Abstract
Hepatocellular carcinoma (HCC) stands as the prevailing form of primary liver cancer, characterized by a poor prognosis and high mortality rate. A pivotal factor in HCC tumorigenesis is epigenetics, specifically the regulation of gene expression through methylation. This process relies significantly on the action of proteins that modify methylation, including methyltransferases, their associated binding proteins, and demethylases. These proteins are crucial regulators, orchestrating the methylation process by regulating enzymes and their corresponding binding proteins. This orchestration facilitates the reading, binding, detection, and catalysis of gene methylation sites. Methylation ences the development, prolisignificantly influferation, invasion, and prognosis of HCC. Furthermore, methylation modification and its regulatory mechanisms activate distinct biological characteristics in HCC cancer stem cells, such as inducing cancer-like differentiation of stem cells. They also influence the tumor microenvironment (TME) in HCC, modulate immune responses, affect chemotherapy resistance in HCC patients, and contribute to HCC progression through signaling pathway feedback. Given the essential role of methylation in genetic information, it holds promise as a potential tool for the early detection of HCC and as a target to improve drug resistance and promote apoptosis in HCC cells.
Introduction
Hepatocellular carcinoma (HCC), the predominant form of liver cancer, presents a significant global health challenge and is the fourth leading cause of cancer-related deaths. According to global cancer statistics, mortality from liver cancer accounts for 8.2% of all cancer-related deaths. The five-year survival rate for HCC and intrahepatic ductal carcinoma is 20.8%.Citation1–3 Recent advancements in HCC management guidelines have significantly improved monitoring for high-risk groups and optimized tumor staging, leading to innovative approaches for early detection and improved prognosis. Despite the absence of uniform standards, personalized multimodal treatment strategies are increasingly recommended.Citation3–5
Epigenetics involves heritable gene expression patterns that do not alter the DNA sequence.Citation6 The dysregulation of the epigenome becomes a key driver in the early development of HCC.Citation7 Methylation regulation is an essential component of epigenetics, which is related to embryonic formation. In cancer, it encompassing DNA methylation, RNA methylation, histone methylation, and their respective modifiers. Various enzymes and binding proteins are essential for maintaining different methylation states.Citation8–10 The combined use of databases with bisulfite detection and sequencing (BSP) for methylation studies is commonly used in experimental research, although some studies have also used techniques like MassARRAYEpiTYPER and Methylation-specific PCR (MSP).Citation11–13 Recent studies have underscored the potential of reversing epigenetic modifications through modifier inhibitors to restore normal cancer gene expression. This strategy offers promising therapeutic avenues to combat HCC.Citation14 However, research into epigenetic methylation modification and targeted therapeutic effects in HCC remains largely experimental, with no clinical trial applications reported.
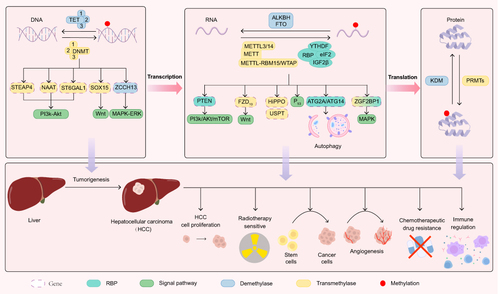
Aberrant methylation levels may occur in HCC in certain genes associated with the cell cycle, cancer progression as well as the immune environment.Citation15,Citation16 Targeting these genes induces cancer-specific “synthetic lethality”. Such aberrant methylation has proven beneficial for early cancer detection, particularly in HCC.Citation17 Non-invasive liquid biopsies have been employed to detect epigenetic variants carried by circulating tumor DNA, serving as biomarkers for early HCC detection. Another promising method, enzymatic methyl sequencing (EM-seq), has shown potential to identify early HCC lesions.Citation18 The experimental results have indicated that certain methylated genes may serve as predictive biomarkers for HCC. The types of methylation modifications of these biomarker genes and their roles in HCC are shown in . In brief, this comprehensive review aims to encapsulate recent advances in our understanding of epigenetic methylation modifications in HCC at DNA, RNA, and protein levels.
Table 1 Methylation-Related Biomarkers in HCC
The Role of DNA Associated Methylation in HCC
Regarding DNA methylation, contemporary research has primarily focused on CpG-rich promoters.Citation31 Additional investigations have examined DNA methylation of other genomic regions.Citation32 Methylation at the fifth position of cytosine (5mC) is connected with hepatitis B virus (HBV) infection and altered expression of DNA methylation related enzymes.Citation33 Another common methylation modification, DNA N6-adenine methylation (6mA), observed in human cells, has been linked to tumorigenesis when downregulated.Citation34 The methylation extent appears to correlate with tumor stage and malignancy.Citation35 Distinct genes exhibit methylation modifications across different HCC etiologies. For instance, hypomethylation of the F-box protein 43 promoter, glycine dehydrogenase (GLDC) promoter, and signal-transducing adaptor protein-1 (STAP1) promoter is linked to HBV-associated HCC (HBV-HCC).Citation13,Citation36,Citation37 In hepatitis C-associated HCC (HCV-HCC), demethylation of the transmembrane protein 164 (TMEM164) gene is closely connected.Citation38 In non-alcoholic steatohepatitis -associated (NASH-HCC), DNA hypermethylation of the zinc finger C3H1 domain-containing protein (ZFC3H1) gene’s CpG island influences the cancer’s multistage development process.Citation39
As the earliest epigenetic alteration, DNA methylation aberrations precede changes in gene expression and the occurrence of diseases, making it significant in the diagnosis and screening of early stage of HCC. Genes associated with the role of DNA methylation modification, such as RDH16,Citation11 DLC1Citation12 and STAP1,Citation13 etc, can be used as biomarkers for early detection of HCC. More detailed reflections of early HCC methylation marker genes and their mechanisms are shown in . Several studies have shown that combining Cell-free DNA (cfDNA) methylation-specific high-throughput or MSP testing with serology enhances the accuracy of serological assays of alpha-fetoprotein (AFP) alone during early screening for HCC.Citation40
In targeted therapy of HCC, DNA methyltransferases (DNMT) and DNA demethylases (ten-eleven translocation, TET) in methylation modification may play an important role. In cancer stem-like cells (CSCs), coordinated activation of DNMT3a and TET2 triggers and maintains drug resistance, which would be a therapeutic target for refractory HCC.Citation41
Promoter Region Methylation
Methylation at these CpG sites can be categorized into high, low, or demethylation states. Hypermethylation of DNA promoter regions can significantly influence the transcriptional activity of oncogenes or tumor suppressor genes (TSGs), thereby playing a crucial role in tumor recurrence, particularly in advanced tumor stages.Citation42 The promoter hypermethylation of the gene VIPR1 can suppress the proliferation and invasion of HCC cells through interactions with lncRNA-AC079061.1.Citation43 Additionally, Promoter hypermethylation affects HCC by modulating the PI3K/Akt or the PI3K/AKT/mTOR signaling pathway.Citation17,Citation44–46 It also downregulates the Wnt pathway as well as its target genes to suppress cell proliferation in HCC.Citation47 Promoter hypermethylation of the APC, REPK and UTRN2-1 promoters of oncogenes has been observed to elevate their expression, thereby inhibiting tumor growth, angiogenesis, and invasion, leading to extended relapse-free survival in patients.Citation30,Citation42,Citation48,Citation49 The hypermethylation status impacts the expression of tumor suppressors or cell proliferation-associated proteins, thereby reducing the development of HCC.Citation50,Citation51
Low promoter methylation levels enhance gene self expression.Citation16,Citation52 Hypomethylation of potential proto-oncogene histone deacetylase 11 (HDAC11) promoters may result in the activation and overexpression of this gen, which leads to the the development of cancer.Citation53 It has been shown that individuals with lower methylation levels in HCC exhibit shorter survival times and increased vascular involvement.Citation54 Promoter hypomethylation can positively influence stem cells, fostering the transformation of liver cancer stem cells into malignant cells, which is mainly regulated by DNA demethylases.Citation55 Hypomethylation of promoters facilitates tumor tissue proliferation, invasion, and migration and serves as an independent prognostic indicator for overall survival and disease progression in liver cancer.Citation30,Citation54,Citation56 The phenomenon of partial promoter hypomethylation plays a pivotal role in HCC by influencing cell cycle dynamics. For instance, Liu et al have shown that hypomethylation of the Cyclin-D1 (CCND1) promoter, specific to the G1/S phase, enhances the development of HBV-HCC. This discovery posits that it could be a potential diagnostic marker for patients with AFP negative HBV-HCC and AFP-positive chronic hepatitis B (CHB).Citation57 Besides, hypomethylation of the ZCCHC13 promoter in HCC tissues, leading to its overexpression, promotes cell cycle progression from the G1 phase to the S phase in HCC cells. This aberrant activation is linked with the ATK/ERK/c-MYC/CDK pathway.Citation58 Futhermore, demethylases have been detected to be overexpressed in HCC cells and liver cancer stem cells, which promote liver fibrosis and reduce radiosensitivity of HCC.Citation17,Citation55 These findings emphasize the catalytic evoke of promoter hypo- or demethylation on the differentiation of hepatocytes from cancer stem cells (CSCs), thus promoting cell cycle growth and shaping the immune microenvironment.
