Abstract
Vitamin D deficiency and sepsis are both significant global health problems. Insufficient vitamin D is considered to be a pathogenically relevant factor of sepsis-related deaths; however, a causal relationship has not yet been demonstrated. Recently, vitamin D has been an exciting field of research owing to the identification of vitamin D receptors on many extra skeletal tissues and cells, suggesting an unexpected role on body physiology, beyond its effects on bone homeostasis. However, while the role of vitamin D on bone health is widely understood and has been successfully translated into clinical applications and public health policies, recent evidence supporting its role in other physiological and pathological processes has not been fully established. In sepsis, there is an induction of local intracellular vitamin D activity by most immune cells, including lymphocytes, macrophages, neutrophils, and dendritic cells, as well as vascular endothelial cells, to ensure efficient clearance of infective microorganisms and mediate anti-inflammatory and tolerogenic effects. The literature suggests an association between low vitamin D levels and sepsis, but clinical trials have yielded contradictory results. A greater understanding of this role may improve disease management. This article reviews the available knowledge regarding vitamin D in immune function, emerging literature regarding the association between its deficiency and sepsis, as well as presenting its potential effect on platelet leukocyte aggregations (PLAs), a significant pathology in sepsis. It also summarizes clinical trials involving vitamin D supplementation during critical illness and sepsis and addresses the impact of relevant factors of sepsis pathogenesis on the efficacy of vitamin D supplementation, which could contribute to the reported inconsistencies. Looking ahead, further studies are required to uncover the possible modulatory relationship between vitamin D and sepsis to define better cut-offs for its levels, proper timing of its administration, and the optimum dosage for best management.
Background
Vitamin D (VD), is a steroid hormone and a crucial nutrient that is reported to control a wide range of physiological processes.Citation1 Several sources of VD are available in the form of D2, known as ergocalciferol, and D3, known as cholecalciferol. VD2 is produced from ergosterol found mainly in fungi and also in some plants upon ultraviolet irradiation. Approximately 80% of VD3 is synthesized endogenously in the human skin as 7-dehydrocholesterol in response to ultraviolet ray exposure from sunlight, and about 20% is provided through diet.Citation2–Citation4 Inside the body, VD2 and VD3 undergo two consecutive steps of hydroxylation in the liver and then kidneys to be converted into their active compounds, 25(OH)D3 calcidiol (a clinical marker of plasmaV D level), and 1,25(OH)2D3 calcitriol, respectively.Citation5
In addition to its well-known effects on calcium and phosphate metabolism to ensure bone health, VD has an emerging immune modulatory effect. VD is involved immune system regulation; it regulates the action of suppressor T lymphocytes, the synthesis of cytokines, and acts by modulating the processes of cellular apoptosis.Citation6 In the 19th century, prior to the development of effective antibiotics, VD was serendipitously used to cure infections, such as tuberculosis, through sunlight exposure and administration of cod liver oil, which are the main sources of VD.Citation7–Citation11 The Nobel Prize for medicine or physiology in 1903 was awarded to Finsen for his contribution in treating lupus vulgaris, a skin disease caused by Mycobacterium tuberculosis with ultraviolet (UV) light.Citation12–Citation14 Since then, some cross-sectional studies have suggested an inverse correlation between lower levels of VD and increased infections, such as tuberculosis (TB) and upper respiratory tract infections. In 1977, it was reported that children with malnutritional rickets were more prone to lung infections associated with an apparent radiographic pulmonary abnormalities called “rachitic lung.”Citation15 However, little attention has been paid to these studies owing to the subsequent discovery and application of antibiotic therapy for infections.
Over the past three decades, Finsen’s work has received renewed attention as a consequence of multiple epidemiological studies showing a strong correlation between VD deficiency and the incidence of different infectious diseases, including pneumonia and sepsis.Citation16–Citation19 A significantly higher rate of such infections were reported during winter when exposure to sunlight, the major source of VD, is reduced.Citation20,Citation21 Since then, extensive studies of VD and incidence of infection have been published. Most of them focused on respiratory tract infections and consistently revealed the link between low VD plasma level (25(OH)D3 and the risk of acute respiratory infections.Citation22,Citation23 These findings were further confirmed by several randomized clinical trials (RCTs) that reported the protective effect of VD supplementation in reducing the risk of acute respiratory infections by 25% at doses of 400–1000 IU per day for 12 months, particularly in those with a baseline of <25 nmol/l.Citation24,Citation25
During sepsis, there is growing evidence that VD deficiency is strongly associated with sepsis risk, pathogenesis, and outcomes as described later,Citation26–Citation29 but to date, these data could not be applied clinically. Several clinical trials aimed at analyzing the effects of supplementing VD on the outcomes of critical illness including sepsis have reported contradictory results as shown in .
Table 1 Randomized Clinical Trial of Vitamin D Reinforcement in Critical Ill and Sepsis
Given the fact that sepsis is the most common cause of critical illness,Citation30 all RCTs investigating the effect of VD supplementation for critically ill and septic adult patients controlled with a placebo were retrieved. The databases of Pumped, Scopus, Medline, Embase, Web of Science, and Clinical Trials.gov were used to search for the following words: RCT, administration, supplementation, vitamin D, vitamin D2, vitamin D3, cholecalciferol, ergocalciferol, calcitriol, calcidiol, 25-hydroxyvitamin D3, 25(OH)D3, 1,25-dihydroxyvitamin D3, 1,25(OH)2D3, sepsis, critical ill, intensive care unit, septic shock. All critical and septic cases with low VD plasma levels (25(OH)D3 ≤50 nmol/l) at admission were eligible for inclusion.
In some studies, the administration of VD resulted in significant increases in leukocyte mRNA expression of cathelicidin (LL-37, antimicrobial peptide)Citation31 and plasma cathelicidin, significant reductions in IL-1β and 1L-6 among septic patients,Citation32 and showed a reduction in 30-day ICU readmission in septic cases, a lower hospital death rate among critically ill patients with severe VD deficiency (25(OH)D3 ≤30 nmol/l),Citation33 a significant decrease in the duration of hospital stay,Citation34 a reduction in the duration of respiratory support with mechanical ventilators and hospitalization, and a reduction in mortality rate in the ICU among the critically ill.Citation35 However, other studies demonstrated the ineffectiveness of VD supplementation on the mortality rate and duration of hospital stay.Citation31,Citation33,Citation36,Citation37
VD deficiency and sepsis are very common,Citation20,Citation26 and both conditions frequently coexist clinically. However, neither the effects of its deficiency on the pathogenesis of the disease and outcomes nor the effects of the disease itself on the correct assessment of VD status have yet been estimated. Such effects may contribute to the confusion about positivity and negativity of VD effects in the reported results so far. For the first time, this paper discusses the role of VD in the pathogenesis of sepsis with particular focus on platelet leukocyte aggregations (PLAs) and how it may reduce aggregate formation as well as their adherence to the endothelium to mitigate sepsis progression. It also addresses the impact of relevant factors of sepsis pathogenesis on the effectiveness of VD supplementation, which could contribute to clarification of the controversy in the reported results.
