Abstract
Purpose
Solitary pulmonary inflammatory nodules (SPINs) are frequently misdiagnosed as malignancy. We aimed to investigate CT features and pathological findings of SPINs for improving diagnosis strategies.
Patients and Methods
In this retrospective study, 225 and 310 consecutive patients with confirmed SPINs and lung cancerous nodules were enrolled from January 2013 to December 2020. Nodules were classified into different types based on the key CT features: I, homogeneous and well-defined nodules with smooth (Ia), coarse (Ib), or spiculated margins (Ic); II, nodules with blurred boundaries, peripheral patches, or both; III, nodules exhibiting heterogeneous density; and IV, polygonal nodules. The pathological findings of SPINs were simultaneously studied and summarized.
Results
Among the 225 SPINs, type I (Ia, Ib, and Ic), II, III, and IV were 137 (60.9%) (47 [20.9%], 33 [14.7%], and 57 [25.3%]), 62 (27.6%), 12 (5.3%) and 14 (6.2%), respectively. Correspondingly, those in 310 cancerous nodules were 275 (88.7%) (119 [38.4%], 70 [22.6%], and 86 [27.7%]), 20 (6.5%), 15 (4.8%), and 0, respectively. Compared with lung cancers, type I nodules were less common but type II and IV nodules were more common in SPINs (each P < 0.0001). Though the frequencies of subtype I (P = 0.095) and type III (P = 0.796) nodules were similar between two groups, their specific CT features were significantly different. The main pathological findings of each type of SPINs were most extensively identical (82.2 – 100%).
Conclusion
Between cancerous nodules and SPINs, differences in overall or specific CT features exist. The type II and IV nodules are highly indicative of SPINs, and each type of SPINs have almost similar pathological findings.
Introduction
Solitary pulmonary nodules are usually detected incidentally or during screening for lung cancer.Citation1,Citation2 Solitary pulmonary nodules arise mainly from tumors (benign or malignant), infectious lesions, and noninfectious lesions.Citation2,Citation3 The treatment and prognosis for benign nodules differ significantly from those for malignant nodules.Citation3–Citation7 Therefore, accurate diagnosis of pulmonary nodules is of great importance in clinical practice. Currently, computed tomographic (CT) scanning is the best diagnostic imaging tool for pulmonary nodules. However, benign and malignant nodules have many similar CT features.Citation2,Citation4–Citation7 For example, most well-defined solitary pulmonary nodules with smooth margins have been benign, but 21% of malignant nodules also shared such features.Citation2,Citation8 Thus, distinguishing their CT characteristics of benign and malignant nodules is of great significance.
Solitary pulmonary nodules may be solid or subsolid. Previous studies had confirmed that ground-glass nodules, especially the mixed ones, had an extremely high probability of being malignant.Citation9–Citation12 However, the pathological nature of solid nodules is diverse, correctly differentiating them is crucial but difficult. In fact, a confident diagnosis of benignity can be made only for completely calcified or fat-containing nodules.Citation13 The majority of incidental and screen-detected pulmonary solid nodules are inflammatory lesions (both healed and active) and benign tumors.Citation13 CT manifestations of pulmonary benign tumors are usually similar, whereas those of inflammatory nodules and lung cancers are often complex, and such lesions need to be further understood and distinguished.
Previous studies have focused on differentiating all kinds of benign and malignant pulmonary nodules,Citation14–Citation16 but no study specifically for the CT manifestations of solitary pulmonary inflammatory nodules (SPINs) has been reported. Furthermore, we have found no report about the systematic correlation between CT features and pathological findings of SPINs. The aim of this study was to summarize and clarify the CT features of SPINs by comparing inflammatory and cancerous nodules. In addition, the pathological characteristics of SPINs were studied for better understanding their CT findings.