Promoter methylation is mainly known for its role in gene silencing,Citation59 although specific cases have shown it can increase gene expression.Citation48 Both gene silencing and increased gene expression contribute to the initiation and progression of HCC. The review also discusses the influence of DNA methylation in HCC on epithelial-mesenchymal transition (EMT), neoangiogenesis, and the tumor microenvironment (TME). DNMT, a key regulator of DNA methylation, influences the proliferation, migration, and invasion of HCC cells via EMT through its promoter methylation status.Citation60 Alternatively, DNMT may affect HCC progression by altering its transcriptional activity.Citation46 EMT is a key mechanism contributing to cancer progression, enhancing the metastatic capabilities of tumor cells through various pathways, including organ fibrosis.Citation61 Furthermore, some studies further affirming the impact of methylation on tumor-associated angiogenesis.Citation54 DNA methylation also exerts an influence on immune cells in the TME. Studies have revealed that the hypermethylation of the Natural Killer Group 2D (NKG2D) gene promoter in HCC can markedly affect the disease by modulating immune system responses, thereby establishing its potential as a diagnostic marker for HCC.Citation62 Notable correlations have been observed between lysyl oxidase-like protein 3 (LOXL3) and methyltransferases. Specifically, LOXL3 expression is positively correlates with the infiltration of diverse immune cells and the expression of immune checkpoint genes in HCC. High LOXL3 expression has been linked to adverse outcomes in HCC patients.Citation63 Down regulation of triggered by high methylation evoked by DNMT1, is associated with increased microvessel density (MVD) in HCC.Citation64 Dysregulated DNA methylation, observed in almost all types of cancer, has been associated with the infiltration characteristics of hepatocellular immune cells, the TME, and its potential impact on immunotherapy, as well as the degree of immune system activation and the prognosis of HCC prognosis.Citation65 Experimental evidences have demonstrated that promoter methylation modifications of target genes at distinct chromosomal sites may exert disparate effects on HCC ().
Table 2 Effect of DNA Promoter Methylation Modification of Diverse Genes on HCC
Figure 1 HCC methylation occurs in DNA, RNA, and protein. M6A modifications are regulated by a family of methyltransferases (writer), demethylases (eraser), and specific RNA binding proteins(reader). The methyltransferase family includes METTL family and WTAP. Demethylases include FTO, ALKBH5 meanwhile others, which directly remove m6A modifications from mRNAs. Specific RNA binding proteins consist of YTH domain proteins, the IGF binding protein 2 family, eukaryotic initiation factor 3, and nuclear heterogeneous protein2β1.
Methylation of Other Genomic Regions
In other cancer studies, hypermethylation in intron CpG-rich regions have been observed, potentially leading to oncogenic alterations in tumors due to changes in protein abundance.Citation83,Citation84 Intron demethylation status in tumour suppressor genes can either amplify or repress the gene, while also regulating the gene’s transcript. Histone methylation inhibits or initiates transcription when modifying these genes.Citation85 LncRNAs may act as transcripts in the intronic regions of genes, where high expression can impact HCC prognosis by mediating metabolic processes.Citation86 Additionally, the retention of introns (RI) is regulated by protein arginine methyltransferases (PRMTs),Citation87 and RNA methylation at exon-intron boundaries may impair the splicing of pre-mRNAs.Citation30 MIR elements in genes and enhancers, sensitive to changes in DNA methylation activity, are implicated in hematological cancers.Citation88 Currently, the DNA methylation of other regions of these genes in HCC remains an area for further study.
Role of RNA Associated Methylation in HCC
RNA methylation (RM), a dynamically reversible epigenetic modification during translation, involves RNA processing, transport, translation, and metabolism.Citation89,Citation90 RM mainly includes modifications such as N1-methyladenosine (m1A), 5-methylcytosine (m5C), N3-methylcytidine (m3C), N6-methyladenosine (m6A), and 2’-O-methylation (2′-O-M). These methylation modifications occur at specific positions within RNA base.Citation91 M6A, m5C, and m1A are associated with specific gene clusters and risk models in liver cancer.Citation92 The 2’-O- M of ribosomal RNA (rRNA) regulates autophagy and promotes malignant progression of cancer.Citation93 RNA methylation is mediated by RNA modifying proteins, including writers (methyltransferases) that catalyze methylation formation, readers (RNA binding proteins) that interpret methylation modification information, and erasers that detect RNA methylation modification. Expression levels of these proteins are significantly upregulated in HCC samples.Citation79,Citation94,Citation95
Methyltransferases regulate the interplay between the transcriptome and epitranscriptome during RNA methylation. They facilitate transcriptional dormancy through interconnected mechanisms relevant to the dormancy of adult stem cells and cancer.Citation96 Furthermore, RNAs can affect gene methylation or directly impact HCC by acting on the methylation level of the corresponding gene.Citation97–100 RNA methyltransferases (METTs) and RNA-binding proteins (RBPs) offer promising therapeutic targets in HCC. In m6A, METTL3 depends on the YTH domain family 2 (YTHDF2) of RBPs to regulate the proliferation, migration, and invasion of HCC cells. RBPs’ YTHDF1 binds NOTCH1 mRNA in the m6A-modified NOTCH pathway to drive HCC stemness and resistance.Citation41,Citation101 The METT-FTSJ3 induced 2′-O-M process inhibits immune escape and is a promising therapeutic approach.Citation102 All of these provide new avenues for HCC-targeted therapies.