Association Between Vitamin D Deficiency and Sepsis
Sepsis is a life-threatening organ dysfunction caused by a dysregulated host response to an infection.Citation38 It is a fast-growing international health issue that imposes a substantial economic burden. Worldwide, it accounts for 48.9 million cases annually, resulting in more than 11 million deaths.Citation39 In the UK, around 250,000 cases and 44,000 deaths of sepsis are reported every year.Citation40,Citation41 In the United States, 1.7 million cases are diagnosed and 270,000 of these cases die from sepsis.Citation42 Very little is known about the estimates of the incidence and outcome of sepsis from developing countries, especially Saudi Arabia. However, all the available data confirm that sepsis is a serious cause of morbidity and mortality all over the world. Approximately two-thirds of septic patients are treated in intensive care units (ICU) with an annual estimated cost of £15.6 billion in the UKCitation41 and $24 billion in the United States.Citation39 The current management guidelines applied for sepsis, including antibiotics and fluid replacement, are crucial and lead to significant improvement in clinical outcomes and reduction in mortality.Citation43 However, some patients may still die and this maybe owing to the difficulty in assigning a patient’s disease course to the relative over- or under inflammation that may occur,Citation44 or microbial adaptation within the host in the form of acquiring resistance genes to the applied antibiotics (bacterial sepsis).Citation45 Development of new adjunctive therapy to improve the disease might be helpful in those cases when standard care is not sufficient.
Although there is no consensus in the literature about 25(OH)D plasma concentrations used to define VD deficiency, it is a highly prevalent condition worldwide.Citation46–Citation48 The minimum agreement to date is that maintaining a plasma level of 25(OH)D above 30 nmol/l shields against VD deficiency-associated bone disorders; a lower 25(OH)D level could be used to define VD deficiency and should be prevented and treated.Citation46 Based on the 25(OH)D cutoff of <30 nmol/l, VD deficiency is very common worldwideCitation49 with a reported prevalence of 13% in Europe, 5.9% in the United States, 7.4% in Canada, and more than 20% in many developing countries.Citation50 The reported worldwide prevalence is much higher when VD deficiency is defined as <50 nmol/l. It is estimated as 40% in Europe, 24% in the United States, 37% in Canada,Citation50 34–22% in Africa,Citation48 and 60% in Saudi Arabia.Citation51
Low VD plasma levels have been observed in 79% to 98% of critical care unit patients, involving septic cases.Citation52–Citation54 The risk of sepsis and its consequential outcomes such as death rate, length of hospital stay, and organ failure are positively correlated with VD deficiency.Citation52,Citation55 Trongtrakul and Feemuchang found that three-quarters of patients who are diagnosed with severe sepsis had a low plasma level of VD and a higher death rate, especially in cases where VD plasma levels were severely deficient (25(OH)D <30 nmol/l).Citation29 In the developed world, the number of new cases of sepsis as well as its related deaths are elevated during winter when low plasma levels of VD are detected,Citation17 albeit that various seasonal factors are also implicated.Citation56 Thus, restoring the VD to optimal plasma levels could have a valuable impact on sepsis development and outcomes. To date, the suggested recommended plasma concentrations of 25(OH)D that are considered sufficient or optimal vary in different settings. Concentrations of >50 nmol/l are sufficient for bone health maintenance, although concentrations of 60–75 nmol/l are suggested for optimal beneficial effects on bone health. However, several studies note that higher levels of above 75 nmol/l are needed for optimal function of the immune system.Citation57,Citation58 Thus, it is very important to conduct further studies to determine optimal plasma levels for VD to exert its extra skeletal functions. In the developing world, data regarding the link between VD deficiency and sepsis is very limited. However, with the high reported prevalence of VD deficiency, increasing hospital admissions of septic patients with VD deficiency are to be expected. Future studies to estimate the VD levels among patients with sepsis and to correlate plasma level with various inflammatory mediators and disease outcomes are required.
Vitamin D Mechanisms to Interfere with Sepsis Development
In (bacterial) sepsis, damage and stress signals resulting from invading microorganisms and associated inflammatory responses stimulate intracellular VD activity locally to ensure efficient clearance of the microorganisms and mediate anti-inflammatory and tolerogenic effects.Citation59 Stimulation of the TLR 2/1 pathway by binding to various pathogen-associated molecular patterns (PAMPs) results in induction of VDR and 1-α-hydroxylase (CYP27B1) genes. Inflammatory mediators such as IFNγ, IL-15, and IL-17A contribute to 1-α-hydroxylase (CYP27B1) gene activation.Citation60,Citation61 The majority of immune cells, such as lymphocytes, macrophages, neutrophils, and dendritic cells, as well as vascular endothelial cells have 25-hydroxyvitamin D-1α-hydroxylase (1α-OHase), which acts locally to convert 25-hydroxy-vitamin D (circulating form of VD), to its active compound (1,25(OH)2D).Citation62 Unlike the conventional renal activation of VD, this conversion is regulated by 25-hydroxy-vitamin D levels; thus, low concentration of 25-hydroxy-vitamin D interferes with its extra-skeletal actions.Citation63,Citation64 After activation, VD is translocated intracellularly, in association with a protein called VD binding protein and attach to its nuclear receptors (VDR) forming a complex. Subsequently, this complex attaches to the VD response element on DNA to regulate target gene transcription.Citation60,Citation65,Citation66
VD receptor is expressed by most immune system cells. Thus, signaling through the VD receptor intensifies the local innate immune response by augmenting the release of antimicrobial peptides (AMPs) such as cathelicidin and LL-37, the active form of cathelicidin.Citation67 Such AMPs induce a broad spectrum of antimicrobial activity mediated by cytokine release, including chemotaxis, phagocytosis, and programmed cell death.Citation68 VD reduces antigen presentation processes by decreasing the expression level of major histocompatibility complex (MHC) class II and co-stimulatory molecules, CD40, CD80, CD86, on antigen presenting cells (APCs) such as dendritic cells (DC), resulting in a more tolerogenic, immature state.Citation69 It also has various effects on the activation status of cells mediating adaptive immunity.Citation70–Citation72 VD suppresses T lymphocytes (Th1) and their release of IL-2 and interferon gamma (IFNγ).Citation73,Citation74 Furthermore, the intracellular downstream signaling initiated by the VD-VDR complex in the vascular endothelium reduces cell activity and inflammatory response.Citation75 Moreover, VD has an anti-inflammatory effect. Four hours pre-incubation of immune cells extracted from blood of normal individuals with 100 nM 1,25(OH)2D3, reduced the production of several pro-inflammatory mediators (such as IL-1β, TNF-α, and IFN-γ and IL-8), a 53-fold in response to their 24 h-treatment with bacterial stimulus [heat-killed pneumococcal serotype 19F (HK19F)].Citation76 Another study found that activating blood immune cells isolated from VD deficient samples with TLR stimuli released a wide range of proinflammatory mediators, which were significantly decreased after VD treatment.Citation77
VD exerts its anti-inflammatory actions by suppressing the gene expression of Toll-like receptor-2 and Toll-like receptor-4, reducing p38 and p42/42 phosphorylation and its downstream signaling, as well as decreasing the release of reactive oxygen species.Citation78 In addition, VD acts as a transcription factor to regulate the gene expression of several biological processes controlling immune response, such as cellular proliferation, differentiation, apoptosis, and angiogenesis.Citation79 Furthermore, VD deficiency results in disturbance of the gut microbiome,Citation80 which has an emerging role in sepsis pathogenesis as it increases the susceptibility to sepsis and enhances subsequent multiorgan failure.Citation81,Citation82 VD and VDR both play a significant role in maintaining the normal balance of gut microbiota,Citation83 which in turn boosts immunity against enteric and systemic pathogens.Citation84 Currently, multiple sepsis interventional approaches aimed at restoration of a balanced gut microbiota are under investigation.Citation82 Thus, VD could be used as an adjuvant to enhance their therapeutic effects.