Patients and Methods
Patients
This retrospective observational study was conducted in the First Affiliated Hospital of Chongqing Medical University between January 2013 and December 2020 in China. Patients with surgically resected (video-assisted thoracic surgery segmentectomy or lobectomy) and pathologically confirmed SPINs (main pathological findings included inflammatory cells infiltration and fibrous tissue proliferation) and lung cancers were consecutively enrolled in this study. All the patients underwent chest CT examination within a week before surgery. Inclusion criteria were as follows: (1) The lesion was a nodule (diameter, ≤3 cm); (2) the lesion was solid; and (3) the patients’ clinical and pathological data were complete. The exclusion criterion was CT images containing breath artifact. In all, 225 patients with SPINs and 310 cases with lung cancers were enrolled in this study.
CT Protocol
All studies were performed on a SOMATOM Definition Flash CT scanner (Siemens Healthineers, Erlangen, Germany) with following parameters for non-contrast chest scan: 120 kV, 100 mAs, rotation time of 0.5 s, pitch of 1, 128×0.6 mm collimation, and 5-mm slice thickness and 5-mm interval for axial images. During CT examination, all the patients were uniformly scanned in the craniocaudal direction and placed in a supine position with both hands placed near the head. The scanning range included the whole chest from the level of the thoracic inlet to immediately below the costophrenic angle. Contiguous transverse images were reconstructed with a slice thickness of 0.6 mm and a standard filtered back projection algorithm involving a kernel of high spatial resolution (lung images: widths of 1200–1600 HU and levels of −500–−700 HU) and a soft-tissue kernel (mediastinal images: widths of 350–450 HU and levels of 20–40 HU), respectively.
Image and Pathological Findings Analysis
All patients’ CT data were initially reviewed on a workstation (Advantage Workstation 4.6; GE Healthcare, Chicago) by two senior chest radiologists (reviewer 1 has 12 years of experience, reviewer 2 has 25 years of experience) who were unaware of the pathological results of the nodules. Interpretation discrepancy, if any, was resolved by consensus.
On CT image, the following characteristics were evaluated: nodule distribution in different lobes, lesion size (mean of the longest diameter and perpendicular diameter on axial images), locations (whether abutting pleura or not, wide or narrow base connected to pleura), shape (round, oval, polygonal, or irregular), nodule–lung interface (well-defined or blurred), margins (smooth, coarse, or spiculated), density on lung window images (homogeneous or heterogeneous), internal signs (calcification, cavity, vacuole, and air bronchogram), and changes in the surrounding lung field (clear, patchy, or fibrotic). Wide base indicated the diameter of nodule-pleura contact surface was greater than or equal to that of nodule, or it was narrow base. Peripheral patch indicated that there was ground glass opacity surrounding nodules or locating at one side. Spiculation was further described as intensive if spiculations densely distributed around the nodules or as sparse, and short (length of spiculation ≤ diameter of nodule) or long (length of spiculation > diameter of nodule).
Based on the key CT features (order of categorization basis: shape [polygonal or not], density [heterogeneous or not], boundary [blurred or not], changes in peripheral lung fields [peripheral patch or not], and margin [smooth, coarse, or spiculated]), nodules were classified into different types: I, homogeneous and well-defined nodules with smooth (Ia), coarse (Ib), or spiculated margins (Ic); II, nodules with blurred boundaries, peripheral patches, or both; III, nodules exhibiting heterogeneous density; and IV, polygonal nodules.
The pathological findings of nodules were reviewed and summarized by pathologist with 10 years of experience. The main pathological components were determined for each type of SPINs.
Statistical Analysis
Patients’ clinical data and CT features of nodules were statistically analyzed. Continuous variables were expressed as means ± standard deviations, whereas categorical variables were expressed as absolute numbers and percentages. Statistical differences were analyzed using the Wilcoxon rank sum test for patient age and mean diameter of nodules. Patients’ clinical data, types of nodules on CT images, and CT features of nodules were compared between two groups by using the Pearson χ2 test and Fisher exact test, as appropriate. The к statistic was used to calculate the interobserver variability in classifying nodules. Statistical analysis was performed with the SPSS 20.0 software package (IBM, Chicago). A p value of less than 0.05 was considered significant.