M6A Modification
The most prevalent RNA methylation modification is M6A, predominantly occurring within the stop codon or 3’UTR of mammalian genes.Citation91 M6A involves the methylation of the sixth nitrogen atom of adenine This modification is notably prevalent in the mammalian transcriptome.Citation17 The dynamics of m6A methylation are regulated by various enzymes and RBPs in vivo (). The molecular regulatory networks M6A-modulated exhibit differentiable phenotypes in cancer patients. M6A-score models constructed via phenotypic clustering divergences revealed their predictive potential for HCC prognosis and immunotherapy responsiveness, potentially bolstering clinical decision-making or delivering superior prognoses.Citation103
Methyltransferases such as METTL3, ZC3H13, and RBPs YTHDF1 and YTHDF2 have been identified to be associated with immune checkpoints and are observed to be overexpressed in HCC.Citation104 While the expression levels of most m6A-related genes in HCC are significantly higher compared to adjacent non-cancerous tissues, exceptions include zinc finger CCCH type 13 (ZC3H13) and METTL14. The interplay of m6A modification with pathways such as P53, Wnt/β-Catenin, and PI3K/AKT/mTOR may collaboratively influence the tumor microenvironment and immune responses across different HCC clusters.Citation105–107
By affecting assorted RNA methylation and acting on multiple cytokine axes, m6A and related regulators promote growth, proliferation and invasion in HCC. In coding RNAs, m6A methylation modifications lead to reduced translational capacity, increased instability, and diminished expression of gene responsive proteins.Citation108 These alterations can affect the progression of HCC via diverse cell signaling pathways.Citation105 Among ncRNAs, m6A primarily targets lncRNAs, microRNAs (miRNAs), and circular RNAs (circRNAs). M6A related lncRNAs predicted the prognosis of HCC, in which LncRNA FAM111A-DT and LncRNA LEAWBIH could be used as potential therapeutic targets or diagnostic markers.Citation109 LncRNAs may affect HCC through the Wnt/β-catenin axis.Citation110,Citation111 CircRNAs primarily affect the growth, proliferation, and invasion of HCC,Citation112–114 with circMEMO1 and circRERE, etc, acting as a sponge, functioning with m6A modification, and forming an epigenetic model with positive feedback.Citation115 Analysis and validation of circRNA-miRNA networks regulated by m6A RNA methylation regulators have revealed that the CircMAP2K4/miR-139-5p/YTHDF1 axis is involved in the proliferation of HCC.Citation114
M5C Modification
M5C methylation in human RNA occurs through the transfer of methyl groups to cytosine by methyltransferases, using S-adenosylmethionine as a donor. This process is primarily catalyzed by members of the nucleolar protein family (NOL1, NOL2), the SUN family, and DNMT2 and is integral to RNA stability and functionality.Citation116 In HCC tissues, the prevalence of mRNA m5C is notably higher compared to the overall level in adjacent tissues. M5C is predominantly enriched downstream of the translation initiation site in the mRNA coding sequence (CDS). Additionally, genes in the Ras pathway, such as GRB2, MAPK3, and PIK3R, exhibit increased m5C in HCC tissues.Citation117 The expression level of m5C-modified lncRNA H19 is significantly higher in HCC tissues than in non-cancerous tissues. It may contribute to tumor development and progression by recruiting oncogenic proteins and is closely linked to HCC malignancy, making it a potential target or biomarker for HCC diagnosis and treatment.Citation29
M1A Modification
M1A, the methylation of the first nitrogen atom of adenosine in RNA, is a prevalent RNA modification that plays a significant role in tumorigenesis, with tRNA being the most modified class of RNA.Citation118 The formation of M1A is catalyzed by methyltransferases, with these “writers” specifically recognizing m1A sites as well as inducing downstream effects.Citation97 Genetic variations in m1A regulators may be linked to mutations in oncogenes, playing a role in the carcinogenesis or metabolic reprogramming of HCC.Citation90 While M1A regulates the PI3K/AKT/mTOR pathway in gastrointestinal cancers, its specific mechanisms in HCC remain to be fully elucidated.Citation119 In HCC patient tissues, m1A levels in tRNA are notably increased, and m1A methylation is elevated in CSCs. TRMT6/TRMT61A-mediated m1A methylation is essential for liver tumorigenesis, enhancing m1A methylation in tRNA and activating Hedgehog signaling, thereby driving self-renewal and tumorigenesis in liver CSCs.Citation120
Role of Protein Associated Methylation in HCC
Protein methylation comprises two main types: histone methylation and regulator associated methylation, which involve methyltransferases, demethylases, and their corresponding binding proteins. Histone methylation predominantly affects RNA coding and transcription by methylating amino acids at specific sites, thereby influencing the immunophenotype and aggressiveness of HCC. These modification patterns differentially predict TME infiltration, homologous recombination defects (HRD), intratumoral heterogeneity, proliferative activity, mRNA stemness index, and prognosis.Citation116
Multimodal Histone Methylation
Histone methylation on chromosome components mainly targets lysine or arginine residues in H3 or H4 histones, with S-adenosylmethionine as the donor molecule for methyltransferase activity.Citation121 Methylation of specific residues in histones, such as H3K4, H3K27, H3K36, H3K79, H4K20, H3K23, H3K63, and H4K12, plays an important role in RNA regulation.Citation122 Histone methylation primarily influences RNA coding and transcription through the methylation of amino acids at specific sites, thus regulating the immunophenotype and aggressiveness of HCC.Citation123
Histone methylation is diverse and can be classified as monomethylated, bimethylated and trimethylated. Reduced methylation in the H3-lysine monomethylation alteration leads to decreased expression levels of tumour suppressors encoded by the motifs.Citation26 Dimethylation and trimethylation of H3-lysine related to hypoxia-inducible factor 1α (HIF-1α) activation.Citation124 Trimethylation at the lysine site is frequently observed with histone H3 lysine 9 trimethylation (H3K9me3) and histone 3 trimethylation at the lysine 27 (H3K27me3) site. Trimethylation reprogramming that has been altered may lead to chromatin compression, gene silence, and transcriptional repression, all of which can encourage EMT and the spread of cancerous cells.Citation125–127
Most cancer associated pathway factors with phenotypes are primarily enhanced in the histone H4 hypermethylated group (H4M). H4M modification plays a key role in TME remodeling that is significantly associated with HCC immunophenotyping, while H4M regulators effect HCC epitranscriptome patterns and tumor microenvironment invasion characterization.Citation123,Citation128 Arginine methylation exhibits the capacity to undergo mono- and bimethylation alterations in response to the of PRMTs.
Role of Histone Methylation Modifiers
Investigations into the effects of histone methylation modulators on HCC have predominantly focused on methyltransferases in conjunction with demethylases. The malignant process of tumourigenesis is frequently accompanied by aberrant modifications of protein arginine methylation, in which PRMTs play a pivotal role. PRMTs comprise a category of methyltransferases that exhibit marked expression alterations in HCC tissues and cell lines, with their isoforms being associated with the sensitivity of specific anticancer drugs.Citation18 The PARMTs family demonstrates significant correlations with the inflammatory response, glucose metabolism, as well as tumor progression, and is required for the regulation of RNA processing factors, signalling mono- or asymmetric dimethylation.Citation129,Citation130 PRMTs impose post-transcriptional regulation on retained introns (RIs), whereby their inhibition results in altered splicing rates. By protein arginine methylation, PRMTs regulate the post-transcriptional processing of nuclear retention introns.Citation87 Additionally, PRMTs demonstrate a synergistic relationship with the poly ADP-ribose polymerase (PARP DNA repair enzyme), which is responsible for blocking the defective DNA replication induced in response to stress.Citation131 Type I-PRMTs regulate the intrinsic antiviral immune response by altering RNA splicing. And inhibition of type I-PRMT enhances antitumour immune properties in the refractory setting. Furthermore, arginine methylation further ameliorates oxidative stress by effecting serine levels in HCC patients, thus constituting a potential HCC therapeutic modality.Citation93
Histone methylation is also subject to remodeling by Lysine-specific demethylase (KDM, also named LSD). KDM, a protein capable of suppressing the expression of specific genes, regulates cancer progression and is used in anti-cancer therapy for various cancers, including HCC.Citation132 KDM can repress hepatic CSCs through demethylation in the promoter regions of stemness-associated transcription factors, further affecting stem cell differentiation direction stemness.Citation133
In summary, regulators of histone methylation primarily affect HCC resistance to chemotherapy drugs and cancer stem cell pluripotency through the modification of methyltransferases and demethylases. A number of studies have indicated that, during protein methylation modification arginine, methyltransferases PRMTs may be suitable as targets for the development of drugs to treat cancers by inhibiting epigenetic methylation. The concept has been validated in other cancer studies. In cellular experiments, inhibitors of type I-PRMTs were demonstrated to have anti-tumour effects in a melanoma xenograft model, indicating that the compounds warrant further investigation as a potential anti-cancer agent.Citation134 Moreover, targeting of type I-PRMT has the potential to enhance the efficacy of immunotherapy in patients with triple-negative breast cancer (TNBC).Citation135 Further studies are required to elucidate the specific role of PRMT in HCC and to identify effective targeted therapies.
