Abstract
Purpose
Lipopolysaccharides (LPS) induce inflammation by binding to the Toll-like receptor (TLR) 4 complex, including LPS-binding protein (LBP). The anti-inflammatory effects of linagliptin in LPS-induced inflammation in the TLR4-independent pathway have not been examined before. We examined the anti-inflammatory effects of linagliptin in the TLR4- and the LBP-independent pathway.
Methods
U937 cells were cultured in the medium supplemented with 10% fetal bovine serum (FBS) and treated with 100 nM phorbol myristate acetate for 48 h. Cells were then left untreated or were treated with 10 μg/mL anti-TLR4 antibodies alone or in combination with linagliptin for 1 h in media supplemented with or without 10% FBS. The cells were divided into 5 groups: a) control cells (untreated) b) cells treated with LPS c) cells treated with 10 μg/mL anti-TLR4 antibodies d) cells treated with LPS and 10 μg/mL anti-TLR4 antibodies and e) cells treated with LPS, 10 μg/mL anti-TLR4 antibodies, and linagliptin. The LPS concentrations used were 50 pg/mL or 100 pg/mL for cells treated in the presence of 10% FBS and 100 pg/mL or 1 μg/mL for cells treated in the absence of FBS. Linagliptin concentrations of 1 nM, 10 nM, and 100 nM were used for treatment. The supernatants were analyzed for interleukin (IL)-6 production after 24 h of various treatments.
Results
LPS increased IL-6 production compared to the untreated control cells, and anti-TLR4 antibody suppressed LPS-induced increased IL-6 levels. Linagliptin suppressed LPS-induced IL-6 production in a concentration-dependent manner in the presence of FBS. However, only 100 nM linagliptin could suppress LPS-induced IL-6 production in the absence of FBS.
Conclusion
Concentration-dependent and -independent inflammatory suppression was observed following linagliptin treatment after LPS induction in an experimental model of TLR4 inhibition by anti-TLR4 antibodies. Our results showed that linagliptin may inhibit inflammation through multiple mechanisms centered around the TLR-4-mediated pathway.
Plain Language Summary
Lipopolysaccharides (LPS) induce inflammation by binding to the Toll-like receptor (TLR) 4 complex, including LPS-binding protein (LBP). We have not examined the anti-inflammatory effects of linagliptin, a dipeptidyl peptidase-4 (DPP-4) inhibitor, in LPS-induced inflammation in the TLR4-independent pathway. We examined the anti-inflammatory effects of linagliptin in the TLR4- and the LBP-independent pathway.
We cultured Human U937 cells in the medium with phorbol myristate acetate and analyzed for interleukin (IL)-6 production in the supernatants after treatment with LPS, anti-TLR4 antibodies and linagliptin. LPS increased IL-6 production compared to the untreated control cells, and anti-TLR4 antibody suppressed LPS-induced increased IL-6 levels. Linagliptin suppressed LPS-induced IL-6 production in a concentration-dependent manner in the presence of FBS. However, only 100 nM linagliptin could suppressed LPS-induced IL-6 production in the absence of FBS.
We observed concentration-dependent and -independent inflammatory suppression following linagliptin treatment after LPS induction in an experimental model of TLR4 inhibition by anti-TLR4 antibodies. Our results showed that linagliptin may inhibit inflammation through multiple mechanisms centered around the TLR-4-mediated pathway.