Results
Study Population
Patients’ clinical data are summarized in . Compared with patients with lung cancers, those with SPINs were younger (P < 0.0001), and there were less smokers (P = 0.033), less cases with symptoms (P = 0.001) but more with pulmonary basic diseases (P < 0.0001).
Table 1 Patients’ Clinical Data
Types of SPINs and Lung Cancers on CT Images
Numbers of nodules distributed in the upper, middle, and lower lobe of right lung and upper and lower lobe of left lung were 83 (36.9%), 19 (8.4%), 54 (24.0%), 39 (17.3%) and 30 (13.3%) and 106 (34.2%), 30 (9.7%), 51 (16.5%), 74 (23.9%), and 49 (15.8%) in SPIN and lung cancer groups, respectively. Of the 225 SPINs (mean diameter: 14.7 ± 6.3 mm, range: 4–30 mm) and 310 lung cancers (mean diameter: 17.3 ± 6.5 mm, range: 4–30 mm), 155 (68.9%) and 271 (87.4%) were round or oval, 14 (6.2%) and 0 were polygonal, and 56 (24.9%) and 39 (12.6%) were irregular, respectively. The lobulated sign was detected 141 (45.5%) lung cancers and 32 (14.2%) SPINs. Compared with lung cancerous nodules, the SPINs were smaller (P < 0.0001), more irregular (P < 0.0001), and less lobulated (P < 0.0001).
The classifications of SPINs and lung cancers based on key CT features are shown in and , respectively. There was perfect agreement between the two observers on classifying SPINs (к = 0.908) and lung cancers (к = 0.924). Among the 225 SPINs, type I (Ia, Ib, and Ic) (), II (), III (), and IV () nodules were 137 (60.9%) (47 [20.9%], 33 [14.7%], and 57 [25.3%]), 62, (27.6%), 12 (5.3%) and 14 (6.2%), respectively. Correspondingly, type I (Ia, Ib, and Ic) (–), II ( and ), III ( and ), and IV in lung cancer group were 275 (88.7%) (119 [38.4%], 70 [22.6%], and 86 [27.7%]), 20 (6.5%), 15 (4.8%), and 0, respectively. Compared with lung cancers, type I nodules were less common (P < 0.0001) but type II and IV nodules were more common (each P < 0.0001) in SPINs, while the frequencies of all subtypes I (P = 0.095) and type III (P = 0.796) nodules were similar between two groups.
Table 2 Classification of SPINs Based on Key CT Features
Table 3 Classification of Lung Cancers Based on Key CT Features
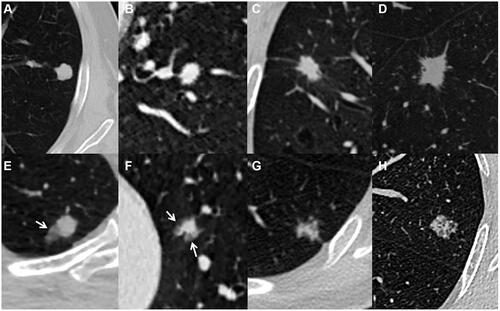
Figure 1 Solid SPINs with smooth margin (type Ia) (A), coarse margin (type Ib) (B), sparse and long spiculations (type Ic) (C), and sparse and short spiculations (type Ic) (D). Pathologically, they have similar manifestations including significant fibrous tissue proliferation, hyaline change and few chronic inflammatory cells infiltration (E–H).
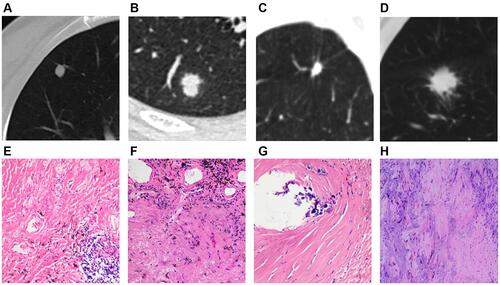
Figure 2 Solid SPINs with blurred margin (A) and peripheral patch (C) (type II). Pathologically, they have similar manifestations including more acute and chronic inflammatory cells infiltration and fibrous tissue proliferation (B and D).