Discussion
The occurrence of dynamic methylation overlap is observed throughout the development of HCC. The three methylation modes (DNA, RNA, and protein methylation) cross-talk with each other and are closely related to chromosome remodelling and gene expression, which likely plays a pivotal role in tumor development and transformation. Aberrant DNA methylation is a primary alteration that drives tumorigenesis, while RNA and protein methylation predominantly influence tumor progression through transcription and translation. All three methylation modifications can affect tumour progression. Epigenetic methylation modifications can act via pathways such as Wnt/beta-catenin, PI3K/Akt/mTOR and ATK/ERK/c-MYC/CDK. Furthermore, we have provided a summary of the target genes that are crucial for the methylation modification process, as well as the relationship between the target genes and the signalling pathways in .
In recent years, studies have shed light on the significant role of methylation in the HCC tumor microenvironment. Methylation has been implicated in HCC development and drug resistance, however, its precise effects on HCC remain elusive as well as require further investigation. Emerging evidences suggest a potential connection between methylation, ferroptosis, and autophagy.Citation136 Since modifications of the m6A RNA can regulate autophagy to modulate HCC growth or development.Citation137 Recent discovery that H4 forms doubly modified acetylmethyllysine, which has all the characteristics of post-translational modification. It has great significance for further study on the pathogenesis of cancer. Notably, Investigating the interference of methylation at the HCC locus in autophagy and exploring the potential of methylation inhibitors or inducers to overcome drug resistance in HCC are areas that warrant further exploration. However, given the significant role of methylation in genetic information, it holds promise as a potential tool for the early detection of HCC and may serve as a new target for improving drug resistance in HCC cells and promoting apoptosis. Exploring the possibility of incorporating methylation indicators into individualized testing parameters requires further investigation. Epigenetic liquid biopsies, which detect abnormal immune environments by examining methylation, fragmentation, and histone labeling patterns of decellularized DNA in blood,Citation138 offer a promising avenue for investigation. Moreover, several experimental studies have demonstrated that targeted inhibition of aberrant DNA methylation in HCC may affect tumorigenesis. Modifying methylation-associated enzymes and their binding proteins to target the transcription-translation axis presents a promising therapeutic strategy. In brief, the prospect of methylation in HCC research, particularly the feasibility of using agonists or inhibitors targeting enzymes and binding proteins involved in methylation processes to treat HCC patients, requires further investigation.
In conclusion, this review confirms that methylated genes have the potential to be biomarkers for the diagnosis and prognosis of HCC and demonstrates that different types of methylation modifications affect the cancer cell phenotype and the development of HCC through the regulation of signalling pathways by different enzymes and binding proteins. However, further research is needed in HCC targeted therapy in the future.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests in this work.
Additional information
Funding
References
- Bray F, Laversanne M, Sung H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74(3):229–263. doi:10.3322/caac.21834
- Devarbhavi H, Asrani SK, Arab JP, Nartey YA, Pose E, Kamath PS. Global burden of liver disease: 2023 update. J Hepatol. 2023;79(2):516–537. doi:10.1016/j.jhep.2023.03.017
- Cho Y, Kim BH, Park JW. Overview of Asian clinical practice guidelines for the management of hepatocellular carcinoma: an Asian perspective comparison. Clin Mol Hepatol. 2023;29(2):252–262. doi:10.3350/cmh.2023.0099
- Wen N, Cai Y, Li F, et al. The clinical management of hepatocellular carcinoma worldwide: a concise review and comparison of current guidelines: 2022 update. Biosci Trends. 2022;16(1):20–30. doi:10.5582/bst.2022.01061
- Xie D, Shi J, Zhou J, Fan J, Gao Q. Clinical practice guidelines and real-life practice in hepatocellular carcinoma: a Chinese perspective. Clin Mol Hepatol. 2023;29(2):206–216. doi:10.3350/cmh.2022.0402
- Jones PA, Baylin SB. The epigenomics of cancer. Cell. 2007;128(4):683–692. doi:10.1016/j.cell.2007.01.029
- Hlady RA, Robertson KD. Epigenetic memory of environmental exposures as a mediator of liver disease. Hepatology. 2023. doi:10.1097/HEP.0000000000000414
- Nepal C, Andersen JB. Alternative promoters in CpG depleted regions are prevalently associated with epigenetic misregulation of liver cancer transcriptomes. Nat Commun. 2023;14(1):2712. doi:10.1038/s41467-023-38272-4
- Gou D, Liu R, Shan X, et al. Gluconeogenic enzyme PCK1 supports S-adenosylmethionine biosynthesis and promotes H3K9me3 modification to suppress hepatocellular carcinoma progression. J Clin Invest. 2023;133(13). doi:10.1172/JCI161713
- Madkour MM, Ramadan WS, Saleh E, El-Awady R. Epigenetic modulations in cancer: predictive biomarkers and potential targets for overcoming the resistance to topoisomerase I inhibitors. Ann Med. 2023;55(1):2203946. doi:10.1080/07853890.2023.2203946
- Kim SC, Kim DW, Cho EJ, et al. A circulating cell-free DNA methylation signature for the detection of hepatocellular carcinoma. Mol Cancer. 2023;22(1):164. doi:10.1186/s12943-023-01872-1
- Pan J, Li D, Fan X, et al. Aberrant DNA Methylation Patterns of Deleted in Liver Cancer 1 Isoforms in Hepatocellular Carcinoma. DNA Cell Biol. 2023;42(3):140–150. doi:10.1089/dna.2022.0384
- Sun L, Lu J, Li K, et al. Diagnostic and prognostic value of STAP1 and AHNAK methylation in peripheral blood immune cells for HBV-related hepatopathy. Front Immunol. 2022;13:1091103. doi:10.3389/fimmu.2022.1091103
- Costa P, Sales SLA, Pinheiro DP, et al. Epigenetic reprogramming in cancer: from diagnosis to treatment. Front Cell Dev Biol. 2023;11:1116805. doi:10.3389/fcell.2023.1116805
- Zhi R, Hao P, Li W, Zhao H. Expression of CKS2 in Hepatocellular Carcinoma: correlation with Survival Outcomes and Immune Microenvironment. J Hepatocell Carcinoma. 2023;10:1767–1784. doi:10.2147/JHC.S427624
- Zhen J, Pan J, Zhou X, et al. FARSB serves as a novel hypomethylated and immune cell infiltration related prognostic biomarker in hepatocellular carcinoma. Aging. 2023;15(8):2937–2969. doi:10.18632/aging.204619
- Guo P, Zheng H, Li Y, et al. Hepatocellular carcinoma detection via targeted enzymatic methyl sequencing of plasma cell-free DNA. Clin Clin Epigenet. 2023;15(1):2. doi:10.1186/s13148-022-01420-6
- Gou D, Liu R, Shan X, et al. Gluconeogenic enzyme PCK1 supports S-adenosylmethionine biosynthesis and promotes H3K9me3 modification to suppress hepatocellular carcinoma progression. J Clin Invest. 2023;133(13).
