsCNS Neurosci Ther201723868068528612398
- LiuZRenSFuKRestoration of motor function after operative reconstruction of the acutely transected spinal cord in the canine modelSurgery2018163597698329223327
- AndersonMABurdaJERenYAstrocyte scar formation aids central nervous system axon regenerationNature2016532759819520027027288
- KimCYPEG-assisted reconstruction of the cervical spinal cord in rats: effects on motor conduction at 1hSpinal Cord20165491091227215738
- EstradaVBrazdaNSchmitzCLong-lasting significant functional improvement in chronic severe spinal cord injury following scar resection and polyethylene glycol implantationNeurobiol Dis20146716517924713436
- MacayaDSpectorMInjectable hydrogel materials for spinal cord regeneration: a reviewBiomed Mater201271012001
- OdaYTaniKIsozakiAEffects of polyethylene glycol administration and bone marrow stromal cell transplantation therapy in spinal cord injury miceJ Vet Med Sci201476341542124270802
- HuangZFilipovicZMpNAC105 Increases extracellular magnesium delivery and reduces excitotoxic glutamate exposure within injured spinal cords in ratsJ Neurotrauma201634368569427503053
- AgnesEHInesM-LMartinOBiomaterials for revascularization and immunomodulation after spinal cord injuryBiomed Mater201813404410529359704
- JainAKimYTMcKeonRJBellamkondaRVIn situ gelling hydrogels for conformal repair of spinal cord defects, and local delivery of BDNF after spinal cord injuryBiomaterials200627349750416099038
- ComolliNNeuhuberBFischerILowmanAIn vitro analysis of PNIPAAm-PEG, a novel, injectable scaffold for spinal cord repairActa Biomater2009541046105519054721
- CaiLDewiREHeilshornSCInjectable hydrogels with in situ double network formation enhance retention of transplanted stem cellsAdv Funct Mater20152591344135126273242
- TylerJYXuXMChengJXNanomedicine for treating spinal cord injuryNanoscale20135198821883623945984
- KimYTCaldwellJMBellamkondaRVNanoparticle-mediated local delivery of methylprednisolone after spinal cord injuryBiomaterials200930132582259019185913
- KangCEBaumannMDTatorCHShoichetMSLocalized and sustained delivery of fibroblast growth factor-2 from a nanoparticle-hydrogel composite for treatment of spinal cord injuryCells Tissues Organs20131971556322796886
- HottaRChengLGrahamHKDelivery of enteric neural progenitors with 5-HT4 agonist-loaded nanoparticles and thermosensitive hydrogel enhances cell proliferation and differentiation following transplantation in vivoBiomaterials20168811126922325
- VeroneseFMMeroAThe impact of PEGylation on biological therapiesBioDrugs200822531532918778113
- GefenTVayaJKhatibSThe impact of PEGylation on protein immunogenicityInt Immunopharmacol201315225425923306102
- YanKu SWangFSunYYangYYeNLThe blood-brain barrier penetration and distribution of PEGylated fluorescein-doped magnetic silica nanoparticles in rat brainBiochem Biophys Res Commun2010394487187620206605
- RichterAWAkerblomEAntibodies against polyethylene glycol produced in animals by immunization with monomethoxy polyethylene glycol modified proteinsInt Arch Allergy Appl Immunol19837021241316401699
- WunderlichDAMacdougallMMierzDVGeneration and characterization of a monoclonal IgG antibody to polyethylene glycolHybridoma (Larchmt)200726316817217600499
- ShimizuTIchiharaMYoshiokaYIshidaTNakagawaSKiwadaHIntravenous administration of polyethylene glycol-coated (PEGylated) proteins and PEGylated adenovirus elicits an anti-PEG immunoglobulin M responseBiol Pharm Bull20123581336134222863934
- SwartzlanderMDBarnesCABlakneyAKKaarJLKyriakidesTRBryantSJLinking the foreign body response and protein adsorption to PEG-based hydrogels using proteomicsBiomaterials201541263625522962
- LynnADBlakneyAKKyriakidesTRBryantSJTemporal progression of the host response to implanted poly(ethylene glycol)-based hydrogelsJ Biomed Mater Res A201196462163121268236
- GarayRPEl-GewelyRArmstrongJKGarrattyGRichettePAntibodies against polyethylene glycol in healthy subjects and in patients treated with PEG-conjugated agentsExpert Opin Drug Deliv20129111319132322931049
- MimaYHashimotoYShimizuTKiwadaHIshidaTAnti-PEG IgM Is a major contributor to the accelerated blood clearance of polyethylene glycol-conjugated proteinMolecular Pharmaceutics20151272429243526070445
- YangQLaiSKAnti-PEG immunity: emergence, characteristics, and unaddressed questionsWiley Interdiscip Rev Nanomed Nanobiotechnol20157565567725707913
- IshidaTWangXShimizuTNawataKKiwadaHPEGylated liposomes elicit an anti-PEG IgM response in a T cell-independent mannerJ Control Release2007122334935517610982
- ShimizuTMimaYHashimotoYAnti-PEG IgM and complement system are required for the association of second doses of PEGylated liposomes with splenic marginal zone B cellsImmunobiology2015220101151116026095176
