Abstract
Purpose
To compare the efficacy and safety of transarterial chemoembolization (TACE) plus donafenib with immune checkpoint inhibitors (ICIs) (T+D+I) versus TACE plus donafenib (T+D) as the first-line treatment for patients with unresectable hepatocellular carcinoma (HCC).
Methods
This retrospective study included patients with unresectable HCC who received T+D+I or T+D between June 2021 and February 2023. The tumor response was analyzed according to the modified Response Evaluation Criteria in Solid Tumors. The objective response rate (ORR), disease control rate (DCR), progression-free survival (PFS), overall survival (OS), and treatment-related adverse events (TRAEs) in the two groups were compared before and after propensity score matching (PSM). Cox’s proportional-hazards regression model was used to analyze factors affecting PFS and OS.
Results
This study included 69 patients: 41 patients in the T+D group and 28 patients in the T+D+I group. After PSM, 26 patients in each group were analyzed. Patients in the T+D+I group had a higher DCR (96.2% vs 73.1%, P = 0.021), longer median PFS (13.1 vs 7.2 months, P = 0.017), and longer median OS (23.1 vs 14.7 months, P = 0.021) than those in the T+D group. The ORR in the two groups was similar (53.8% vs 50.0%, P = 0.781). Multivariate analyses revealed that T+D+I treatment and total bilirubin levels of <20 μmol/L were independent prognostic factors for long PFS. T+D+I treatment, Child–Pugh class A, and single-lobe tumor distribution were independent prognostic factors for long OS. The incidence of TRAEs in the two groups was similar (P > 0.05).
Conclusion
In comparison with TACE plus donafenib, TACE plus donafenib with ICIs could significantly improve DCR, PFS, and OS as a potential first-line treatment for unresectable HCC with an acceptable safety profile.
Introduction
Primary liver cancer is the sixth most common cancer and the third most common cause of cancer-related death worldwide.Citation1 China accounts for more than 50% of new liver cancer cases and deaths caused by liver cancer globally.Citation1,Citation2 Hepatocellular carcinoma (HCC) represents 75–85% of cases of liver cancer.Citation3
Molecular targeted therapies and immunotherapies are the standard treatments for advanced HCC in first-line settings.Citation4 Sorafenib was the first molecular targeted agent approved for the treatment of unresectable HCC and remains the standard first-line therapy.Citation3,Citation5 Donafenib is a modified form of sorafenib with enhanced molecular stability and improved pharmacokinetics.Citation5 Donafenib is a novel oral small-molecule multikinase inhibitor that inhibits multiple-receptor tyrosine kinases, such as vascular endothelial growth factor (VEGF) receptor and platelet-derived growth factor (PDGF) receptor, and various Raf kinases and thereby suppresses tumor cell proliferation and angiogenesis.Citation5 Donafenib is a first-line treatment that was approved for unresectable or metastatic HCC as a result of the ZGDH3 trial and can improve overall survival (OS) in comparison with sorafenib.Citation6
The IMbrave150 and ORIENT-32 studies demonstrated that combinations of antiangiogenic drugs and immune checkpoint inhibitors (ICIs) as first-line treatments for unresectable HCC provided a better survival benefit in terms of progression-free survival (PFS) and OS in comparison with sorafenib.Citation7,Citation8 ICIs in combination with antiangiogenic drugs alter the tumor endothelium to enhance drug penetration and immune cell infiltration, which has shown promising prospects.Citation9,Citation10
Transarterial chemoembolization (TACE) is recommended as a first-line treatment for patients with intermediate-stage HCC, while it is also widely used in unresectable HCC.Citation4,Citation11,Citation12 TACE can induce immunogenic death of tumor cells, which results in the initiation of immune responses by antigen presentation.Citation9,Citation13 However, TACE may also promote tumor angiogenesis by upregulating PDGF and VEGF to induce tumor metastasis/recurrence.Citation14 Therefore, we hypothesized that TACE plus donafenib with ICIs might improve treatment outcomes in patients with unresectable HCC. In this study, we compared the efficacy and safety of the TACE plus donafenib with ICIs (T+D+I) regimen with those of the TACE plus donafenib (T+D) regimen as the first-line treatment for unresectable HCC.