Effect of Vitamin D on Platelet Leukocyte Aggregation
Platelet leukocyte aggregates are liberally generated during sepsis and correlate significantly with diseases severity.Citation85 The engagement of white blood cells (WBCs) with activated platelets producing cellular aggregates (moving freely in blood or attached to endothelial cells) contributed substantially to provoking and exacerbating organ dysfunction in septic cases.Citation86 Thus, targeting this pathology at a specific time point in disease duration may limit the severity of sepsis outcomes such as vascular occlusion, decreased blood supply, and organ failure. PLA formation and their attachment to the endothelial lining of the vascular system may be triggered and propagated by a broad spectrum of activated mediators, such as adhesion molecules, proinflammatory cytokines, chemokines, complements, and procoagulant factors, as illustrated in . This leads to sustained endothelial dysfunction, increased platelet and leukocyte reactivity, and activation of coagulation.Citation87
Figure 1 Vitamin D effects on the cellular interaction of platelets, leukocytes and endothelium. Stimulation of platelets, leukocytes and endothelium by PAMPs, DAMPs and the released mediators in response to such stimulation results in the attachment of platelets to leukocytes and generating platelet leukocyte aggregates moving freely in the circulatory system or fixed to the stimulated endothelium. Several molecular interactions, which could be interfered by the action of VD, mediate this pathological phenomenon including P-Selectin with PSGL-1 and αMβ2 integrin with GPIbα, (TREM-1) with its ligand on neutrophil, platelet JAM-3 with neutrophil αMβ2 integrin, and LFA-1 to its legend on platelet. These interactions induce further cell activation resulting in increased surface expression of adhesion receptors, degranulation, release of CD40L, Angiopoietin 2 (Ang2), inflammatory mediators, reactive oxygen species, tissue factor expression, microparticles release, thrombus and neutrophil extracellular trap formation (NET). Sites of Vitamin D (VD) effects are indicated in green. The figure was created using Biorender.com.
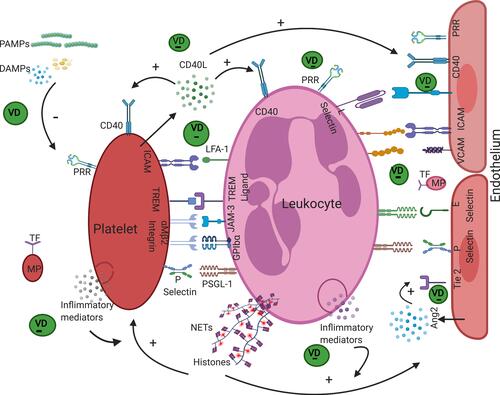
As VDR is expressed genetically by most of the cells implicated in PLA formation, such as lymphocytes, neutrophils, macrophages, and vascular endothelial cells,Citation88 VD may affect the formation of these aggregates. Recently, it was shown that healthy individuals with deficient VD have a high level of circulating PLAs and leukocyte endothelial adhesion.Citation89 However, there is no reported clinical data of the effect of VD administration on the extent of PLAs. It has also been found that VD reduces the expression of adhesion molecules required for platelet activationCitation90 and decreases homotypic platelet aggregation and platelet–platelet complexes.Citation91 Therefore, it becomes crucial to consider the impact of VD and VDR in the formation of PLAs.
The first immune defense after infection is initiated by recognizing pathogen associated molecular patterns (PAMPs) and the released damage associated molecular pattern from tissue (DAMPs) by recognition receptors (RRs) such as toll-like receptors (TLRs). RRs are expressed by leukocytes and to a certain degree by endothelial cells and platelets, and result in the stimulation of intracellular signaling pathways and release of inflammatory cytokines such as IL-1, TNFα, IL-6, IL-8, and IL-12.Citation92–Citation94 VD downregulates the gene expression of TLRs, thus reducing inflammatory responses.Citation77,Citation95 Moreover, VD decreases many proinflammatory mediators such as IL-6, IL-8, and TNFα,Citation96 which themselves induce the stimulation of leukocytes, platelets, and endothelial cells and lead to the formation of PLAs.
The plasma level of VD is inversely correlated with mean platelet volume (MPV), a marker for platelet activity, noting that platelets with larger size are more active.Citation97,Citation98 Activated platelets secrete CD40 ligand (CD40-L) into the blood, which interacts with CD40 receptors present on platelets to enhance its activation,Citation99 and on leukocytes to augment its activation and generation of reactive oxygen species, ROS.Citation100 Furthermore, it may bind to CD40 receptors expressed on vascular endothelial cells amplifying its activation and its expression of several adhesion molecules, such as ICAM and VCAM, on their surface and its production of the chemokine CCL2, thus mediating leukocyte recruitment.
Recently, it has been found that the administration of VD reduces gene expression of the CD40 ligand by blood cells,Citation101 and serum levels of ICAM and VCAM that are secreted by stimulated endothelium.Citation102 Angiopoietin 2 (Ang-2) is produced immediately from endothelial Weibel–Palade bodies upon their activation. Ang-2 interacts with Tie 2 receptors in a competitive manner to inhibit the protective effect of Ang-1,Citation103,Citation104 strengthen the activation of endothelium, and exaggerate the inflammation.Citation105 Additionally, it interacts synergistically with other inflammatory cytokines to boost their actions; for example, sensitizing the vascular endothelium to stimulation with TNFα.Citation106,Citation107 It also induces the direct activation of polymorphonuclear cells towards the proinflammatory state.Citation108 VD administration causes a considerable reduction in the serum level of angiopoietin-2.Citation109 The interaction of triggering receptors expressed on myeloid cells (TREM-1) on platelets with their ligand on leukocytes is another mediator of PLAs. VD has been reported to inhibit the induced expression of TREM-1 in vitro.Citation110
Tissue factor is expressed by activated endothelium, platelets, leukocytes,Citation111–Citation113 and their released microparticles, bearing procoagulant and proinflammatory properties, upon activation.Citation114,Citation115 It stimulates the extrinsic pathway of coagulation and generates thrombin and fibrinogen. These are important for the following reasons: Thrombin enhances adhesion molecule expression on the surface of the vascular endothelium such as E and P selection and production of von Willebrand factor (VWF) as well as several soluble secretory products, including platelet activating factor, Il-8, and angiopoietin 2.Citation116–Citation120 Fibrinogen stabilizes platelet leukocyte endothelial cell interaction by binding to Mac-1 on leukocytic cells and GPIIb/IIIa (αIIbβ3) on platelets and it also binds to CD11b/CD18 on leukocytic cells and intracellular adhesion molecule −1 (ICAM-1) on endothelial cells.Citation121
VD downregulates the expression of tissue factor in vitro. Treatment of an activated (inflamed) endothelial cell line with VD suppresses gene expression for tissue factors and adhesion molecules.Citation122 Furthermore, treatment with VD in patients with chronic kidney disease who have endothelial dysfunction with high serum levels of circulating microparticles leads to a significant reduction in the level of microparticles;Citation123 it has also been found to inhibit microparticle release from a human endothelial cell line after their exposure to oxidative stress.Citation124 Moreover, supplementing high doses of VD decreases thrombin production in severely VD deficient patients.Citation125
Potential Confounders of Vitamin D Supplementation in Sepsis
The current in vitro and observational data, discussed in this review, argue for the usefulness of VD supplementation in sepsis. Several RCTs, as summarized in , have been conducted to evaluate the effect of VD supplementation on the clinical outcome of critical illnesses including sepsis, but their results are contradictory, and the correlation has not been confirmed in all studies.
The results obtained to date warrant further in-depth studies to determine the underlying mechanisms or factors that interfere with yielding the expected protective influences of VD on the progression and outcomes from sepsis. Herein, this part of the review addresses several factors which may confound the effectiveness of VD supplementation.