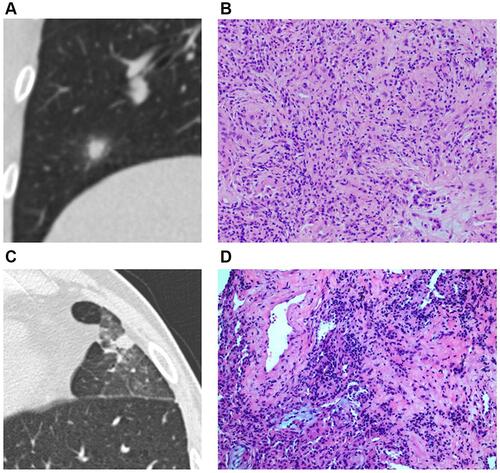
Figure 3 Solid SPIN with heterogeneous density (type III) and spiculations (A) on lung window. Pathologically, it consists of multiple components including fibrous tissue (B), hyaline change (C), calcification (D), and few inflammatory cells (B and C).
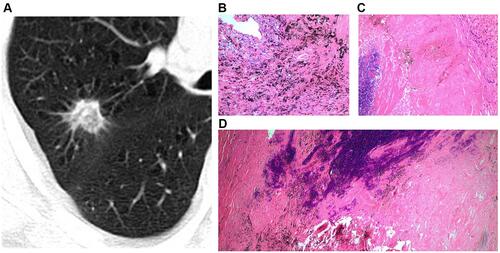
Figure 4 Solid SPIN with polygonal shape (type IV) (A). It has homogeneous density and smooth margin. Pathologically, it consists of fibrous tissue proliferation, hyaline change and a small amount of inflammatory cells infiltration (B).
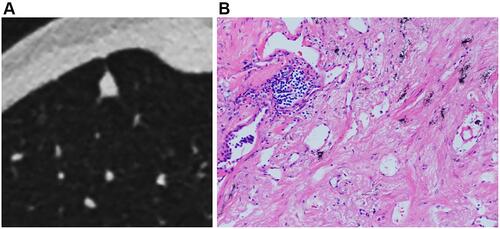
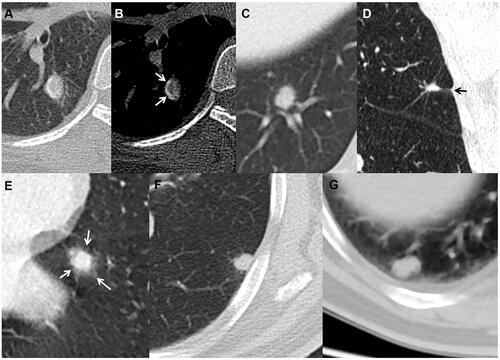
Figure 5 Solid cancerous nodules with smooth and lobulated margin (type Ia) (A), coarse margin (type Ib) (B), lobulated margin and sparse and long spiculations (type Ic) (C), intensive and short spiculations (type Ic) (D), well-defined peripheral patch locating at one side (arrow) (E) or surrounding lesion (arrows) (F) (type II), and heterogeneous density (type III) (G and H).
In the 57 type Ic SPINs, spiculations in 30 (52.6%) lesions were sparse and long, in 23 (40.4%) were sparse and short, in 3 (5.3%) were sparse, short, and long, and in 1 (1.8%) was intensive. In the 86 type Ic lung cancers, spiculations in 43 (50.0%) lesions were sparse and long, in 18 (20.9%) were sparse and short, and in 25 (29.1%) were intensive. Compared with lung cancer, sparse and short spiculations were more common (P = 0.012) but intensive spiculations were less common (P < 0.0001) in type Ic SPINs. For type II nodules, more nodules with blurred boundary (54.8% vs 10%, P < 0.0001) and peripheral patch (90% vs 45.2%, P < 0.0001) were detected in SPINs and lung cancers, respectively. Regarding nodules with peripheral patch, all of the ground glass opacities surrounded nodules (100%) and were ill-defined (100%) in SPINs () but those mostly located at one side of nodules (83.3%) and were always well-defined (100%) in lung cancers ( and ). For type III nodules, the cancerous ones presented as mixed branched () or reticulated () higher density and peripheral slightly lower density (), while these were not found in SPINs.