- Wei H, Yang J, Chen X, et al. BAIAP2L2 is a novel prognostic biomarker related to migration and invasion of HCC and associated with cuprotosis. Sci Rep. 2023;13(1):8692. doi:10.1038/s41598-023-35420-0
- Li C, Jia Y, Li N, Zhou Q, Liu R, Wang Q. DNA methylation-mediated high expression of CCDC50 correlates with poor prognosis and hepatocellular carcinoma progression. Aging. 2023;15(15):7424–7439. doi:10.18632/aging.204899
- Saeki I, Suehiro Y, Yamauchi Y, et al. Methylated SEPT9 assay-based liquid biopsy as a biomarker in molecular targeted agent-treated hepatocellular carcinoma. Hepatol Int. 2023;17(5):1289–1299. doi:10.1007/s12072-023-10488-y
- Yan J, Zhang MY, Lin J, et al. WHSC1 is involved in DNA damage, cellular senescence and immune response in hepatocellular carcinoma progression. J Cell Mol Med. 2023;27(10):1436–1441. doi:10.1111/jcmm.17743
- Wen H, Ji T, Lin L, Cheng N, Zhu K, Cao L. High Expression of Ten Eleven Translocation 1 Is Associated with Poor Prognosis in Hepatocellular Carcinoma. Mediators Inflamm. 2023;2023:2664370. doi:10.1155/2023/2664370
- Zhang Q, Wei T, Yan L, et al. Hypoxia-Responsive lncRNA AC115619 Encodes a Micropeptide That Suppresses m6A Modifications and Hepatocellular Carcinoma Progression. Cancer Res. 2023;83(15):2496–2512. doi:10.1158/0008-5472.CAN-23-0337
- Kuang Y, Cheng Y, Wang J, Li H, Cao X, Wang Y. KIAA1429 mediates epithelial mesenchymal transition in sorafenib-resistant hepatocellular carcinoma through m6A methylation modification. Cancer Med. 2023;12(6):7222–7233. doi:10.1002/cam4.5432
- Liu R, Yin G, Tuo H, et al. METTL3-induced lncRNA GBAP1 promotes hepatocellular carcinoma progression by activating BMP/SMAD pathway. Biol Direct. 2023;18(1):53. doi:10.1186/s13062-023-00409-2
- Pu J, Xu Z, Huang Y, et al. N(6) -methyladenosine-modified FAM111A-DT promotes hepatocellular carcinoma growth via epigenetically activating FAM111A. Cancer Sci. 2023;114(9):3649–3665. doi:10.1111/cas.15886
- Wei H, Huang L, Lu Q, et al. N(6)-Methyladenosine-Modified LEAWBIH Drives Hepatocellular Carcinoma Progression through Epigenetically Activating Wnt/β-Catenin Signaling. J Hepatocell Carcinoma. 2023;10:1991–2007. doi:10.2147/JHC.S433070
- Sun Z, Xue S, Zhang M, et al. Aberrant NSUN2-mediated m(5)C modification of H19 lncRNA is associated with poor differentiation of hepatocellular carcinoma. Oncogene. 2020;39(45):6906–6919. doi:10.1038/s41388-020-01475-w
- Shi Y, Niu Y, Yuan Y, et al. PRMT3-mediated arginine methylation of IGF2BP1 promotes oxaliplatin resistance in liver cancer. Nat Commun. 2023;14(1):1932. doi:10.1038/s41467-023-37542-5
- Chen Y, Zhou P, Deng Y, et al. ALKBH5-mediated m(6) A demethylation of TIRAP mRNA promotes radiation-induced liver fibrosis and decreases radiosensitivity of hepatocellular carcinoma. Clin Transl Med. 2023;13(2):e1198. doi:10.1002/ctm2.1198
- Tang Z, Yang Y, Chen W, Li E, Liang T. Demethylation at enhancer upregulates MCM2 and NUP37 expression predicting poor survival in hepatocellular carcinoma patients. J Transl Med. 2022;20(1):49. doi:10.1186/s12967-022-03249-2
- Liu J, Jiang J, Mo J, et al. Global DNA 5-Hydroxymethylcytosine and 5-Formylcytosine Contents Are Decreased in the Early Stage of Hepatocellular Carcinoma. Hepatology. 2019;69(1):196–208. doi:10.1002/hep.30146
- Xiao CL, Zhu S, He M, et al. N(6)-Methyladenine DNA Modification in the Human Genome. Mol Cell. 2018;71(2):306–18 e7. doi:10.1016/j.molcel.2018.06.015
- Cui B, Fan X, Zhou D, et al. CSF1R methylation is a key regulatory mechanism of tumor-associated macrophages in hepatocellular carcinoma. Oncol Lett. 2020;20(2):1835–1845. doi:10.3892/ol.2020.11726
- Miao LL, Wang JW, Liu HH, Gao S, Fan YC, Wang K. Hypomethylation of glycine dehydrogenase promoter in peripheral blood mononuclear cells is a new diagnostic marker of hepatitis B virus-associated hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2024;23(1):35–42. doi:10.1016/j.hbpd.2023.02.011
- Zhang Y, Wang JW, Su X, et al. F-box protein 43 promoter methylation as a novel biomarker for hepatitis B virus-associated hepatocellular carcinoma. Front Microbiol. 2023;14:1267844. doi:10.3389/fmicb.2023.1267844
- Nishikawa M, Okada H, Kawaguchi K, et al. Identification of a Transmembrane Protein Involved in Shear Stress Signaling and Hepatocarcinogenesis After a Sustained Virological Response to Hepatitis C Virus. Cell Mol Gastroenterol Hepatol. 2023;16(2):263–286. doi:10.1016/j.jcmgh.2023.04.006
- Kuramoto J, Arai E, Fujimoto M, et al. Quantification of DNA methylation for carcinogenic risk estimation in patients with non-alcoholic steatohepatitis. Clin Clin Epigenet. 2022;14(1):168. doi:10.1186/s13148-022-01379-4
- Zhao Y, Zhao L, Jin H, et al. Plasma methylated GNB4 and Riplet as a novel dual-marker panel for the detection of hepatocellular carcinoma. Epigenetics. 2024;19(1):2299044. doi:10.1080/15592294.2023.2299044
- Cheng T, Zhou C, Bian S, Sobeck K, Liu Y. Coordinated activation of DNMT3a and TET2 in cancer stem cell-like cells initiates and sustains drug resistance in hepatocellular carcinoma. Cancer Cell Int. 2024;24(1):110. doi:10.1186/s12935-024-03288-3
- Yu MC, Lee CW, Lin CH, et al. Differential hypermethylation of the VTRNA2-1 promoter in hepatocellular carcinoma as a prognostic factor: tumor marker prognostic study. Int J Surg. 2020;79:282–289. doi:10.1016/j.ijsu.2020.05.016
- Lin XH, Zhang DY, Liu ZY, et al. lncRNA-AC079061.1/VIPR1 axis may suppress the development of hepatocellular carcinoma: a bioinformatics analysis and experimental validation. J Transl Med. 2022;20(1):379. doi:10.1186/s12967-022-03573-7
- Tang Y, Wang Y, Xu X, Sun H, Tang W. STEAP4 promoter methylation correlates with tumorigenesis of hepatocellular carcinoma. Pathol Res Pract. 2022;233:153870. doi:10.1016/j.prp.2022.153870
- Chen Y, Huang W, Ouyang J, Wang J, Xie Z. Identification of Anoikis-Related Subgroups and Prognosis Model in Liver Hepatocellular Carcinoma. Int J Mol Sci. 2023;24(3).