First, variability in the applied intervention methods regarding the dose, form, route, and duration of VD supplementation as well as heterogeneity of the population and sample size seen across the published RCTs could largely contribute to the reported mixed results. Second, there is a lack of clarity and consistency regarding definitions of VD deficiency and sufficiency in the literature, which produce a significant variation in the criteria of VD deficiency among RCTs.Citation50,Citation126,Citation127 Thus, non-VD deficient cases could be involved in RCTs and have influenced the reported results.
Third, there is an inadequacy of prospective studies to determine the optimal VD dose for extra skeletal tissue functions.Citation128 Moreover, the exact plasma concentration of VD to replace an insufficiency is controversial in patients with sepsis and its measurement must be interpreted cautiously. VD is physiologically distributed as free (0.3%), or bound to either VD binding protein (DBP; 85%) or albumin (15%). Both DBP level and polymorphic variants affect the bioavailability of VD (free and bound to albumin) and this could affect the response to supplementation. In some patients, DBP is low due to protein catabolismCitation129 or leakage to the extracellular matrix owing to increased vascular permeability.Citation130 In the laboratory, VD sufficiency is commonly determined by measuring the plasma level of total vitamin 25(OH)D.Citation131 Thus, the bioavailability of free VD (active form) is increased and it can diffuse into most body cells.Citation132,Citation133 Additionally, the higher rate of polymorphism in the gene encoding VDBP (GC gene) results in DBP isoforms with different binding affinities to VD,Citation134,Citation135 which affect the bioavailability of VD. Additional VD supplementation therefore requires attention because it may lead to higher risk of hypercalcemia.Citation63 Approximately 1% of mild hypercalcemia not requiring clinical intervention was reported among septic patients following VD administration.Citation54 At the molecular level, any increase in extracellular (serum) calcium ions affects the inflammatory response. Extracellular Ca2+ act as a danger signal (DAMPs), and amplify inflammation.Citation136 Moreover, septic patients may be classified as VD deficient based on their plasma level of total vitamin 25(OH)D, even if the bioactive free form of VD is within normal values.Citation130 Consequently, supplementation of VD may not produce the expected positive effects.
VD levels are important in the maintenance of circulating immunoglobulins and complement. A large study has documented, for low levels of VD, a positive association with IgG2 and C4 and a negative association for IgA (complement binding), IgG1, and C3.Citation137 These changes are of particular relevance in cases of systemic inflammatory reactions, which draw initially on intact mucosal barriers and a humoral component of the immune response. Depletion of complement components following overactivation and deposition in tissue (consumption in the blood phase) may be a determinant of overall outcome.Citation138,Citation139 Sepsis associated multiple-organ dysfunction, in which complement activation products and complement dysregulation have an undisputed pathogenic role, may well be influenced by specifically targeting pathways or components thereof. However, the timing of such an intervention for it to be effective and efficient may be difficult to judge.Citation140 Septic patients suffer initially from an uncontrolled excessive inflammatory response followed by immune suppression.Citation141 Therefore, proper timing of VD supplementation could significantly influence its supplementation outcomes. Modes of action may include pleiotropic effects on lipid metabolism, which are altered in the context of sepsis.Citation142 VD deficiency is correlated with high levels of cholesterol, triglycerides, and low-density lipoproteins (LDL).Citation143 VD supplementation leads to a reduction in lipid parameters, total cholesterol, very-low-density lipoproteins (VLDL), LDL, and triglycerides,Citation144 which could be falsely interpreted as sepsis, because low lipid profiles are positively associated with poor sepsis outcome.Citation145,Citation146 Thus, it appears that timing and dose of VD supplementation in sepsis influence its effectiveness and this could be a contributing factor to the controversy in the reported results of interventional studies. Detailed understanding of the molecular basis of VD metabolism and the regulation of its bioavailability during the course of sepsis is required to determine its effectiveness.
Conclusion
VD deficiency is prevalent in cases with sepsis;Citation26 thus, its deficiency may affect disease pathogenesis and aggravate the condition. VD has immunomodulatory effects. Restoration of VD plasma levels may limit disease progression through decreasing the abundance of DAMPs and PAMPs, inflammatory mediators, extent of PLAs formation and their adhesion to endothelia, and balancing the gut microbiota to fight systemic and enteric pathogens. However, data available from the literature regarding the influence of VD supplementation are conflicting in patients with sepsis and disagree with current evidence from in vitro and observational studies. Several factors may underestimate the expected positive effects. Further studies are warranted to elucidate the molecular interactions of VD with different players involved in the pathogenesis of sepsis (immune homeostasis). Thus, proper timing of VD supplementation, dose, form, and its sufficient plasma levels to be effective, as well as identifying patients who may benefit the most from supplementation with VD, need to be accurately determined in future clinical trials. Targeting the population with severe VD deficiency below 30 nmol/l is highly suggested for future trials.
Acknowledgments
The author would like to thank Prof. Suhad Bahijri, and Dr. Cordula Stover for her support in reviewing this manuscript.
Disclosure
The author declares no conflicts of interest.
References
- Tintut Y, Demer LL. Potential impact of the steroid hormone, vitamin D, on the vasculature vitamin D-hormones and cardiovascular disease. Am Heart J. 2021;239:147–153. doi:10.1016/j.ahj.2021.05.012
- Christakos S, Dhawan P, Verstuyf A, Verlinden L, Carmeliet G. Vitamin D: metabolism, molecular mechanism of action, and pleiotropic effects. Physiol Rev. 2016;96:365–408. doi:10.1152/physrev.00014.2015
- Tripkovic L, Lambert H, Hart K, et al. Comparison of vitamin D2 and vitamin D3 supplementation in raising serum 25-hydroxyvitamin D status: a systematic review and meta-analysis. Am J Clin Nutr. 2012;95:1357–1364. doi:10.3945/ajcn.111.031070
- Siddiqui M, Manansala JS, Abdulrahman HA, et al. Immune modulatory effects of vitamin D on viral infections. Nutrients. 2020;12:2879. doi:10.3390/nu12092879
- Demer LL, Hsu JJ, Tintut Y. Steroid hormone vitamin D: implications for cardiovascular disease. Circ Res. 2018;122:1576–1585. doi:10.1161/CIRCRESAHA.118.311585
- Dattola A, Silvestri M, Bennardo L, et al. Role of vitamins in skin health: a systematic review. Curr Nutr Rep. 2020;1–10.
- Aranow C. Vitamin D and the immune system. J Investig Med. 2011;59:881–886. doi:10.2310/JIM.0b013e31821b8755
- Grant AH. The effect of Rachitic diets on experimental tuberculosis: III. resistance to tuberculosis decreased by adding codliver oil. Am Rev Tuberc. 1930;21:102–114.
- Banyai AL. Topical application of codliver oil in tuberculosis: a preliminary report. Am Rev Tuberc. 1937;36:250–258.
- Coulter JS, Carter HA. The treatment of pulmonary tuberculosis by ultraviolet radiation. J Am Med Assoc. 1935;105:171–174. doi:10.1001/jama.1935.02760290005003
- Gerstenberger HJ, Burhans CW. Treatment of extensive pulmonary tuberculosis with ultraviolet rays. Am J Dis Child. 1927;33:54–73.
- Finsen NR. Phototherapy: 1. The Chemical Rays of Light and Smallpox, 2. Light as a Stimulant, 3. The Treatment of Lupus Vulgarisby Concentrated Chemical Rays. Arnold; 1901.