Introduction
Lipopolysaccharide (LPS) induces inflammation by binding to the Toll-like receptor (TLR) 4 complex, including LPS-binding protein (LBP).Citation1–Citation7 TLR4 and LBP play an important role in LPS-induced inflammation. But LPS can induce inflammation by binding to TLR 4 without LBP as well.Citation8,Citation9 Therefore, the inflammatory effect of LPS can change in the presence or absence of LBP. LBP is synthesized in the liver and is constantly maintained in the serum.Citation10 Therefore, the presence or absence of LBP largely depends on the presence or absence of fetal bovine serum (FBS) in an in vitro study. We confirmed that only cultures with FBS have LBP in our previous studies.Citation4
Linagliptin, a dipeptidyl peptidase-4 (DPP-4) inhibitor, has anti-inflammatory effects.Citation1–Citation3 Therefore, linagliptin is a critical therapeutic drug for the patient population which inflammation is a prognosis-related factor.Citation11 Moreover, linagliptin is known to induce two types of anti-inflammatory effects as shown in our previous study.Citation4 These two types of anti-inflammatory effects of linagliptin induced by the presence or absence of LBP can be attributed to two different anti-inflammatory mechanisms mediated via TLR4.
The anti-inflammatory mechanism of linagliptin was examined in the presence or absence of LBP in our previous study as well.Citation1–Citation4 However, the experimental model of TLR4 inhibition by anti-TLR4 antibodies was not examined and thus it is important to study the effect of linagliptin in absence of both TLR4 and LBP.
Therefore, in this study, we examined the anti-inflammatory effect of linagliptin in an in vitro model that excludes both TLR4 and LBP, to further examine the anti-inflammatory mechanism of linagliptin.
Methods
Cell
Human U937 monocytes (EC85011440) were purchased from the European Collection of Animal Cell Culture (UK). Linagliptin compounds were provided by Boehringer Ingelheim Pharmaceuticals, Inc. (Ingelheim am Rhein, Rhineland-Palatinate, Germany); Anti-TLR4 antibody by Thermo Fisher Scientific (Waltham, MA USA); FBS from Biosera (NUAILLE, France); LPS from Escherichia coli 055:B5 from Sigma-Aldrich (St. Louis, MO, USA); phorbol 12-myristate 13-acetate (PMA) from Abcam; Roswell Park Memorial Institute medium (RPMI 1640) (Wako Pure Chemical Industries Ltd., Osaka, Japan). We used linagliptin, FBS, LPS, PMA, and RPMI 1640, as described in our previous study.Citation3,Citation4 Cells were cultured in RPMI 1640 media containing 10% FBS, as previously described.Citation3,Citation4 The cell passage number ranged from two to five and each experiment was repeated three times.
Drug Treatment
After 24 h of culture, we replaced the medium with or without 10% FBS, and cells were then left untreated or were treated with 10 μg/mL anti-TLR4 antibody alone or in combination with linagliptin for 1 h, after which the medium was replaced again with or without 10% FBS, and the cells were left untreated or were treated with LPS only; treated with 10 μg/mL anti-TLR4 antibodies only; were treated with LPS and 10 μg/mL anti-TLR4 antibodies or were treated with LPS, 10 μg/mL anti-TLR4 antibodies and linagliptin. Cells were treated with LPS concentrations of 50 pg/mL or 100 pg/mL when cultured in the presence of FBS while only 100 pg/mL or 1 μg/mL LPS concentrations were used for treatment when cells were cultured in the absence of FBS. LPS was able to induce inflammation in cells at all doses used. Linagliptin concentrations of 1 nM, 10 nM, and 100 nM were used for the study. The supernatants were collected and were analyzed for interleukin (IL)-6 production after 24 h of various treatments.
Measurement of IL-6 in the Medium
IL-6 levels were measured using a Human IL-6 Quantikine enzyme-linked immunosorbent assay kit (BioTechne-R&D Systems Inc., Minneapolis, MN, USA) employing a sandwich-type immunoassay, as previously described.Citation1–Citation4
Statistical Analysis
The JMP (version 10) statistical software (SAS Institute, Cary, NC, USA) was used to analyze the results. Data was presented as mean ± standard error. Results of various treatments were compared with untreated control or LPS only or LPS and anti-TLR4 antibody groups using one-way analysis of variance and Dunnett’s tests. A p-value of less than 0.05, was considered significant.