In SPINs, lesions with CT features of more than one type were more common than those in lung cancers (8.9% vs 1.9%, P < 0.0001). Compared with lung cancers, lesions abutting pleura were more common in SPINs (25.8% vs 3.5%, P < 0.0001), while both of them frequently had a wide base attached to pleura (70.7% vs 54.5%, P = 0.484). In addition, sporadic patch in same lobe (12.9% vs 4.2%, P < 0.0001) and intranodular calcification (4.9% vs 1.3%, P = 0.013) were more commonly detected in SPINs than in lung cancers, while frequencies of vacuole or cavity, pleural indentation, and air bronchogram in both groups were similar (each P > 0.05).
Pathological Findings of SPINs
Among the 310 cancerous nodules, 276 (89.0%) were adenocarcinomas, 23 (7.4%) were squamous carcinomas, 7 (2.3%) were neuroendocrine carcinomas, and 4 (1.3%) were adenosquamous carcinoma and mucoepidermoid carcinoma. Among the 225 SPINs, 203 (90.2%) were nonspecific inflammation (–), 17 (7.6%) were tuberculosis (–), and 5 (2.2%) were fungal infection ( and ). The pathological findings of SPINs are summarized in . Different types of SPINs had similar pathological components but with different predominance. In type I SPINs, fibrous tissue proliferation and hyaline change were significant, and infiltration with few chronic inflammatory cells could be detected (128, 93.5%) (). In type II SPINs, more acute and chronic inflammatory cells could be detected, in addition to fibrous tissue proliferation (51, 82.2%, ). In type III SPINs, multiple components could be observed: fibrous tissue proliferation, hyaline change, hemorrhage, mucoid degeneration, calcification, and inflammatory cell infiltration (12, 100%) (). Type IV SPINs, like type I SPINs, exhibited fibrous tissue proliferation, hyaline change, and infiltration with few chronic inflammatory cells (14, 100%) ().
Table 4 The Pathological Findings of SPINs
Figure 6 Tuberculous nodules with smooth margin (A) and curved calcification (arrows) (B), coarse margin (C), sparse and long spiculations and pleural indentation (arrow) (D), and ill-defined peripheral patch (arrows) (E). A cryptococcal nodule abutting pleura with a wide base (F), and an oval aspergillus nodule with clear and smooth margin (G).
Discussion
In this study, solid SPINs exhibited various CT manifestations and some were exclusive, whereas each type had relatively uniform pathological findings. Knowing the pathological characteristics of SPINs as well is helpful for understanding their different CT manifestations. However, CT features for each type were not distinct; different SPINs had CT manifestations of more than one type. Moreover, some types of SPINs also had signs that could be found in lung cancers. Thus, a further understanding the potential differences in their CT features is needed.
With regard to lesion distribution and location, SPINs were found mainly in the superior lobe, which was similar to the location of lung cancers in this and previous studies.Citation16 Some SPINs in this study were closely attached to adjacent pleura and a majority of those had a wide base, while these were rare for lung cancers.Citation4–Citation6,Citation17–Citation20 Peripheral inflammation frequently involving distal subpleural lung tissues may account for these differences. For nodules not abutted pleura, pleural indentations were all infrequent and similar in SPIN and lung cancer group. Thus, pleural indentation could not be used for differentiating them.