- VosQLeesAWuZQSnapperCMMondJJB-cell activation by T-cell-independent type 2 antigens as an integral part of the humoral immune response to pathogenic microorganismsImmunological Reviews20031761154170
- VerhoefJJCarpenterJFAnchordoquyTJSchellekensHPotential induction of anti-PEG antibodies and complement activation toward PEGylated therapeuticsDrug Discov Today201419121945195225205349
- PapastefanakiFJakovcevskiIPouliaNIntraspinal delivery of polyethylene glycol-coated gold nanoparticles promotes functional recovery after spinal cord injuryMol Ther2015236993100225807288
- PapaSFerrariRDe PaolaMPolymeric nanoparticle system to target activated microglia/macrophages in spinal cord injuryJ Control Release2014174152624225226
- JenkinsSIWeinbergDal-ShakliAF“Stealth” nanoparticles evade neural immune cells but also evade major brain cell populations: implications for PEG-based neurotherapeuticsJ Control Release201622413614526780172
- DengLi JYuanJJZonisamide-loaded triblock copolymer nanomicelles as a novel drug delivery system for the treatment of acute spinal cord injuryInt J Nanomedicine2017122443245628408816
- JiangWu JBiHQApamin-mediated actively targeted drug delivery for treatment of spinal cord injury: more than just a conceptMol Pharm20141193210322225098949
- LiJMooneyDJDesigning hydrogels for controlled drug deliveryNat Rev Mater20161121607129657852
- LeeKSilvaEAMooneyDJGrowth factor delivery-based tissue engineering: general approaches and a review of recent developmentsJ R Soc Interface201185515317020719768
- BurdickJAWardMLiangEYoungMJLangerRStimulation of neurite outgrowth by neurotrophins delivered from degradable hydrogelsBiomaterials200627345245916115674
- KongLAskesSHCBonnetSKrosACampbellFTemporal control of membrane fusion through photolabile PEGylation of liposome membranesAngew Chem Int Ed Engl20165541396140026661729
- LampeKJKernDSMahoneyMJBjugstadKBThe administration of BDNF and GDNF to the brain via PLGA microparticles patterned within a degradable PEG-based hydrogel: protein distribution and the glial responseJ Biomed Mater Res A201196A3595607
- LynamDAShahriariDWolfKJBrain derived neurotrophic factor release from layer-by-layer coated agarose nerve guidance scaffoldsActa Biomater20151812813125712385
- Elliott DonaghueIShoichetMSControlled release of bioactive PDGF-AA from a hydrogel/nanoparticle compositeActa Biomater201525354226257128
- GarbayoEAnsorenaELanciegoJLAymerichMSBlanco-PrietoMJSustained release of bioactive glycosylated glial cell-line derived neurotrophic factor from biodegradable polymeric microspheresEur J Pharm Biopharm200869384485118417331
- StanwickJCBaumannMDShoichetMSEnhanced neurotrophin-3 bioactivity and release from a nanoparticle-loaded composite hydrogelJ Control Release2012160366667522510446
- HammerNBrandlFPKirchhofSMessmannVGoepferichAMProtein compatibility of selected cross-linking reactions for hydrogelsMacromol Biosci201515340541325399803
- van de WeteringPMettersATSchoenmakersRGHubbellJAPoly(ethylene glycol) hydrogels formed by conjugate addition with controllable swelling, degradation, and release of pharmaceutically active proteinsJ Control Release2005102361962715681084
- LinC-CMettersATEnhanced Protein Delivery from Photopolymerized Hydrogels Using a Pseudospecific Metal Chelating LigandPharm Res200623361416397740
- CensiRVermondenTvan SteenbergenMJPhotopolymerized thermosensitive hydrogels for tailorable diffusion-controlled protein deliveryJ Control Release2009140323023619527757
- LangerRTirrellDADesigning materials for biology and medicineNature2004428698248749215057821
- HongLTAKimYMParkHHAn injectable hydrogel enhances tissue repair after spinal cord injury by promoting extracellular matrix remodelingNat Commun20178153328912446
- HejclALesnyPPradnyMBiocompatible hydrogels in spinal cord injury repairPhysiol Res200857Suppl 3S12113218481908
- LiuCHuangYPangMTissue-engineered regeneration of completely transected spinal cord using induced neural stem cells and gelatin-electrospun poly (Lactide-Co-Glycolide)/Polyethylene glycol scaffoldsPLoS One2015103e011770925803031
- ChenBKMadiganNNHakimJSGDNF Schwann cells in hydrogel scaffolds promote regional axon regeneration, remyelination and functional improvement after spinal cord transection in ratsJ Tissue Eng Regen Med2018121e398e40728296347
- CaronIRossiFPapaSA new three dimensional biomimetic hydrogel to deliver factors secreted by human mesenchymal stem cells in spinal cord injuryBiomaterials20167513514726497428
- HakimJSEsmaeili RadMGrahnPJPositively charged oligo[Poly(Ethylene Glycol) Fumarate] scaffold implantation results in a permissive lesion environment after spinal cord injury in ratTissue Engineering Part A20152113–142099211425891264