- Liu R, Cao X, Liang Y, et al. Downregulation of ST6GAL1 Promotes Liver Inflammation and Predicts Adverse Prognosis in Hepatocellular Carcinoma. J Inflamm Res. 2022;15:5801–5814. doi:10.2147/JIR.S385491
- Wei B, Chen H, Chen X, Guo D, Hong L, Zheng S. Sox15 Methylation Inhibits Cell Proliferation Through Wnt Signaling in Hepatocellular Carcinoma. Front Oncol. 2022;12:842312. doi:10.3389/fonc.2022.842312
- Liu J, Zhang N, Zeng J, et al. N(6) -methyladenosine-modified lncRNA ARHGAP5-AS1 stabilises CSDE1 and coordinates oncogenic RNA regulons in hepatocellular carcinoma. Clin Transl Med. 2022;12(11):e1107. doi:10.1002/ctm2.1107
- Abo El-Khair SM, Elalfy H, Diasty M, Ebrahim EE, Elsamanoudy AZ. Methylation degree of metalloproteinase inhibitor RECK gene: links to RECK protein level and hepatocellular carcinoma in chronic HCV infection patients. J Biochem Mol Toxicol. 2021;35(10):e22886. doi:10.1002/jbt.22886
- Long M, Zhou Z, Wei X, et al. A novel risk score based on immune-related genes for hepatocellular carcinoma as a reliable prognostic biomarker and correlated with immune infiltration. Front Immunol. 2022;13:1023349. doi:10.3389/fimmu.2022.1023349
- Huang Y, Yu Z, Zheng M, Yang X, Huang H, Zhao L. Methylation‑associated inactivation of JPH3 and its effect on prognosis and cell biological function in HCC. Mol Med Rep. 2022;25(4). doi:10.3892/mmr.2022.12640
- Ahn HR, Baek GO, Yoon MG, et al. Hypomethylation-mediated upregulation of the WASF2 promoter region correlates with poor clinical outcomes in hepatocellular carcinoma. J Exp Clin Cancer Res. 2022;41(1):158. doi:10.1186/s13046-022-02365-7
- Wang W, Ding B, Lou W, Lin S. Promoter Hypomethylation and miR-145-5p Downregulation- Mediated HDAC11 Overexpression Promotes Sorafenib Resistance and Metastasis of Hepatocellular Carcinoma Cells. Front Cell Dev Biol. 2020;8:724. doi:10.3389/fcell.2020.00724
- Chen MM, Zhao RC, Chen KF, et al. Hypomethylation of CTCFL promoters as a noninvasive biomarker in plasma from patients with hepatocellular carcinoma. Neoplasma. 2020;67(4):909–915. doi:10.4149/neo_2020_190819N789
- Kong R, Zhang H, Jia Y, Man Q, Liu S. Integrated analysis revealing the role of TET3-mediated MUC13 promoter hypomethylation in hepatocellular carcinogenesis. Epigenomics. 2022;14(24):1579–1591. doi:10.2217/epi-2022-0395
- Lee YR, Kim G, Lee HW, et al. Long interspersed nuclear element-1 hypomethylation is associated with poor outcomes via the activation of ST18 in human hepatocellular carcinoma. Medicine (Baltimore). 2021;100(16):e25552. doi:10.1097/MD.0000000000025552
- Liu HH, Fang Y, Wang JW, et al. Hypomethylation of the cyclin D1 promoter in hepatitis B virus-associated hepatocellular carcinoma. Medicine (Baltimore). 2020;99(20):e20326. doi:10.1097/MD.0000000000020326
- Li Z, Li Z, Wang L, Long C, Zheng Z, Zhuang X. ZCCHC13-mediated induction of human liver cancer is associated with the modulation of DNA methylation and the AKT/ERK signaling pathway. J Transl Med. 2019;17(1):108. doi:10.1186/s12967-019-1852-0
- Jones PA. Functions of DNA methylation: islands, start sites, gene bodies and beyond. Nat Rev Genet. 2012;13(7):484–492. doi:10.1038/nrg3230
- Liu Y, Cheng H, Cheng C, et al. ZNF191 alters DNA methylation and activates the PI3K-AKT pathway in hepatoma cells via transcriptional regulation of DNMT1. Cancer Med. 2022;11(5):1269–1280. doi:10.1002/cam4.4535
- Lu W, Kang Y. Epithelial-Mesenchymal Plasticity in Cancer Progression and Metastasis. Dev Cell. 2019;49(3):361–374. doi:10.1016/j.devcel.2019.04.010
- Zhao NH, Qian Y, Wu CS, et al. Diagnostic value of NKG2D promoter methylation in hepatitis B virus-associated hepatocellular carcinoma. Biomarker Med. 2019;13(13):1093–1105. doi:10.2217/bmm-2019-0102
- Wang N, Zhou X, Tang F, Wang X, Zhu X. Identification of LOXL3-associating immune infiltration landscape and prognostic value in hepatocellular carcinoma. Virchows Arch. 2021;479(6):1153–1165. doi:10.1007/s00428-021-03193-4
- Song G, Zhu X, Xuan Z, et al. Hypermethylation of GNA14 and its tumor-suppressive role in hepatitis B virus-related hepatocellular carcinoma. Theranostics. 2021;11(5):2318–2333. doi:10.7150/thno.48739
- Song D, Zhou Z, Wu J, et al. DNA methylation regulators-related molecular patterns and tumor immune landscape in hepatocellular carcinoma. Front Oncol. 2022;12:877817. doi:10.3389/fonc.2022.877817
- Peng X, Lei C, He A, Luo R, Cai Y, Dong W. Upregulation of phosphatidylinositol glycan anchor biosynthesis class C is associated with unfavorable survival prognosis in patients with hepatocellular carcinoma. Oncol Lett. 2021;21(3):237. doi:10.3892/ol.2021.12498
- Heidari Z, Asemi-Rad A, Moudi B, Mahmoudzadeh-Sagheb H. mRNA expression and epigenetic-based role of chromodomain helicase DNA-binding 5 in hepatocellular carcinoma. J Int Med Res. 2022;50(7):3000605221105344. doi:10.1177/03000605221105344
- Peng H, Du X, Zhang Y. RAB42 is a Potential Biomarker that Correlates With Immune Infiltration in Hepatocellular Carcinoma. Front Mol Biosci. 2022;9:898567. doi:10.3389/fmolb.2022.898567
- Hur K, Cejas P, Feliu J, et al. Hypomethylation of long interspersed nuclear element-1 (LINE-1) leads to activation of proto-oncogenes in human colorectal cancer metastasis. Gut. 2014;63(4):635–646. doi:10.1136/gutjnl-2012-304219
- Lu S, Lu H, Jin R, Mo Z. Promoter methylation and H3K27 deacetylation regulate the transcription of VIPR1 in hepatocellular carcinoma. Biochem Biophys Res Commun. 2019;509(1):301–305. doi:10.1016/j.bbrc.2018.12.129
- Xiang L, Chen LM, Zhai YJ, et al. Hypermethylation of secreted frizzled related protein 2 gene promoter serves as a noninvasive biomarker for HBV-associated hepatocellular carcinoma. Life Sci. 2021;270:119061. doi:10.1016/j.lfs.2021.119061
- Zhou Y, Qiu XP, Li ZH, et al. Clinical significance of aberrant cyclin-dependent kinase-like 2 methylation in hepatocellular carcinoma. Gene. 2019;683:35–40. doi:10.1016/j.gene.2018.10.009
- Yan X, Wu T, Tang M, et al. Methylation of the ataxia telangiectasia mutated gene (ATM) promoter as a radiotherapy outcome biomarker in patients with hepatocellular carcinoma. Medicine (Baltimore). 2020;99(4):e18823. doi:10.1097/MD.0000000000018823
- Wang J, Yu H, Dong W, et al. N6-Methyladenosine-Mediated Up-Regulation of FZD10 Regulates Liver Cancer Stem Cells’ Properties and Lenvatinib Resistance Through WNT/β-Catenin and Hippo Signaling Pathways. Gastroenterology. 2023;164(6):990–1005. doi:10.1053/j.gastro.2023.01.041
- Fan H, Zhang M, Liu W. Hypermethylated KCNQ1 acts as a tumor suppressor in hepatocellular carcinoma. Biochem Biophys Res Commun. 2018;503(4):3100–3107. doi:10.1016/j.bbrc.2018.08.099
- Luo H, Xia X, Huang LB, et al. Pan-cancer single-cell analysis reveals the heterogeneity and plasticity of cancer-associated fibroblasts in the tumor microenvironment. Nat Commun. 2022;13(1):6619. doi:10.1038/s41467-022-34395-2
- Wu M, Lan H, Ye Z, Wang Y. Hypermethylation of the PZP gene is associated with hepatocellular carcinoma cell proliferation, invasion and migration. FEBS Open Bio. 2021;11(3):826–832. doi:10.1002/2211-5463.13093
- Yuan XD, Wang JW, Fang Y, et al. Pathol Res Pract.2020;216Methylation status of the T-cadherin gene promotor in peripheral blood mononuclear cells is associated with HBV-related hepatocellular carcinoma progression. Pathology, Research and Practice. 2020;216(5):152914. doi:10.1016/j.prp.2020.152914
- Shi S, Wang B, Wan J, et al. TMEM106A transcriptionally regulated by promoter methylation is involved in invasion and metastasis of hepatocellular carcinoma. Acta Biochim Biophys Sin (Shanghai). 2022;54(7):1008–1020. doi:10.3724/abbs.2022069
- Huang F, Yang G, Jiang H, et al. Role of Plasma methylated SEPT9 for Predicting Microvascular Invasion and Tumor Proliferation in Hepatocellular Carcinoma. Technol Cancer Res Treat. 2022;21:15330338221144510. doi:10.1177/15330338221144510
- Li B, Huang H, Huang R, et al. SEPT9 Gene Methylation as a Noninvasive Marker for Hepatocellular Carcinoma. Dis Markers. 2020;2020:6289063. doi:10.1155/2020/6289063
- Wang X, Chen M, Liang X, et al. RNF135 Promoter Methylation Is Associated With Immune Infiltration and Prognosis in Hepatocellular Carcinoma. Front Oncol. 2021;11:752511. doi:10.3389/fonc.2021.752511
- Galetzka D, Böck J, Wagner L, et al. Hypermethylation of RAD9A intron 2 in childhood cancer patients, leukemia and tumor cell lines suggest a role for oncogenic transformation. EXCLI J. 2022;21:117–143. doi:10.17179/excli2021-4482
- Khanal T, Rajan N, Li W, Liyanarachchi S, Ringel MD. The RCAN1.4 Metastasis Suppressor Is Hypermethylated at Intron 1 in Thyroid Cancer. Thyroid. 2023;33(8):965–973. doi:10.1089/thy.2022.0687
- Cui X, Zhang C, Xu Z, et al. Dual CRISPR interference and activation for targeted reactivation of X-linked endogenous FOXP3 in human breast cancer cells. Mol Cancer. 2022;21(1):38. doi:10.1186/s12943-021-01472-x
- Yang Y, Yan Y, Yin J, et al. O-GlcNAcylation of YTHDF2 promotes HBV-related hepatocellular carcinoma progression in an N(6)-methyladenosine-dependent manner. Signal Transduct Target Ther. 2023;8(1):63. doi:10.1038/s41392-023-01316-8
- Maron MI, Casill AD, Gupta V, et al. Type I and II PRMTs inversely regulate post-transcriptional intron detention through Sm and CHTOP methylation. Elife. 2022;11.