Materials and Methods
Patients’ Selection
This retrospective study was performed in compliance with the Declaration of Helsinki and was approved by the Ethics Committee of the Sichuan Cancer Hospital. The participants provided their written informed consent to participate in this study. The diagnosis of HCC was confirmed according to the guidelinesCitation3,Citation11 or histological confirmation between June 2021 and February 2023.
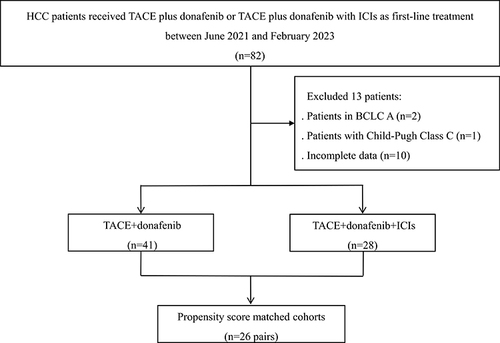
The inclusion criteria were: 1) patients received TACE plus donafenib or TACE plus donafenib with ICIs as the first-line treatment; 2) Eastern Cooperative Oncology Group performance status (ECOG PS) score of 0 or 1; 3) age of ≥18 years; 4) Child–Pugh class A or B; 5) Barcelona Clinic Liver Cancer (BCLC) stage B or C; and 6) patients had ≥1 measurable lesion. The exclusion criteria were: 1) patients with histories of HCC-related treatment such as surgery, ablation, TACE, radiotherapy, or systemic therapies; 2) ECOG PS score of >1 or Child–Pugh class C; 3) patients with coagulation disorders; 4) patients with other malignancies; and 5) incomplete data.
Data Collection
Patients’ baseline characteristics were recorded within 7 days before the first treatment. Clinical, laboratory, and radiological data were collected from medical record systems. These included age, gender, ECOG PS score, BCLC stage, hepatitis B surface antigen status, Child–Pugh class, alpha-fetoprotein level, tumor distribution, tumor size, tumor number, vascular invasion, extrahepatic metastasis, and hematological and biochemical indices.
Treatment
TACE Procedure
Hepatic arterial angiography was performed with a 5 Fr RH catheter to determine the location, number, size, and blood supply of the target tumors. Subsequently, a microcatheter was inserted into the feeding artery of the tumors.
Conventional TACE was performed by intra-arterial injection of 40–60 mg epirubicin (Pharmorubicin; Pfizer, Wuxi, China) mixed with 5–15 mL lipiodol (Jiangsu Hengrui Medicine Co., Ltd, Lianyungang, China). When needed, an embosphere (100–300 μm or 300–500 μm) was used for further embolization to achieve stasis.
Drug-eluting bead TACE was performed using CalliSpheres beads (Jiangsu Hengrui Medicine Co., Ltd, Lianyungang, China) (100–300 μm) loaded with doxorubicin (40–60 mg). CalliSpheres beads and a nonionic contrast agent were mixed in a ratio of 1:1 and injected at a speed of 1 mL/min. The injection was completed when contrast agent stasis was achieved.
TACE was repeated “on demand” according to the result of discussion in our multidisciplinary team (MDT), which depended on evidence of viable tumors or intrahepatic recurrence revealed by contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI).
Administration of Donafenib and ICIs
Oral donafenib (200 mg) was administered twice a day and was discontinued for 2 days before and after the TACE treatment session. Intravenous administration of 200 mg camrelizumab (Hengrui Medical, Lianyungang, China) or sintilimab (Innovent Biologics Co., Ltd, Suzhou, China) was conducted every 3 weeks. Under the guidance of the manufacturers’ instructions, the administration of donafenib and ICIs was reduced or discontinued according to the severity of toxic side effects.
Follow-Up
All patients were regularly followed up at intervals of 4–6 weeks after the first treatment. The results of the follow-up (CT or MR images and laboratory test results) were evaluated by our MDT to determine the status of tumor lesions (presence or absence of tumor progression). The last follow-up was carried out on May 31, 2023.