Compared with lung cancers, irregular nodules were relatively common but lobulated ones were infrequent in SPIN group. This may be related to the proliferation of fibrosis and infiltration of inflammatory cells in SPINs rather than the concurrence of different rates of cell growth and restriction caused by adjacent interstitium in lung cancers.Citation4,Citation11,Citation16,Citation21–Citation23 Additionally, a few nodules with flat edges manifesting as polygonal shapes were only found in SPINs, which was probably caused by obstruction of adjacent structures. Thus, lobulated sign and polygonal shapes are meaningful for discriminating solid nodules.
Heterogeneous attenuation can be detected in both inflammatory and malignant nodules, but different pathological processes are responsible for this appearance.Citation5,Citation9,Citation12 Heterogeneous SPINs usually had multiple pathological components; however, the heterogeneous density in solid cancerous nodules usually indicated degeneration or uneven distribution of tumor cells.Citation19 In lung cancers, heterogeneous lesions would become homogeneous as growing due to tumor cell proliferation, but this change would not happen in inflammatory nodules.Citation19,Citation24–Citation26 Therefore, follow-up for monitoring density change is useful for differentiating heterogeneous nodules.
Spiculations are closely associated with lung cancers.Citation2,Citation4,Citation8,Citation12,Citation16 However, the present study showed the occurrences of nodules with spiculations were similar in both groups but their CT features were different. The intensive spiculations were almost only found in lung cancers, while sparse and short ones were frequently detected in SPINs. These differences may be due to the hyperplasia of fibrous tissue and infiltration of inflammatory cells or tumor cells. Therefore, nodules with intensive spiculations were more likely to be tumor, and other CT features should be considered for differentiating nodules with other patterns of spiculations.
The halo sign (HS) can be seen in a large number of diverse conditions, which is the radiological correlate of infiltration (hemorrhage, neoplastic or inflammatory).Citation27 In the present study, both the SPINs and lung cancers showed this sign but they were different. The HS (peripheral patch) in SPINs was ill-defined while most of that in lung cancers was well-defined, which was consistent with previous results.Citation28–Citation30 The pathological findings revealed that ill-defined HS detected in SPINs was closely related to the infiltration of massive inflammatory cells. Moreover, sporadic patches were detected in the same lobe with SPINs in some cases, which may be additional evidence of SPINs. Therefore, blurred boundaries and ill-defined peripheral patches are more typical of SPINs and this help distinguish them from lung cancers.
Regarding other CT characteristics, intranodular calcification was more commonly detected in SPINs than in lung cancers, while frequencies of vacuole or cavity, pleural indentation, and air bronchogram in both groups were similar. Compared with previous studies, the calcification in SPINs was far less common than that in benign nodules,Citation2,Citation4 but it was a potential sign for evaluating nodule and distinguishing them.
This study had several limitations. First, the key CT features for dividing SPINs into different types were not exclusive; some SPINs had features of more than one type. In addition, some SPINs and lung cancers (such as types I and III) shared same CT features, which still could not be well differentiated. However, after studying the pathological findings, it revealed that types I and III SPINs may not grow significantly due to significant fibrous tissue proliferation and hyaline change. Thus, follow-up could provide more information for the likely diagnosis because most of malignant solid nodules will increase in size and/or density,Citation31 and such information should be added in an affected patient’s flowchart for discriminating SPINs from cancerous nodules.
In conclusion, SPINs share different CT features that are closely correlated with pathological findings. There are differences in overall or specific CT features between cancerous nodules and SPINs. Solid pulmonary nodules should be highly suspected of being inflammatory nodules if they have blurred boundaries, peripheral patches, or polygonal shapes on CT images. In contrast, nodules with intensive spiculations or lobulated sign have a high possibility of malignancy. For nodules without distinct CT features, follow-up may be helpful for discriminating by monitoring changes related to different pathological bases.
Ethics Statement
The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of The First Affiliated Hospital of Chongqing Medical University (No. 2019-062), which absolved the need for written informed consent because of the retrospective study. All personal identification data were anonymized and de-identified before analysis.
Disclosure
The authors report no conflicts of interest in this work.