- Telonis AG, Yang Q, Huang HT, Figueroa ME. MIR retrotransposons link the epigenome and the transcriptome of coding genes in acute myeloid leukemia. Nat Commun. 2022;13(1):6524. doi:10.1038/s41467-022-34211-x
- Roundtree IA, Evans ME, Pan T, He C. Dynamic RNA Modifications in Gene Expression Regulation. Cell. 2017;169(7):1187–1200. doi:10.1016/j.cell.2017.05.045
- Kim SC, Kim J, Kim DW, et al. Methylation-sensitive high-resolution melting analysis of the USP44 promoter can detect early-stage hepatocellular carcinoma in blood samples. BMB Rep. 2022;55(11):553–558. doi:10.5483/BMBRep.2022.55.11.110
- Sun X, Fu S, Yuan X, et al. RNA N6-methyladenosine (m6A) modification in HNSCC: molecular mechanism and therapeutic potential. Cancer Gene Ther. 2023;30(9):1209–1214. doi:10.1038/s41417-023-00628-9
- Huang X, Wang H, Xu F, et al. Overexpression of chaperonin containing TCP1 subunit 7 has diagnostic and prognostic value for hepatocellular carcinoma. Aging. 2022;14(2):747–769. doi:10.18632/aging.203809
- Wang K, Luo L, Fu S, et al. PHGDH arginine methylation by PRMT1 promotes serine synthesis and represents a therapeutic vulnerability in hepatocellular carcinoma. Nat Commun. 2023;14(1):1011. doi:10.1038/s41467-023-36708-5
- Li W, Liu J, Ma Z, Zhai X, Cheng B, Zhao H. m(6)A RNA Methylation Regulators Elicit Malignant Progression and Predict Clinical Outcome in Hepatocellular Carcinoma. Dis Markers. 2021;2021:8859590. doi:10.1155/2021/8859590
- Deng M, Fang L, Li SH, et al. Expression pattern and prognostic value of N6-methyladenosine RNA methylation key regulators in hepatocellular carcinoma. Mutagenesis. 2021;36(5):369–379. doi:10.1093/mutage/geab032
- Collignon E, Cho B, Furlan G, et al. m(6)A RNA methylation orchestrates transcriptional dormancy during paused pluripotency. Nat Cell Biol. 2023;25(9):1279–1289. doi:10.1038/s41556-023-01212-x
- Yu Y, Lu X, Yan Y, et al. The lncRNA KIF9-AS1 Accelerates Hepatocellular Carcinoma Growth by Recruiting DNMT1 to Promote RAI2 DNA Methylation. J Oncol. 2022;2022:3888798. doi:10.1155/2022/3888798
- Hou Y, Chen K, Liao R, Li Y, Yang H, Gong J. LINC01419-mediated epigenetic silencing of ZIC1 promotes metastasis in hepatocellular carcinoma through the PI3K/Akt signaling pathway. Lab Invest. 2021;101(5):570–587. doi:10.1038/s41374-021-00539-z
- Dong ZR, Ke AW, Li T, et al. CircMEMO1 modulates the promoter methylation and expression of TCF21 to regulate hepatocellular carcinoma progression and sorafenib treatment sensitivity. Mol Cancer. 2021;20(1):75. doi:10.1186/s12943-021-01361-3
- Lv HC, Lv YY, Wang G, et al. Mechanism of miR-424-5p promoter methylation in promoting epithelial-mesenchymal transition of hepatocellular carcinoma cells. Kaohsiung J Med Sci. 2022;38(4):336–346. doi:10.1002/kjm2.12499
- Ma S, Chen F, Lin C, et al. MiR-186-5p prevents hepatocellular carcinoma progression by targeting methyltransferase-like 3 that regulates m6A-mediated stabilization of follistatin-like 5. Heliyon. 2024;10(5):e26767. doi:10.1016/j.heliyon.2024.e26767
- Zhuang Q, Dai Z, Xu X, et al. RNA Methyltransferase FTSJ3 Regulates the Type I Interferon Pathway to Promote Hepatocellular Carcinoma Immune Evasion. Cancer Res. 2024;84(3):405–418. doi:10.1158/0008-5472.CAN-23-2049
- Zhao K, Wei B, Zhang Y, Shi W, Zhang G, Wang Z. M6A regulator-mediated immune infiltration and methylation modification in hepatocellular carcinoma microenvironment and immunotherapy. Front Pharmacol. 2022;13:1052177. doi:10.3389/fphar.2022.1052177
- Gebauer F, Schwarzl T, Valcárcel J, Hentze MW. RNA-binding proteins in human genetic disease. Nat Rev Genet. 2021;22(3):185–198. doi:10.1038/s41576-020-00302-y
- Kim GW, Imam H, Khan M, et al. HBV-Induced Increased N6 Methyladenosine Modification of PTEN RNA Affects Innate Immunity and Contributes to HCC. Hepatology. 2021;73(2):533–547. doi:10.1002/hep.31313
- Du Y, Ma Y, Zhu Q, et al. An m6A-Related Prognostic Biomarker Associated With the Hepatocellular Carcinoma Immune Microenvironment. Front Pharmacol. 2021;12:707930. doi:10.3389/fphar.2021.707930
- Dominissini D, Moshitch-Moshkovitz S, Schwartz S, et al. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature. 2012;485(7397):201–206. doi:10.1038/nature11112
- Slobodin B, Han R, Calderone V, et al. Transcription Impacts the Efficiency of mRNA Translation via Co-transcriptional N6-adenosine Methylation. Cell. 2017;169(2):326–37 e12. doi:10.1016/j.cell.2017.03.031
- Zhu S, Ye H, Xu X, et al. Involvement of TRPC7-AS1 Expression in Hepatitis B Virus-Related Hepatocellular Carcinoma. J Oncol. 2021;2021:8114327. doi:10.1155/2021/8114327
- Zhao J, Li L, Guo L, et al. Nano-Gold PCR in Detection of TERT Methylation and Its Correlation with Hepatitis B-Related Hepatocellular Carcinoma. J Biomed Nanotechnol. 2021;17(7):1284–1292. doi:10.1166/jbn.2021.3103
- Chen MH, Fu LS, Zhang F, Yang Y, Wu XZ. LncAY controls BMI1 expression and activates BMI1/Wnt/β-catenin signaling axis in hepatocellular carcinoma. Life Sci. 2021;280:119748. doi:10.1016/j.lfs.2021.119748
- Lin YH, Zhang BY, Chen ZS. circRERE regulates the expression of GBX2 through miR-1299 and ZC3H13/N(6)-methyladenosine (m(6)A) to promote growth and invasion of hepatocellular carcinoma cells. J Biosci. 2022;47.