- Lang PO, Aspinall R. Vitamin D status and the host resistance to infections: what it is currently (not) understood. Clin Ther. 2017;39:930–945. doi:10.1016/j.clinthera.2017.04.004
- Semon HC. The treatment of lupus erythematosus by krysolgan. Br Med J. 1927;2(3475):258. doi:10.1136/bmj.2.3475.258
- Khajavi A, Amirhakimi G. The rachoitic lung: pulmonary findings in 30 infants and children with malnutritional rickets. Clin Pediatr (Phila). 1977;16:36–38. doi:10.1177/000992287701600106
- Laaksi I, Ruohola J-P, Tuohimaa P, et al. An association of serum vitamin D concentrations< 40 nmol/L with acute respiratory tract infection in young Finnish men. Am J Clin Nutr. 2007;86:714–717.
- Upala S, Sanguankeo A, Permpalung N. Significant association between vitamin D deficiency and sepsis: a systematic review and meta-analysis. BMC Anesthesiol. 2015;15:1–11. doi:10.1186/s12871-015-0063-3
- Zhou W, Mao S, Wu L, Yu J. Association between vitamin D status and sepsis. Clin Lab. 2018;64:451–460. doi:10.7754/Clin.Lab.2017.170919
- McNally JD, Leis K, Matheson LA, Karuananyake C, Sankaran K, Rosenberg AM. Vitamin D deficiency in young children with severe acute lower respiratory infection. Pediatr Pulmonol. 2009;44:981–988. doi:10.1002/ppul.21089
- Kempker JA, Han JE, Tangpricha V, Ziegler TR, Martin GS. Vitamin D and sepsis: an emerging relationship. Dermato-Endocrinology. 2012;4:101–108. doi:10.4161/derm.19859
- Yamshchikov A, Desai N, Blumberg H, Ziegler T, Tangpricha V. Vitamin D for treatment and prevention of infectious diseases: a systematic review of randomized controlled trials. Endocr Pract. 2009;15:438–449. doi:10.4158/EP09101.ORR
- F Gunville C, M Mourani P, A Ginde A. The role of vitamin D in prevention and treatment of infection. Inflamm Allergy Drug Targets. 2013;12:239–245.
- Ginde AA, Mansbach JM, Camargo CA. Association between serum 25-hydroxyvitamin D level and upper respiratory tract infection in the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2009;169:384–390. doi:10.1001/archinternmed.2008.560
- Martineau AR, Jolliffe DA, Hooper RL, et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ. 2017;356.
- Jolliffe DA, Camargo CA Jr, Sluyter JD, et al. Vitamin D supplementation to prevent acute respiratory infections: a systematic review and meta-analysis of aggregate data from randomised controlled trials. Lancet Diabetes Endocrinol. 2021;9(5):276–292. doi:10.1016/S2213-8587(21)00051-6
- Shojaei M, Sabzeghabaei A, Barhagh HV, Soltani S. The correlation between serum level of vitamin D and outcome of sepsis patients; a cross-sectional study. Arch Acad Emerg Med. 2019;7.
- El-Gendy FM, Khattab AA, Naser RG, Abdel-Aziz AA. Association between vitamin D deficiency and sepsis in pediatric ICU. Menoufia Med J. 2021;34:210. doi:10.4103/mmj.mmj_210_19
- Li Y, Ding S. Serum 25-Hydroxyvitamin D and the risk of mortality in adult patients with Sepsis: a meta-analysis. BMC Infect Dis. 2020;20:1–10.
- Trongtrakul K, Feemuchang C. Prevalence and association of vitamin D deficiency and mortality in patients with severe sepsis. Int J Gen Med. 2017;10:415. doi:10.2147/IJGM.S147561
- Loftus TJ, Mira JC, Ozrazgat-Baslanti T, et al. Sepsis and critical illness research center investigators: protocols and standard operating procedures for a prospective cohort study of sepsis in critically ill surgical patients. BMJ Open. 2017;7:e015136. doi:10.1136/bmjopen-2016-015136
- Leaf DE, Raed A, Donnino MW, Ginde AA, Waikar SS. Randomized controlled trial of calcitriol in severe sepsis. Am J Respir Crit Care Med. 2014;190:533–541. doi:10.1164/rccm.201405-0988OC
- Quraishi SA, De Pascale G, Needleman JS, et al. Effect of cholecalciferol supplementation on vitamin D status and cathelicidin levels in sepsis: a randomized, placebo-controlled trial. Crit Care Med. 2015;43:1928. doi:10.1097/CCM.0000000000001148
- Amrein K, Schnedl C, Holl A, et al. Effect of high-dose vitamin D3 on hospital length of stay in critically ill patients with vitamin D deficiency: the VITdAL-ICU randomized clinical trial. JAMA. 2014;312:1520–1530. doi:10.1001/jama.2014.13204
- Han JE, Jones JL, Tangpricha V, et al. High dose vitamin D administration in ventilated intensive care unit patients: a pilot double blind randomized controlled trial. J Clin Transl Endocrinol. 2016;4:59–65. doi:10.1016/j.jcte.2016.04.004
- Miri M, Kouchek M, Dahmardeh AR, Sistanizad M. Effect of high-dose vitamin D on duration of mechanical ventilation in ICU patients. Iran J Pharm Res. 2019;18:1067.
- Amrein K, Sourij H, Wagner G, et al. Short-term effects of high-dose oral vitamin D3 in critically ill vitamin D deficient patients: a randomized, double-blind, placebo-controlled pilot study. Crit Care. 2011;15:1–7. doi:10.1186/cc10120
- National Heart, L.; Network, B.I.P.C.T. Early high-dose vitamin D3 for critically ill, vitamin D–deficient patients. N Engl J Med. 2019;381:2529–2540. doi:10.1056/NEJMoa1911124
- Marik PE, Taeb AM. SIRS, qSOFA and new sepsis definition. J Thorac Dis. 2017;9:943. doi:10.21037/jtd.2017.03.125
- Rudd KE, Johnson SC, Agesa KM, et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the Global Burden of Disease Study. Lancet. 2020;395:200–211. doi:10.1016/S0140-6736(19)32989-7
- Dave M, Barry S, Coulthard P, et al. An evaluation of sepsis in dentistry. Br Dent J. 2021;230:351–357. doi:10.1038/s41415-021-2724-6
- Daniels R. Sepsis: the silent killer we should be stopping. Int J First Aid Educ. 2018;1:23. doi:10.21038/ijfa.2018.0005
- Rhee C, Dantes R, Epstein L, et al. Incidence and trends of sepsis in US hospitals using clinical vs claims data, 2009–2014. JAMA. 2017;318:1241–1249. doi:10.1001/jama.2017.13836
- Plevin R, Callcut R. Update in sepsis guidelines: what is really new? Trauma Surg Acute Care Open. 2017;2:e000088. doi:10.1136/tsaco-2017-000088
- Hotchkiss RS, Monneret G, Payen D. Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat Rev Immunol. 2013;13:862–874. doi:10.1038/nri3552
- Khademi SH, Sazinas P, Jelsbak L. Within-host adaptation mediated by intergenic evolution in Pseudomonas aeruginosa. Genome Biol Evol. 2019;11:1385–1397. doi:10.1093/gbe/evz083
- Roth DE, Abrams SA, Aloia J, et al. Global prevalence and disease burden of vitamin D deficiency: a roadmap for action in low-and middle-income countries. Ann N Y Acad Sci. 2018;1430:44. doi:10.1111/nyas.13968
- Giustina A, Adler R, Binkley N, et al. Consensus statement from 2 nd International Conference on Controversies in Vitamin D. Rev Endocr Metab Disord. 2020;21:89.
- Mogire RM, Mutua A, Kimita W, et al. Prevalence of vitamin D deficiency in Africa: a systematic review and meta-analysis. Lancet Glob Health. 2020;8:e134–e142. doi:10.1016/S2214-109X(19)30457-7
- Pilz S, Zittermann A, Trummer C, et al. Vitamin D testing and treatment: a narrative review of current evidence. Endocr Connect. 2019;8:R27–R43.