Evaluation of Treatment Response
Tumor responses were evaluated by two radiologists with more than 10 years’ experience using the modified Response Evaluation Criteria in Solid Tumors.Citation15 The objective response rate (ORR) and disease control rate (DCR) were assessed. Treatment-related adverse events (TRAEs) were assessed using the Common Terminology Criteria for Adverse Events (version 5.0).
PFS was defined as the time from the first day of inpatient treatment to disease progression or death from any cause, whichever occurred first. OS was defined as the time from the first day of inpatient treatment to the time of death or the follow-up deadline.
Statistical Analysis
Statistical analysis was carried out using SPSS 25.0 software (IBM, Armonk, NY, USA). To address potential imbalances in confounders between the two groups, propensity score matching (PSM) analysis was performed using the one-to-one nearest-neighbor method without replacement with a caliper width of 0.03. The propensity score model employed the following variables: age, sex, and Child–Pugh class. Before and after PSM, quantitative data were presented as the mean ± standard deviation, frequency, or median with a 95% confidence interval (CI). The continuity correction and independent-samples t-test, Mann–Whitney U-test, chi-squared test, and Fisher’s exact test were used to determine significant differences in categorical variables between the two groups. Survival curves for PFS and OS were analyzed by the Kaplan–Meier method using the Log rank test. Univariate and multivariate analyses used Cox’s proportional-hazards regression model to determine the prognostic factors. All statistically significant (P < 0.1) factors identified by the univariate analysis were entered into a Cox’s proportional-hazards regression model to identify independent predictors. A two-sided significance level of P < 0.05 was considered to be statistically significant.
Results
Patients’ Characteristics
A total of 69 patients with HCC at BCLC B or C stage were included in this study: 41 patients in the T+D group and 28 patients in the T+D+I group (). Following PSM, 52 patients in the two groups (n = 26 in each group) were analyzed (). In the T+D+I group, 16 patients received sintilimab and 12 patients received camrelizumab. The baseline characteristics of the two groups before and after PSM were similar (P > 0.05) (). The subsequent treatments, 4 patients received surgical resection and 1 patient received radiofrequency ablation in the T+D+I group; and 2 patients received surgical resection in the T+D group.
Table 1 Baseline Characteristics of Patients in the Two Groups Before and After PSM
Treatment Outcomes
Tumor Response Evaluation
In this study, no patient achieved a complete response. The DCR was higher in the T+D+I group than in the T+D group both before PSM (96.4% vs 75.6%, P = 0.020) and after PSM (96.2% vs 73.1%, P = 0.021) (). The ORR in the two groups was similar before PSM (53.6% vs 43.9%, P = 0.430) and after PSM (53.8% vs 50.0%, P = 0.781) ().
Table 2 Summary of Response Rates Before and After PSM
Survival Analysis
The median follow-up time in this study was 11.6 months (95% CI 9.3–14.0). During the follow-up, 41.5% (17/41) patients in the T+D group and 25.0% (7/28) patients in the T+D+I group died.
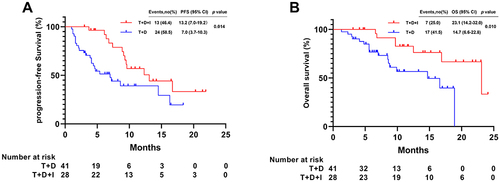
Before PSM, the median PFS in the T+D+I group (13.2 months [95% CI 7.0–19.2]) was longer than that in the T+D group (7.0 months [95% CI 3.7–10.3]; P = 0.014) (). The median OS was longer in the T+D+I group (23.1 months [95% CI 14.2–32.0]) than in the T+D group (14.7 months [95% CI 6.6–22.8]; P = 0.010) (). In T+D+I group, the median PFS (9.6 months [95% CI 3.2–16.0] vs 13.1 months [95% CI 7.7–18.5], P = 0.751) and OS (23.1 months vs not reached, P = 0.435) were similar for patients received camrelizumab and sintilimab, respectively.