- Wu A, Hu Y, Xu Y, et al. Methyltransferase-Like 3-Mediated m6A Methylation of Hsa_circ_0058493 Accelerates Hepatocellular Carcinoma Progression by Binding to YTH Domain-Containing Protein 1. Front Cell Dev Biol. 2021;9:762588. doi:10.3389/fcell.2021.762588
- Chi F, Cao Y, Chen Y. Analysis and Validation of circRNA-miRNA Network in Regulating m(6)A RNA Methylation Modulators Reveals CircMAP2K4/miR-139-5p/YTHDF1 Axis Involving the Proliferation of Hepatocellular Carcinoma. Front Oncol. 2021;11:560506. doi:10.3389/fonc.2021.560506
- Liu L, Gu M, Ma J, et al. CircGPR137B/miR-4739/FTO feedback loop suppresses tumorigenesis and metastasis of hepatocellular carcinoma. Mol Cancer. 2022;21(1):149. doi:10.1186/s12943-022-01619-4
- Wang W, Huang Q, Liao Z, et al. ALKBH5 prevents hepatocellular carcinoma progression by post-transcriptional inhibition of PAQR4 in an m6A dependent manner. Exp Hematol Oncol. 2023;12(1):1. doi:10.1186/s40164-022-00370-2
- Song D, An K, Zhai W, et al. NSUN2-mediated mRNA m(5)C Modification Regulates the Progression of Hepatocellular Carcinoma. Genomics Proteomics Bioinf. 2022;21(4):823–833. doi:10.1016/j.gpb.2022.09.007
- Zheng Q, Yu X, Zhang Q, He Y, Guo W. Genetic characteristics and prognostic implications of m1A regulators in pancreatic cancer. Biosci Rep. 2021;41(4). doi:10.1042/BSR20210337
- Zhao Y, Zhao Q, Kaboli PJ, et al. m1A Regulated Genes Modulate PI3K/AKT/mTOR and ErbB Pathways in Gastrointestinal Cancer. Transl Oncol. 2019;12(10):1323–1333. doi:10.1016/j.tranon.2019.06.007
- Wang Y, Wang J, Li X, et al. N(1)-methyladenosine methylation in tRNA drives liver tumourigenesis by regulating cholesterol metabolism. Nat Commun. 2021;12(1):6314. doi:10.1038/s41467-021-26718-6
- Husmann D, Gozani O. Histone lysine methyltransferases in biology and disease. Nat Struct Mol Biol. 2019;26(10):880–889. doi:10.1038/s41594-019-0298-7
- Guccione E, Richard S. The regulation, functions and clinical relevance of arginine methylation. Nat Rev Mol Cell Biol. 2019;20(10):642–657. doi:10.1038/s41580-019-0155-x
- Yu L, Ji T, Liao W, et al. H4-methylation regulators mediated epitranscriptome patterns and tumor microenvironment infiltration characterization in hepatocellular carcinoma. Clin Clin Epigenet. 2023;15(1):43. doi:10.1186/s13148-023-01460-6
- Qian Y, Li Y, Zheng C, et al. High methylation levels of histone H3 lysine 9 associated with activation of hypoxia-inducible factor 1α (HIF-1α) predict patients’ worse prognosis in human hepatocellular carcinomas. Cancer Genet. 2020;245:17–26. doi:10.1016/j.cancergen.2020.04.077
- Ghobashi AH, Vuong TT, Kimani JW, Ladaika CA, Hollenhorst PC, O’Hagan HM. Activation of AKT induces EZH2-mediated β-catenin trimethylation in colorectal cancer. iScience. 2023;26(9):107630. doi:10.1016/j.isci.2023.107630
- Guo S, Chen F, Li L, et al. Intracellular Fusobacterium nucleatum infection increases METTL3-mediated m6A methylation to promote the metastasis of esophageal squamous cell carcinoma. J Adv Res. 2023. doi:10.1016/j.jare.2023.08.014
- Chen M, Fang Y, Liang M, et al. The activation of mTOR signalling modulates DNA methylation by enhancing DNMT1 translation in hepatocellular carcinoma. J Transl Med. 2023;21(1):276. doi:10.1186/s12967-023-04103-9
- Fatemiyan N, Davie JR. Broad histone H4 monomethylation marks expressed genes involved in translation. Genome. 2023;66(8):224–234. doi:10.1139/gen-2023-0011
- Wong TL, Ng KY, Tan KV, et al. CRAF Methylation by PRMT6 Regulates Aerobic Glycolysis-Driven Hepatocarcinogenesis via ERK-Dependent PKM2 Nuclear Relocalization and Activation. Hepatology. 2020;71(4):1279–1296. doi:10.1002/hep.30923
- Zhao J, O’Neil M, Schonfeld M, Komatz A, Weinman SA, Tikhanovich I. Hepatocellular Protein Arginine Methyltransferase 1 Suppresses Alcohol-Induced Hepatocellular Carcinoma Formation by Inhibition of Inducible Nitric Oxide Synthase. Hepatol Commun. 2020;4(6):790–808. doi:10.1002/hep4.1488
- Li Y, Dobrolecki LE, Sallas C, et al. PRMT blockade induces defective DNA replication stress response and synergizes with PARP inhibition. Cell Rep Med. 2023;4(12):101326. doi:10.1016/j.xcrm.2023.101326
- Kim SY, Hwang S, Lee BR, Hong JA, Sung YH, Kim I. Inhibition of histone demethylase KDM4 by ML324 induces apoptosis through the unfolded protein response and Bim upregulation in hepatocellular carcinoma cells. Chem Biol Interact. 2022;353:109806. doi:10.1016/j.cbi.2022.109806
- Lin Q, Wu Z, Yue X, et al. ZHX2 restricts hepatocellular carcinoma by suppressing stem cell-like traits through KDM2A-mediated H3K36 demethylation. EBioMedicine. 2020;53:102676. doi:10.1016/j.ebiom.2020.102676
- Liu Z, Lin M, Liu C, et al. Development of (2-(Benzyloxy)phenyl)methanamine Derivatives as Potent and Selective Inhibitors of CARM1 for the Treatment of Melanoma. J Med Chem. 2024;67(8):6313–6326. doi:10.1021/acs.jmedchem.3c02265
- Zhang S, Guo L, Zhang Z, et al. Type-I protein arginine methyltransferase inhibition primes anti-programmed cell death protein 1 immunotherapy in triple-negative breast cancer. Cancer. 2024;130(S8):1415–1423. doi:10.1002/cncr.35142
- Hao M, Jiang Y, Zhang Y, Yang X, Han J. Ferroptosis regulation by methylation in cancer. Biochim Biophys Acta Rev Cancer. 2023;1878(6):188972. doi:10.1016/j.bbcan.2023.188972
- Mao X, Xu J, Wang W, et al. Crosstalk between cancer-associated fibroblasts and immune cells in the tumor microenvironment: new findings and future perspectives. Mol Cancer. 2021;20(1):131. doi:10.1186/s12943-021-01428-1
- Fox-Fisher I, Shemer R, Dor Y. Epigenetic liquid biopsies: a novel putative biomarker in immunology and inflammation. Trends Immunol. 2023;44(5):356–364. doi:10.1016/j.it.2023.03.005