- Amrein K, Scherkl M, Hoffmann M, et al. Vitamin D deficiency 2.0: an update on the current status worldwide. Eur J Clin Nutr. 2020;74:1498–1513. doi:10.1038/s41430-020-0558-y
- Al-Alyani H, Al-Turki HA, Al-Essa ON, Alani FM, Sadat-Ali M. Vitamin D deficiency in Saudi Arabians: a reality or simply hype: a meta-analysis (2008–2015). J Family Community Med. 2018;25:1.
- Kempker JA, Tangpricha V, Ziegler TR, Martin GS. Vitamin D in sepsis: from basic science to clinical impact. Crit Care. 2012;16:316. doi:10.1186/cc11252
- Azim A, Ahmed A, Yadav S, et al. Prevalence of vitamin D deficiency in critically ill patients and its influence on outcome: experience from a tertiary care centre in North India (an observational study). J Intensive Care. 2013;1:1–5. doi:10.1186/2052-0492-1-14
- Amrein K, Papinutti A, Mathew E, Vila G, Parekh D. Vitamin D and critical illness: what endocrinology can learn from intensive care and vice versa. Endocr Connect. 2018;7:R304–R315. doi:10.1530/EC-18-0184
- de Haan K, Groeneveld AJ, de Geus HR, Egal M, Struijs A. Vitamin D deficiency as a risk factor for infection, sepsis and mortality in the critically ill: systematic review and meta-analysis. Crit Care. 2014;18:660. doi:10.1186/s13054-014-0660-4
- Fares A. Factors influencing the seasonal patterns of infectious diseases. Int J Prev Med. 2013;4:128.
- Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–1930. doi:10.1210/jc.2011-0385
- Helde Frankling M, Norlin A-C, Hansen S, Wahren Borgström E, Bergman P, Björkhem-Bergman L. Are vitamin D3 tablets and oil drops equally effective in raising S-25-hydroxyvitamin D concentrations? A post-hoc analysis of an observational study on immunodeficient patients. Nutrients. 2020;12:1230. doi:10.3390/nu12051230
- Chirumbolo S, Bjørklund G, Sboarina A, Vella A. The role of vitamin D in the immune system as a pro-survival molecule. Clin Ther. 2017;39:894–916. doi:10.1016/j.clinthera.2017.03.021
- Medrano M, Carrillo-Cruz E, Montero I, Perez-Simon JA. Vitamin D: effect on haematopoiesis and immune system and clinical applications. Int J Mol Sci. 2018;19:2663. doi:10.3390/ijms19092663
- Shin DM, Yuk JM, Lee HM, et al. Mycobacterial lipoprotein activates autophagy via TLR2/1/CD14 and a functional vitamin D receptor signalling. Cell Microbiol. 2010;12:1648–1665.
- Lagishetty V, Liu NQ, Hewison M. Vitamin D metabolism and innate immunity. Mol Cell Endocrinol. 2011;347:97–105. doi:10.1016/j.mce.2011.04.015
- Nair P, Venkatesh B, Center JR. Vitamin D deficiency and supplementation in critical illness—the known knowns and known unknowns. Crit Care. 2018;22:276. doi:10.1186/s13054-018-2185-8
- Ahari MH, Pishbin E. Vitamin D and sepsis. Rev Clin Med. 2014;1:225–228.
- Christakos S, Dhawan P, Benn B, et al. Vitamin D: molecular mechanism of action. Ann N Y Acad Sci. 2007;1116:340–348. doi:10.1196/annals.1402.070
- Jeon S-M, Shin E-A. Exploring vitamin D metabolism and function in cancer. Exp Mol Med. 2018;50:20. doi:10.1038/s12276-018-0038-9
- Chung C, Silwal P, Kim I, Modlin RL, Jo E-K. Vitamin D-cathelicidin axis: at the crossroads between protective immunity and pathological inflammation during infection. Immune Netw. 2020;20. doi:10.4110/in.2020.20.e12
- Van Harten RM, Van Woudenbergh E, Van Dijk A, Haagsman HP. Cathelicidins: immunomodulatory antimicrobials. Vaccines. 2018;6:63. doi:10.3390/vaccines6030063
- Prietl B, Treiber G, Pieber TR, Amrein K. Vitamin D and immune function. Nutrients. 2013;5:2502–2521. doi:10.3390/nu5072502
- Hewison M. Antibacterial effects of vitamin D. Nat Rev Endocrinol. 2011;7:337. doi:10.1038/nrendo.2010.226
- Gombart AF. The vitamin D–antimicrobial peptide pathway and its role in protection against infection. Future Microbiol. 2009;4:1151–1165. doi:10.2217/fmb.09.87
- Youssef DA, Miller CW, El-Abbassi AM, et al. Antimicrobial implications of vitamin D. Dermato-Endocrinology. 2011;3:220–229. doi:10.4161/derm.3.4.15027
- Wei R, Christakos S. Mechanisms underlying the regulation of innate and adaptive immunity by vitamin D. Nutrients. 2015;7:8251–8260. doi:10.3390/nu7105392
- Sheikh V, Kasapoglu P, Zamani A, Basiri Z, Tahamoli-Roudsari A, Alahgholi-Hajibehzad M. Vitamin D3 inhibits the proliferation of T helper cells, downregulate CD4+ T cell cytokines and upregulate inhibitory markers. Hum Immunol. 2018;79:439–445. doi:10.1016/j.humimm.2018.03.001
- Bozic M, Álvarez Á, de Pablo C, et al. Impaired vitamin D signaling in endothelial cell leads to an enhanced leukocyte-endothelium interplay: implications for atherosclerosis development. PLoS One. 2015;10:e0136863. doi:10.1371/journal.pone.0136863
- Hoe E, Nathanielsz J, Toh ZQ, et al. Anti-inflammatory effects of vitamin D on human immune cells in the context of bacterial infection. Nutrients. 2016;8:806. doi:10.3390/nu8120806
- Ojaimi S, Skinner NA, Strauss BJ, Sundararajan V, Woolley I, Visvanathan K. Vitamin D deficiency impacts on expression of toll-like receptor-2 and cytokine profile: a pilot study. J Transl Med. 2013;11:1–7. doi:10.1186/1479-5876-11-176
- Calton EK, Keane KN, Newsholme P, Soares MJ. The impact of vitamin D levels on inflammatory status: a systematic review of immune cell studies. PLoS One. 2015;10:e0141770. doi:10.1371/journal.pone.0141770
- Umar M, Sastry KS, Chouchane AI. Role of vitamin D beyond the skeletal function: a review of the molecular and clinical studies. Int J Mol Sci. 2018;19:1618. doi:10.3390/ijms19061618
- Haak BW, Wiersinga WJ. The role of the gut microbiota in sepsis. Lancet Gastroenterol Hepatol. 2017;2:135–143. doi:10.1016/S2468-1253(16)30119-4
- Haak BW, Prescott HC, Wiersinga WJ. Therapeutic potential of the gut microbiota in the prevention and treatment of sepsis. Front Immunol. 2018;9:2042. doi:10.3389/fimmu.2018.02042
- Miller WD, Keskey R, Alverdy JC. Sepsis and the microbiome: a vicious cycle. J Infect Dis. 2021;223:S264–S269.