Figure 2 Kaplan-Meier analyses of progression-free survival (A) and overall survival (B) according to two groups before PSM.
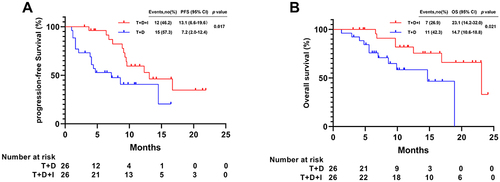
After PSM, the median PFS in the T+D+I group (13.1 months [95% CI 6.6–19.6]) was longer than that in the T+D group (7.2 months [95% CI 2.0–12.4]; P = 0.017) (). The median OS was longer in the T+D+I group (23.1 months [95% CI 14.2–32.0]) than in the T+D group (14.7 months [95% CI 10.6–18.8]; P = 0.021) ().
Figure 3 Kaplan-Meier analyses of progression-free survival (A) and overall survival (B) according to two groups after PSM.
Analysis of Prognostic Factors
The results of univariate and multivariate analyses of the matched cohorts were shown in . Cox’s proportional-hazards model showed that total bilirubin (≥20 vs <20 μmol/L) (hazard ratio [HR] = 2.73 [95% CI 1.20–6.20]; P = 0.017) and treatment option (T+D+I vs T+D) (HR = 0.32 [95% CI 0.14–0.74]; P = 0.008) were independent prognostic factors for PFS ().
Table 3 Univariate and Multivariate Predictors of Progression-Free Survival and Overall Survival After PSM
Multivariate analysis showed that tumor distribution (single lobe vs multiple lobes) (HR = 0.15 [95% CI 0.03–0.66]; P = 0.012), Child–Pugh class (B vs A) (HR = 4.95 [95% CI 1.25–19.63]; P = 0.023), and treatment option (T+D+I vs T+D) (HR = 0.32 [95% CI 0.11–0.93]; P = 0.037) were independent prognostic factors for OS ().
Safety
To assess differences in safety between the two groups before PSM, TRAEs were shown in . The incidence of TRAEs in the T+D+I group and the T+D group was similar (any grade: 89.3% vs 87.8%, P = 0.850). In the T+D+I group, 4 (14.3%) patients experienced hypothyroidism (grade 1/2), and 3 (10.7%) patients experienced reactive cutaneous capillary endothelial proliferation (grade 1/2); no patient experienced those symptoms in the T+D group (P < 0.05 for both events) (). No treatment-related mortality was observed, and no TRAE of higher than grade 4 occurred in the two groups. All TRAEs were relieved after symptomatic treatment or discontinuation of treatment.
Table 4 Treatment-Related Adverse Events
Discussion
This study showed that the T+D+I group had a higher DCR (96.2% vs 73.1%, P = 0.021) and a longer median PFS (13.1 vs 7.2 months, P = 0.017) than the T+D group. T+D+I treatment conferred a significant survival benefit in comparison with T+D treatment (median OS: 23.1 vs 14.7 months, P = 0.021). Furthermore, multivariate analyses showed that T+D+I treatment was an independent predictor for prolonged PFS and OS. Thus, our results showed that T+D+I was more effective than T+D as the first-line treatment for unresectable HCC.
In Phase II–III trial,Citation6 patients with HCC at BCLC B or C stage who received donafenib alone had a median PFS and OS of 3.7 and 12.1 months, respectively. These were shorter than the PFS and OS in the T+D group in this study (7.2 and 14.7 months, respectively). In the LAUNCH study,Citation16 patients with HCC at BCLC B or C stage who received TACE in combination with lenvatinib had a longer median PFS (10.6 vs 6.4 months, P < 0.001) and median OS (17.8 vs 11.5 months, P < 0.001) than those who received lenvatinib alone. Thus, TACE in combination with a molecular targeted therapy exhibited a synergistic effect.