Results
Effects of Anti-TLR4 Antibody on LPS-Induced IL-6 Levels in Presence of FBS
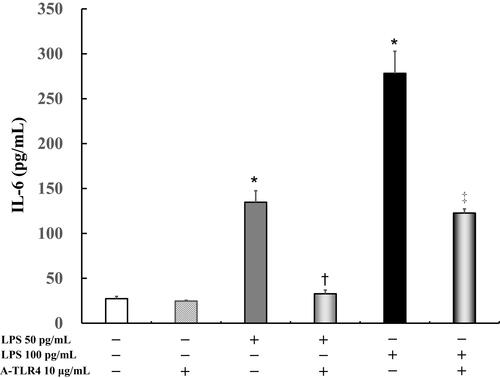
IL-6 levels did not differ significantly in the untreated cells (27.3 ± 2.7 pg/mL) as compared to cells after treatment with anti-TLR4 antibodies (24.7 ± 0.9 pg/mL). In addition, following treatment with 50 pg/mL or 100 pg/mL LPS, IL-6 levels were significantly increased (50 pg/mL LPS: 134.7 ± 12.8 pg/mL, P < 0.0001; 100 pg/mL LPS: 278.2 ± 24.6 pg/mL, P < 0.0001) in the treated cells as compared to the untreated control (27.3 ± 2.7 pg/mL). Anti-TLR4 antibodies suppressed LPS-induced IL-6 levels (50 pg/mL LPS: 32.7 ± 4.2 pg/mL, P < 0.0001; 100 pg/mL LPS: 121.5 ± 5.5 pg/mL, P < 0.0001; ).
Figure 1 Effect of anti-Toll-like receptor (TLR) 4 antibodies on lipopolysaccharide (LPS)-induced interleukin (IL)-6 levels in presence of fetal bovine serum (FBS). Human U937 monocytes were treated with LPS and/or anti-TLR 4 antibodies. IL-6 levels in the supernatants were determined by enzyme-linked immunosorbent assay (ELISA) after 24 h of treatment. *P < 0.0001 vs control; †P < 0.0001 vs LPS 50 pg/mL. ‡P < 0.0005 vs LPS 100 pg /mL.
Effect of Linagliptin on IL-6 Production in LPS (50 pg/mL, 100 pg /mL) Induced and Anti-TLR4 Antibodies Treated Cells in Presence of FBS
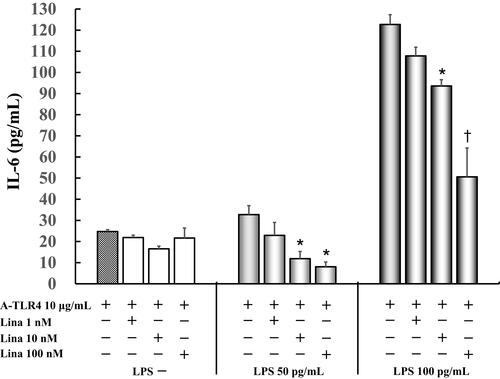
IL-6 levels did not vary significantly in untreated cells as compared to those treated by anti-TLR4 antibodies only (24.7 ± 0.9 pg/mL) after treatment with various concentrations of linagliptin. Linagliptin with anti-TLR4 antibodies suppressed 50 pg/mL LPS-induced IL-6 production (32.7 ± 4.2 pg/mL) in a concentration-dependent manner (1 nM: 22.9 ± 6.1 pg/mL; 10 nM: 11.9 ± 3.4 pg/mL, P < 0.05; 100 nM: 8.0 ± 2.3 pg/mL, P < 0.05 vs anti-TLR4 antibodies 10 μg/mL alone). Moreover, linagliptin with anti-TLR4 antibodies suppressed 100 pg/mL LPS-induced IL-6 levels (122.7 ± 4.6 pg/mL) in a concentration-dependent manner (1 nM: 107.8 ± 4.1 pg/mL; 10 nM: 93.6 ± 3.0 pg/mL, P < 0.05; 100 nM: 50.6 ± 13.7 pg/mL, P < 0.0001 vs anti-TLR4 antibodies 10 μg/mL alone) ().