- Akimbekov NS, Digel I, Sherelkhan DK, Lutfor AB, Razzaque MS. Vitamin D and the host-gut microbiome: a brief overview. Acta Histochem Cytochem. 2020;53:33–42. doi:10.1267/ahc.20011
- Bassetti M, Bandera A, Gori A. Therapeutic potential of the gut microbiota in the management of sepsis. Crit Care. 2020;24:1–7. doi:10.1186/s13054-020-2780-3
- Russwurm S, Vickers J, Meier-Hellmann A, et al. Platelet and leukocyte activation correlate with the severity of septic organ dysfunction. Shock. 2002;17:263–268. doi:10.1097/00024382-200204000-00004
- Wang X, Qin W, Sun B. New strategy for sepsis: targeting a key role of platelet-neutrophil interaction. Burns Trauma. 2014;2:2321–3868.135487
- Alharbi A, Thomas R, Ali M, Thompson J, Stover C. Factors in homo and heterotypic aggregate formation in sepsis. Sepsis. 2016:1–11. https://leicester.figshare.com/articles/chapter/Factors_in_Homo_and_Heterotypic_Aggregate_Formation_in_Sepsis/10235075.
- Mohammad S, Mishra A, Ashraf MZ. Emerging role of vitamin D and its associated molecules in pathways related to pathogenesis of thrombosis. Biomolecules. 2019;9:649. doi:10.3390/biom9110649
- Tay HM, Yeap WH, Dalan R, Wong SC, Hou HW. Increased monocyte‐platelet aggregates and monocyte‐endothelial adhesion in healthy individuals with vitamin D deficiency. FASEB J. 2020;34:11133–11142. doi:10.1096/fj.202000822R
- Yildirim T, Solmaz D, Akgol G, Ersoy Y. Relationship between mean platelet volume and vitamin D deficiency in fibromyalgia. Biomed Res. 2016;27(4).
- Sultan M, Twito O, Tohami T, Ramati E, Neumark E, Rashid G. Vitamin D diminishes the high platelet aggregation of type 2 diabetes mellitus patients. Platelets. 2019;30:120–125. doi:10.1080/09537104.2017.1386298
- Sriskandan S, Altmann D. The immunology of sepsis. J Pathol. 2008;214:211–223. doi:10.1002/path.2274
- Matsuda A, Jacob A, Wu R, et al. Novel therapeutic targets for sepsis: regulation of exaggerated inflammatory responses. J Nippon Med Sch. 2012;79:4–18. doi:10.1272/jnms.79.4
- Cognasse F, Hamzeh H, Chavarin P, Acquart S, Genin C, Garraud O. Evidence of Toll‐like receptor molecules on human platelets. Immunol Cell Biol. 2005;83:196–198. doi:10.1111/j.1440-1711.2005.01314.x
- Sadeghi K, Wessner B, Laggner U, et al. Vitamin D3 down‐regulates monocyte TLR expression and triggers hyporesponsiveness to pathogen‐associated molecular patterns. Eur J Immunol. 2006;36:361–370. doi:10.1002/eji.200425995
- Adegoke SA, Smith OS, Adekile AD, Figueiredo MS. Relationship between serum 25-hydroxyvitamin D and inflammatory cytokines in paediatric sickle cell disease. Cytokine. 2017;96:87–93. doi:10.1016/j.cyto.2017.03.010
- Korzonek-Szlacheta I, Hudzik B, Nowak J, et al. Mean platelet volume is associated with serum 25-hydroxyvitamin D concentrations in patients with stable coronary artery disease. Heart Vessels. 2018;33:1275–1281. doi:10.1007/s00380-018-1182-9
- Park YC, Kim J, Seo MS, Hong SW, Cho ES, Kim J-K. Inverse relationship between vitamin D levels and platelet indices in Korean adults. Hematology. 2017;22:623–629. doi:10.1080/10245332.2017.1318334
- Stokes KY, Granger DN. Platelets: a critical link between inflammation and microvascular dysfunction. J Physiol. 2012;590:1023–1034. doi:10.1113/jphysiol.2011.225417
- Rahman M, Roller J, Zhang S, et al. Metalloproteinases regulate CD40L shedding from platelets and pulmonary recruitment of neutrophils in abdominal sepsis. Inflamm Res. 2012;61:571–579. doi:10.1007/s00011-012-0446-6
- Sharifi A, Vahedi H, Honarvar MR, et al. Vitamin D decreases CD40L gene expression in ulcerative colitis patients: a randomized, double-blinded, placebo-controlled trial. Turk J Gastroenterol. 2020;31:99. doi:10.5152/tjg.2020.181028
- Naeini AE, Moeinzadeh F, Vahdat S, Ahmadi A, Hedayati ZP, Shahzeidi S. The effect of vitamin D administration on intracellular adhesion molecule-1 and vascular cell adhesion molecule-1 levels in hemodialysis patients: a placebo-controlled, double-blinded clinical trial. J Res Pharm Pract. 2017;6:16. doi:10.4103/2279-042X.200994
- David S, Kümpers P, van Slyke P, Parikh SM. Mending leaky blood vessels: the angiopoietin-Tie2 pathway in sepsis. J Pharmacol Exp Ther. 2013;345:2–6. doi:10.1124/jpet.112.201061
- Hegen A, Koidl S, Weindel K, Marmé D, Augustin HG, Fiedler U. Expression of angiopoietin-2 in endothelial cells is controlled by positive and negative regulatory promoter elements. Arterioscler Thromb Vasc Biol. 2004;24:1803–1809.
- El-Banawy HS, Gaber EW, Maharem DA, Matrawy KA. Angiopoietin-2, endothelial dysfunction and renal involvement in patients with systemic lupus erythematosus. J Nephrol. 2012;25:541–550. doi:10.5301/jn.5000030
- Graham SM, Rajwans N, Tapia KA, et al. A prospective study of endothelial activation biomarkers, including plasma angiopoietin-1 and angiopoietin-2, in Kenyan women initiating antiretroviral therapy. BMC Infect Dis. 2013;13:1–11. doi:10.1186/1471-2334-13-263
- Fiedler U, Reiss Y, Scharpfenecker M, et al. Angiopoietin-2 sensitizes endothelial cells to TNF-α and has a crucial role in the induction of inflammation. Nat Med. 2006;12:235–239. doi:10.1038/nm1351
- Mussap M, Cibecchini F, Noto A, Fanos V. In search of biomarkers for diagnosing and managing neonatal sepsis: the role of angiopoietins. J Matern Fetal Neonatal Med. 2013;26:24–26. doi:10.3109/14767058.2013.830411
- Johal P, Kamboj K, Kumar AY, Kumar V, Jha V. SUN-085 effect of cholecalciferol supplementation on serum angiopoietin-2 levels in CKD. Kidney Int Rep. 2020;5:S237. doi:10.1016/j.ekir.2020.02.611
- Zhao Y, Guo Y, Jiang Y, Zhu X, Zhang X. Vitamin D suppresses macrophage infiltration by down-regulation of TREM-1 in diabetic nephropathy rats. Mol Cell Endocrinol. 2018;473:44–52. doi:10.1016/j.mce.2018.01.001
- Ivanov II, Apta BH, Bonna AM, Harper MT. Platelet P-selectin triggers rapid surface exposure of tissue factor in monocytes. Sci Rep. 2019;9:1–10. doi:10.1038/s41598-019-49635-7
- Zelaya H, Rothmeier A, Ruf W. Tissue factor at the crossroad of coagulation and cell signaling. J Thromb Haemost. 2018;16:1941–1952.
- Bouchard BA, Krudysz-Amblo J, Butenas S. Platelet tissue factor is not expressed transiently after platelet activation. Blood. 2012;119:4338–4339.
- Takeshita J, Mohler ER III, Krishnamoorthy P, et al. Endothelial cell‐, platelet‐, and monocyte/macrophage‐derived microparticles are elevated in psoriasis beyond cardiometabolic risk factors. J Am Heart Assoc. 2014;3:e000507. doi:10.1161/JAHA.113.000507
- Creager M, Loscalzo J, Beckman JA. Vascular Medicine E-Book: A Companion to Braunwald’s Heart Disease. Elsevier Health Sciences; 2012.