In the CHANCE001 study,Citation17 patients with HCC at BCLC B or C stage who received TACE with programmed death (ligand)-1 (PD-[L]1) inhibitors plus molecular targeted therapies had a median PFS (9.5 vs 13.1 months) and a median OS (19.2 vs 23.1 months) that were shorter than those in the T+D+I group in this study. The reasons could be summarized as follows: 1) The CHANCE001 study was a multicenter study, and this study was a single study; 2) The CHANCE001 study used several molecular targeted therapies (tyrosine kinase inhibitors or anti-VEGF agents) (sorafenib, lenvatinib, donafenib, regorafenib, apatinib, anlotinib, and bevacizumab), and in this study patients only received donafenib; 3) The CHANCE001 study used several PD-(L)1 inhibitors (atezolizumab, pembrolizumab, nivolumab, camrelizumab, sintilimab, tislelizumab, and toripalimab), and this study used only two PD-1 inhibitors (camrelizumab and sintilimab); 4) In the CHANCE001 study, 32.5% of the patients had a history of HCC-related treatment, and no patient had a history of HCC-related treatment in this study.
The improved efficacy and outcomes among patients receiving T+D+I in this study may also have been due to synergistic effects of TACE plus donafenib with ICIs. Firstly, TACE leads to intrahepatic tumor necrosis, which elicits an anticancer immune response that may be further boosted with ICIs.Citation18,Citation19 TACE can also stimulate the cytokine spectrum and increase levels of CD4+ and CD8+ T cells among peripheral blood mononuclear cells in HCC patients while reducing the population of T reg cells.Citation20 Secondly, donafenib can inhibit PDGF receptor and VEGF receptor and also block the Raf/MEK/ERK signaling pathway, and it can thus achieve vascular normalization.Citation21 Thirdly, VEGF is a key regulatory factor in tumor angiogenesis that can directly influence immune cells and facilitate immune evasion and indirectly influence immunity by increasing vessel permeability.Citation22 Targeting VEGF can restore antitumor activity and enhance the efficacy of ICIs.Citation22
The incidence of TRAEs in the T+D+I group and the T+D group was similar (any grade: 89.3% vs 87.8%, P = 0.850). These TRAEs were manageable, and no fatal TRAE was found. The incidence rate of TRAEs was consistent with those reported in previous studies.Citation6,Citation23,Citation24 These results suggested that T+D+I treatment did not increase the risk of TRAEs with respect to T+D treatment.
There were several limitations in this study. Firstly, this study was a retrospective analysis and thus might have been subject to selection bias, and the PSM model was used to eliminate the effects of confounding factors. Secondly, no subgroup analysis was performed because of the small sample size. Thirdly, donafenib and the ICIs used in this study are recommended for HCC in Chinese guidelines. A randomized clinical trial is required to validate the findings from this study.
In conclusion, in comparison with TACE plus donafenib, TACE plus donafenib with ICIs showed significantly better DCR, PFS, and OS as a potential first-line treatment for unresectable HCC with an acceptable safety profile.
Disclosure
Guohui Xu and Xuegang Yang contributed equally to this work and share last authorship. The authors declare that there is no conflict of interest.