Additional information
Funding
References
- Choi SM, Heo EY, Lee J, et al. Characteristics of benign solitary pulmonary nodules confirmed by diagnostic video-assisted thoracoscopic surgery. Clin Respir J. 2016;10:181–188. doi:10.1111/crj.12200
- Erasmus JJ, Connolly JE, McAdams HP, Roggli VL. Solitary pulmonary nodules: Part I. Morphologic evaluation for differentiation of benign and malignant lesions. Radiographics. 2000;20:43–58. doi:10.1148/radiographics.20.1.g00ja0343
- Hodnett PA, Ko JP. Evaluation and management of indeterminate pulmonary nodules. Radiol Clin North Am. 2012;50:895–914. doi:10.1016/j.rcl.2012.06.005
- Snoeckx A, Reyntiens P, Desbuquoit D, et al. Evaluation of the solitary pulmonary nodule: size matters, but do not ignore the power of morphology. Insights Imaging. 2018;9:73–86. doi:10.1007/s13244-017-0581-2
- Zwirewich CV, Vedal S, Miller RR, Müller NL. Solitary pulmonary nodule: high-resolution CT and radiologic-pathologic correlation. Radiology. 1991;179:469–476. doi:10.1148/radiology.179.2.2014294
- Li BG, Ma DQ, Xian ZY, et al. The value of multislice spiral CT features of cavitary walls in differentiating between peripheral lung cancer cavities and single pulmonary tuberculous thick-walled cavities. Br J Radiol. 2012;85:147–152. doi:10.1259/bjr/79051309
- Honda O, Tsubamoto M, Inoue A, et al. Pulmonary cavitary nodules on computed tomography: differentiation of malignancy and benignancy. J Comput Assist Tomogr. 2007;31:943–949. doi:10.1097/RCT.0b013e3180415e20
- Sim YT, Poon FW. Imaging of solitary pulmonary nodule-a clinical review. Quant Imaging Med Surg. 2013;3:316–326. doi:10.3978/j.issn.2223-4292.2013.12.08
- Park CM, Goo JM, Lee HJ, Lee CH, Chun EJ, Im JG. Nodular ground-glass opacity at thin-section CT: histologic correlation and evaluation of change at follow-up. Radiographics. 2007;27:391–408. doi:10.1148/rg.272065061
- Nambu A, Araki T, Taguchi Y, et al. Focal area of ground-glass opacity and ground-glass opacity predominance on thin-section CT: discrimination between neoplastic and non-neoplastic lesions. Clin Radiol. 2005;60:1006–1017. doi:10.1016/j.crad.2005.06.006
- Qiu ZX, Cheng Y, Liu D, et al. Clinical, pathological, and radiological characteristics of solitary ground-glass opacity lung nodules on high-resolution computed tomography. Ther Clin Risk Manag. 2016;12:1445–1453. doi:10.2147/TCRM.S110363
- Borghesi A, Michelini S, Nocivelli G, et al. Solid indeterminate pulmonary nodules less than or equal to 250 mm3: application of the updated Fleischner society guidelines in clinical practice. Radiol Res Pract. 2019;2019:7218258. doi:10.1155/2019/7218258
- Gould MK, Fletcher J, Iannettoni MD, et al. Evaluation of patients with pulmonary nodules: when is it lung cancer? ACCP evidence-based clinical practice guidelines (2nd edition). Chest. 2007;132:108S–130S. doi:10.1378/chest.07-1353
- Satoh S, Kitazume Y, Ohdama S, Kimula Y, Taura S, Endo Y. Can malignant and benign pulmonary nodules be differentiated with diffusion-weighted MRI? AJR Am J Roentgenol. 2008;191:464–470. doi:10.2214/AJR.07.3133
- You X, Sun X, Yang C, Fang Y. CT diagnosis and differentiation of benign and malignant varieties of solitary fibrous tumor of the pleura. Medicine. 2017;96:e9058. doi:10.1097/MD.0000000000009058