- Sugama Y, Tiruppathi C, Andersen T, Fenton J, Malik A. Thrombin-induced expression of endothelial P-selectin and intercellular adhesion molecule-1: a mechanism for stabilizing neutrophil adhesion. J Cell Biol. 1992;119:935–944. doi:10.1083/jcb.119.4.935
- Meiring M, Allers W, Le Roux E. Tissue factor: a potent stimulator of Von Willebrand factor synthesis by human umbilical vein endothelial cells. Int J Med Sci. 2016;13:759. doi:10.7150/ijms.15688
- Ueno A, Murakami K, Yamanouchi K, Watanabe M, Kondo T. Thrombin stimulates production of interleukin‐8 in human umbilical vein endothelial cells. Immunology. 1996;88:76–81. doi:10.1046/j.1365-2567.1996.d01-635.x
- Heller R, Bussolino F, Ghigo D, et al. Nitrovasodilators inhibit thrombin-induced platelet-activating factor synthesis in human endothelial cells. Biochem Pharmacol. 1992;44:223–229. doi:10.1016/0006-2952(92)90004-3
- Huang Y-Q, Li -J-J, Hu L, Lee M, Karpatkin S. Thrombin induces increased expression and secretion of angiopoietin-2 from human umbilical vein endothelial cells. Blood. 2002;99:1646–1650.
- Rossaint J, Zarbock A. Platelets in leucocyte recruitment and function. Cardiovasc Res. 2015;107:386–395. doi:10.1093/cvr/cvv048
- Cimmino G, Morello A, Conte S, et al. Vitamin D inhibits tissue factor and CAMs expression in oxidized low-density lipoproteins-treated human endothelial cells by modulating NF-κB pathway. Eur J Pharmacol. 2020;885:173422. doi:10.1016/j.ejphar.2020.173422
- Lundwall K, Mörtberg J, Mobarrez F, Jacobson SH, Jörneskog G, Spaak J. Changes in microparticle profiles by vitamin D receptor activation in chronic kidney disease–a randomized trial. BMC Nephrol. 2019;20:1–10. doi:10.1186/s12882-019-1445-4
- Jia X, Xu J, Gu Y, Gu X, Li W, Wang Y. Vitamin D suppresses oxidative stress-induced microparticle release by human umbilical vein endothelial cells. Biol Reprod. 2017;96:199–210. doi:10.1093/biolre/bio142604
- Blondon M, Biver E, Braillard O, Righini M, Fontana P, Casini A. Thrombin generation and fibrin clot structure after vitamin D supplementation. Endocr Connect. 2019;8:1447–1454. doi:10.1530/EC-19-0429
- Guessous I. Role of vitamin D deficiency in extraskeletal complications: predictor of health outcome or marker of health status? Biomed Res Int. 2015;2015:1–13. doi:10.1155/2015/563403
- Lips P, Cashman KD, Lamberg-Allardt C, et al. Current vitamin D status in European and Middle East countries and strategies to prevent vitamin D deficiency: a position statement of the European Calcified Tissue Society. Eur J Endocrinol. 2019;180:P23–P54.
- Bouillon R, Rosen CJ, Mulder JE. Vitamin D and extraskeletal health. UpToDate. 2015;7.
- Takeuti FA, Souza-Fonseca-Guimaraes F, Guimaraes PS. Applications of vitamin D in sepsis prevention. Discov Med. 2018;25:291–297.
- Reijven P, Soeters P. Vitamin D: a magic bullet or a myth? Clin Nutr. 2020;39(9):2663–2674. doi:10.1016/j.clnu.2019.12.028
- Bikle DD, Schwartz J. Vitamin D binding protein, total and free vitamin D levels in different physiological and pathophysiological conditions. Front Endocrinol (Lausanne). 2019;10. doi:10.3389/fendo.2019.00317
- Bikle D. Vitamin D: production, metabolism, and mechanisms of action. In: Feingold KR, Anawalt B, Boyce A, et al., editors. Endotext [Internet]. South Dartmouth (MA): MDText. com, Inc.; 2000. Available from: https://www.ncbi.nlm.nih.gov/books/NBK278935/
- Xie Z, Wang X, Bikle DD. Vitamin D binding protein, total and free vitamin D levels in different physiological and pathophysiological conditions. Front Endocrinol (Lausanne). 2020;11:40. doi:10.3389/fendo.2020.00040
- Cho M-C, Kim JH, Jung MH, et al. Analysis of vitamin D-binding protein (VDBP) gene polymorphisms in Korean women with and without endometriosis. Clin Exp Reprod Med. 2019;46(3):132. doi:10.5653/cerm.2019.00122
- Mehramiz M, Khayyatzadeh SS, Esmaily H, et al. Associations of vitamin D binding protein variants with the vitamin D-induced increase in serum 25-hydroxyvitamin D. Clin Nutr. 2019;29:59–64. doi:10.1016/j.clnesp.2018.12.005
- Rossol M, Pierer M, Raulien N, et al. Extracellular Ca 2+ is a danger signal activating the NLRP3 inflammasome through G protein-coupled calcium sensing receptors. Nat Commun. 2012;3:1–9. doi:10.1038/ncomms2339
- Sakem B, Nock C, Stanga Z, et al. Serum concentrations of 25-hydroxyvitamin D and immunoglobulins in an older Swiss cohort: results of the Senior Labor Study. BMC Med. 2013;11:176. doi:10.1186/1741-7015-11-176
- Stover CM, McDonald J, Byrne S, Lambert DG, Thompson JP. Properdin levels in human sepsis. Front Immunol. 2015;6:24. doi:10.3389/fimmu.2015.00024
- Ospina-Caicedo AI, Cardona-Rincón AD, Bello-Gualtero JM, et al. Lower levels of vitamin D associated with disease activity in Colombian patients with systemic lupus erythematosus. Curr Rheumatol Rev. 2019;15:146–153. doi:10.2174/1573397114666181015161547
- Karasu E, Nilsson B, Köhl J, Lambris JD, Huber-Lang M. Targeting complement pathways in polytrauma-and sepsis-induced multiple-organ dysfunction. Front Immunol. 2019;10:543. doi:10.3389/fimmu.2019.00543
- Nedeva C, Menassa J, Puthalakath H. Sepsis: inflammation is a necessary evil. Front Cell Dev Biol. 2019;7:108. doi:10.3389/fcell.2019.00108
- Sharma NK, Ferreira BL, Tashima AK, et al. Lipid metabolism impairment in patients with sepsis secondary to hospital acquired pneumonia, a proteomic analysis. Clin Proteomics. 2019;16:29. doi:10.1186/s12014-019-9252-2
- Bashir NA, Bashir AAM, Bashir HA. Effect of vitamin D deficiency on lipid profile. Am J Lab Med. 2019;4:11–18. doi:10.11648/j.ajlm.20190401.12
- Dibaba DT. Effect of vitamin D supplementation on serum lipid profiles: a systematic review and meta-analysis. Nutr Rev. 2019;77:890–902. doi:10.1093/nutrit/nuz037
- Lee SH, Park MS, Park BH, et al. Prognostic implications of serum lipid metabolism over time during sepsis. Biomed Res Int. 2015;2015:1–8. doi:10.1155/2015/789298
- Schwetz V, Scharnagl H, Trummer C, et al. Vitamin D supplementation and lipoprotein metabolism: a randomized controlled trial. J Clin Lipidol. 2018;12:588–596. e584. doi:10.1016/j.jacl.2018.03.079