Additional information
Funding
References
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi:10.3322/caac.21338
- European Association for the Study of the Liver. Electronic address eee, European Association for the Study of the L. EASL Clinical Practice Guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236. doi:10.1016/j.jhep.2018.03.019
- Reig M, Forner A, Rimola J, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 2022;76(3):681–693. doi:10.1016/j.jhep.2021.11.018
- Li X, Qiu M, Wang S, Zhu H, Feng B, Zheng L. A Phase I dose-escalation, pharmacokinetics and food-effect study of oral donafenib in patients with advanced solid tumours. Cancer Chemother Pharmacol. 2020;85(3):593–604. doi:10.1007/s00280-020-04031-1
- Qin S, Bi F, Gu S, et al. Donafenib versus sorafenib in first-line treatment of unresectable or metastatic hepatocellular carcinoma: a randomized, open-label, parallel-controlled Phase II–III trial. J Clin Oncol. 2021;39(27):3002–3011. doi:10.1200/JCO.21.00163
- Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894–1905. doi:10.1056/NEJMoa1915745
- Ren Z, Xu J, Bai Y, et al. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): a randomised, open-label, Phase 2–3 study. Lancet Oncol. 2021;22(7):977–990. doi:10.1016/S1470-2045(21)00252-7
- Llovet JM, Castet F, Heikenwalder M, et al. Immunotherapies for hepatocellular carcinoma. Nat Rev Clin Oncol. 2022;19(3):151–172. doi:10.1038/s41571-021-00573-2
- Li SJ, Chen JX, Sun ZJ. Improving antitumor immunity using antiangiogenic agents: mechanistic insights, current progress, and clinical challenges. Cancer Commun. 2021;41(9):830–850. doi:10.1002/cac2.12183
- Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68(2):723–750. doi:10.1002/hep.29913
- Zhou J, Sun H, Wang Z, et al. Guidelines for the Diagnosis and Treatment of Hepatocellular Carcinoma (2019 Edition). Liver Cancer. 2020;9(6):682–720. doi:10.1159/000509424
- Pinato DJ, Murray SM, Forner A, et al. Trans-arterial chemoembolization as a loco-regional inducer of immunogenic cell death in hepatocellular carcinoma: implications for immunotherapy. J Immunother Cancer. 2021;9(9):e003311. doi:10.1136/2Fjitc-2021-003311
- Wang B, Xu H, Gao ZQ, Ning HF, Sun YQ, Cao GW. Increased expression of vascular endothelial growth factor in hepatocellular carcinoma after transcatheter arterial chemoembolization. Acta Radiol. 2008;49(5):523–529. doi:10.1080/02841850801958890
- Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30(1):52–60. doi:10.1055/s-0030-1247132
- Peng Z, Fan W, Zhu B, et al. Lenvatinib combined with transarterial chemoembolization as first-line treatment for advanced hepatocellular carcinoma: a Phase III, Randomized Clinical Trial (LAUNCH). J Clin Oncol. 2023;41(1):117–127. doi:10.1200/JCO.22.00392
- Zhu HD, Li HL, Huang MS, et al. Transarterial chemoembolization with PD-(L)1 inhibitors plus molecular targeted therapies for hepatocellular carcinoma (CHANCE001). Signal Transduct Target Ther. 2023;8(1):58. doi:10.1038/s41392-022-01235-0
- Cheu JW, Wong CC. Mechanistic rationales guiding combination hepatocellular carcinoma therapies involving immune checkpoint inhibitors. Hepatology. 2021;74(4):2264–2276. doi:10.1002/hep.31840
- Chang Y, Jeong SW, Young Jang J, Jae Kim Y. Recent updates of transarterial chemoembolization in hepatocellular carcinoma. Int J Mol Sci. 2020;21(21):8165. doi:10.3390/ijms21218165
- Liao J, Xiao J, Zhou Y, Liu Z, Wang C. Effect of transcatheter arterial chemoembolization on cellular immune function and regulatory T cells in patients with hepatocellular carcinoma. Mol Med Rep. 2015;12(4):6065–6071. doi:10.3892/mmr.2015.4171
- Keam SJ, Duggan S. Donafenib: first approval. Drugs. 2021;81(16):1915–1920. doi:10.1007/s40265-021-01603-0
- Pinter M, Jain RK, Duda DG. The current landscape of immune checkpoint blockade in hepatocellular carcinoma: a review. JAMA Oncol. 2021;7(1):113–123. doi:10.1001/jamaoncol.2020.3381
- Cao F, Yang Y, Si T, et al. The efficacy of TACE combined with lenvatinib plus sintilimab in unresectable hepatocellular carcinoma: a multicenter retrospective study. Front Oncol. 2021;11:783480. doi:10.3389/fonc.2021.783480
- Yang XG, Sun YY, Li DS, Xu GH, Huang XQ. Efficacy and safety of drug-eluting beads transarterial chemoembolization combining immune checkpoint inhibitors in unresectable intrahepatic cholangiocarcinoma: a propensity score matching analysis. Front Immunol. 2022;13:940009. doi:10.3389/fimmu.2022.940009