- Chu ZG, Sheng B, Liu MQ, Lv FJ, Li Q, Ouyang Y. Differential diagnosis of solitary pulmonary inflammatory lesions and peripheral lung cancers with contrast-enhanced computed tomography. Clinics. 2016;71:555–561. doi:10.6061/clinics/2016(10)01
- Xu C, Hao K, Song Y, Yu L, Hou Z, Zhan P. Early diagnosis of solitary pulmonary nodules. J Thorac Dis. 2013;5:830–840. doi:10.3978/j.issn.2072-1439.2013.11.19
- Li M, Ito H, Wada H, Tanaka F. Pit-fall sign on computed tomography predicts pleural involvement and poor prognosis in non-small cell lung cancer. Lung Cancer. 2004;46:349–355. doi:10.1016/j.lungcan.2004.05.017
- Yang ZG, Sone S, Takashima S, et al. High-resolution CT analysis of small peripheral lung adenocarcinomas revealed on screening helical CT. AJR Am J Roentgenol. 2001;176:1399–1407. doi:10.2214/ajr.176.6.1761399
- Hsu JS, Jaw TS, Yang CJ, et al. Convex border of peripheral non-small cell lung cancer on CT images as a potential indicator of pleural invasion. Medicine. 2017;96:e7323. doi:10.1097/MD.0000000000007323
- Fan L, Liu SY, Li QC, Yu H, Xiao XS. Multidetector CT features of pulmonary focal ground-glass opacity: differences between benign and malignant. Br J Radiol. 2012;85:897–904. doi:10.1259/bjr/33150223
- Gurney JW, Lyddon DM, McKay JA. Determining the likelihood of malignancy in solitary pulmonary nodules with Bayesian analysis. Part II. Application. Radiology. 1993;186:415–422. doi:10.1148/radiology.186.2.8421744
- Wang YX, Gong JS, Suzuki K, Morcos SK. Evidence based imaging strategies for solitary pulmonary nodule. J Thorac Dis. 2014;6:872–887. doi:10.3978/j.issn.2072-1439.2014.07.26
- Chu ZG, Zhang Y, Li WJ, Li Q, Zheng YN, Lv FJ. Primary solid lung cancerous nodules with different sizes: computed tomography features and their variations. BMC Cancer. 2019;19(1):1060. doi:10.1186/s12885-019-6274-0
- Xu DM, van Klaveren RJ, de Bock GH, et al. Role of baseline nodule density and changes in density and nodule features in the discrimination between benign and malignant solid indeterminate pulmonary nodules. Eur J Radiol. 2009;70:492–498. doi:10.1016/j.ejrad.2008.02.022
- Oda S, Awai K, Murao K, et al. Volume-doubling time of pulmonary nodules with ground glass opacity at multidetector CT: assessment with computer-aided three-dimensional volumetry. Acad Radiol. 2011;18:63–69. doi:10.1016/j.acra.2010.08.022
- Ray A, Mittal A, Vyas S. CT Halo sign: a systematic review. Eur J Radiol. 2020;124:108843. doi:10.1016/j.ejrad.2020.108843
- Pinto PS. The CT Halo sign. Radiology. 2004;230:109–110. doi:10.1148/radiol.2301020649
- Kim HY, Shim YM, Lee KS, Han J, Yi CA, Kim YK. Persistent pulmonary nodular ground-glass opacity at thin-section CT: histopathologic comparisons. Radiology. 2007;245:267–275. doi:10.1148/radiol.2451061682
- Aoki T, Nakata H, Watanabe H, et al. Evolution of peripheral lung adenocarcinomas: CT findings correlated with histology and tumor doubling time. AJR Am J Roentgenol. 2000;174:763–768. doi:10.2214/ajr.174.3.1740763
- Zhang R, Tian P, Qiu Z, Liang Y, Li W. The growth feature and its diagnostic value for benign and malignant pulmonary nodules met in routine clinical practice. J Thorac Dis. 2020;12(5):2019–2030. doi:10.21037/jtd-19-3591