Figure 2 Effect of linagliptin on IL-6 production in LPS (50 pg/mL, 100 pg /mL) induced and anti-TLR4 antibodies treated cells in presence of FBS. Human U937 monocytes were treated with linagliptin and anti-TLR4 antibodies after induction with LPS (50 pg/mL, 100 pg/mL). IL-6 levels in the supernatants were determined by enzyme-linked immunosorbent assay (ELISA) after 24 h of treatment. *P < 0.05 vs A-TLR4 10 μg/mL alone; †P< 0.0001 vs A-TLR4 10 μg/mL alone.
Effect of Anti-TLR4 Antibodies on LPS-Induced IL-6 Levels in Absence of FBS
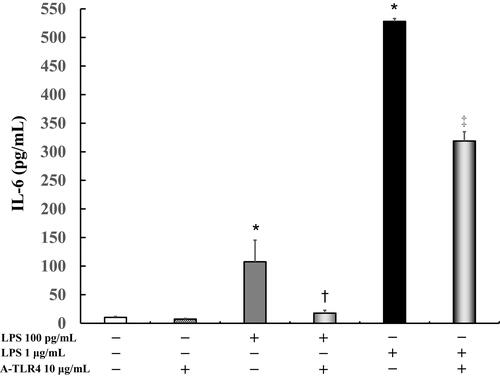
IL-6 levels did not vary significantly in the untreated control cells (10.3 ± 1.6 pg/mL) as compared to those after treatment with anti-TLR4 antibodies alone (7.3 ± 1.2 pg/mL). In addition, following treatment with 100 pg/mL or 1 μg/mL LPS, IL-6 levels were significantly increased (100 pg/mL LPS: 107.6 ± 37.8 pg/mL, P < 0.0001; 1 μg/mL LPS: 528.2 ± 5.0 pg/mL, P < 0.0001) as compared to that in the control. Anti-TLR4 antibodies suppressed LPS-induced IL-6 levels (100 pg/mL LPS: 17.8 ± 5.1 pg/mL, P < 0.05; 1 μg/mL LPS: 318.9 ± 16.2 pg/mL, P < 0.0005; ).
Figure 3 Effect of anti- TLR 4 antibodies on IL-6 production in LPS-induced U937 cells cultured in absence of FBS. Human U937 monocytes were treated with LPS and/or anti-TLR 4 antibodies. IL-6 levels in the supernatants were determined by enzyme-linked immunosorbent assay (ELISA) after 24 h of treatment. *P < 0.0001 vs control; †P < 0.05 vs LPS 100 pg/mL. ‡P < 0.0005 vs LPS 1 μg/mL.
Effect of Linagliptin on IL-6 Production in LPS (100 pg/mL, 1 μg/mL) Induced and Anti-TLR4 Antibodies Treated Cells in Absence of FBS
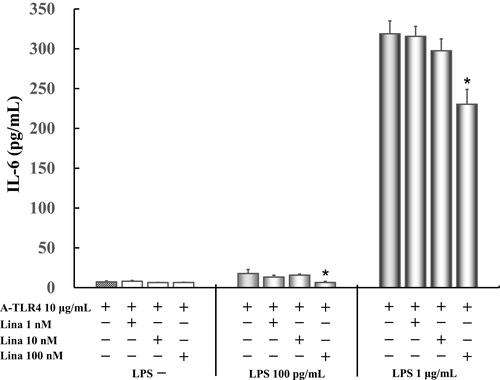
IL-6 levels did not vary significantly in untreated control cells as compared to those treated with anti-TLR4 antibodies only (7.3 ± 1.2 pg/mL) after treatment with various concentrations of linagliptin. Only 100 nM linagliptin (6.5 ± 1.4 pg/mL) suppressed significantly 50 pg/mL LPS-induced IL-6 levels (17.8 ± 5.1 pg/mL). Moreover, only 100 nM linagliptin (230.4 ± 18.5 pg/mL) suppressed significantly 1 μg/mL LPS-induced IL-6 levels (318.9 ± 16.2 pg/mL) ().
Figure 4 Effect of linagliptin on IL-6 production in LPS (100 pg /mL, 1 μg/mL) induced and anti-TLR4 antibodies treated cells in absence of FBS. Human U937 monocytes were treated with linagliptin and anti-TLR4 antibodies after induction with LPS (100 pg /mL, 1 μg/mL). IL-6 levels in the supernatants were determined by enzyme-linked immunosorbent assay (ELISA) after 24 h of treatment. *P < 0.05 vs A-TLR4 10 μg/mL alone.
Discussion
The anti-inflammatory effect of linagliptin was also observed under the TLR4 suppressive condition in this study, as was observed in our previous studies as well.Citation1–Citation4 We reaffirmed that linagliptin mediated anti-inflammatory effect and mechanisms are independent of TLR4 suppression.
Caveolin-1, a binding protein of dipeptidyl peptidase-4 (DPP-4), is involved in LPS-induced TLR4 mediated inflammation.Citation12 Hiromura et al reported that teneligliptin, a DPP-4 inhibitor, could not inhibit LPS induced inflammation in caveolin-knockout mice and thus the study implied that teneligliptin inhibits LPS mediated inflammation by interacting with Caveolin-1.Citation13 In our study, we also propose that linagliptin might inhibit LPS mediated inflammation by interacting with Caveolin-1.
In the presence of LBP, linagliptin inhibited LPS-induced inflammation in a concentration-dependent manner under TLR4 suppressive conditions. LPS forms a complex with TLR4 and CD14 with LBP.Citation5 Under such firm complex formation, the anti-inflammatory effect of linagliptin via Caveolin-1 inhibition was an anti-inflammatory effect that increased in a concentration-dependent manner in proportion to the concentration of linagliptin.
On the other hand, under the condition without LBP, linagliptin suppresses the LPS-induced inflammation in a concentration independent manner, and only high concentrations of linagliptin could inhibit LPS-induced IL-6 levels. In the absence of LBP, there was no concentration dependence, as shown in our previous report.Citation1–Citation3 LBP is not always required for LPS-induced inflammation via TLR4.Citation9 In the absence of LBP, linagliptin inhibited LPS-induced inflammation via Caveolin-1 like on/off switching and suppressed LPS-induced IL-6 levels in a concentration independent manner. Moreover, LPS can bind to receptor of advanced glycation end products (RAGE), a receptor other than TLR4 to induce inflammation.Citation8 Linagliptin might inhibit RAGE as well as TLR4.
Following administration of 5 mg linagliptin, the maximum blood concentration of linagliptin was 7.32 to 22.6 nM in human (Tradjenta® (linagliptin), Boehringer Ingelheim Pharmaceuticals, Inc.).Citation14 Therefore, we decided to use doses from 1 to 100 nM linagliptin in patients whose blood concentration of linagliptin are equivalent to that of actual clinical treatment.
As a limitation of this study, we only used one concentration of anti-TLR4 antibody (10 µg/mL) as described in a previous study.Citation15 In this study, treatment with 10 µg/mL anti- TLR4 antibody did not completely suppress inflammation induced by LPS. Further studies with different anti-TLR4 antibody concentrations are required to fill the gaps and understand the mechanism completely. As the other limitation of this study, we did not use the Western blot assay and another method in our study. And only one single immunological parameter was studied. It is important to use other methods and parameters for measurement. Moreover, it is important to examine the images of morphological changes of cells in different groups, future studies should address this issue.
Conclusion
Concentration-dependent and -independent inflammatory suppression was observed following linagliptin treatment for LPS-induced pro-inflammatory responses in an experimental model of TLR4 suppression by anti-TLR4 antibodies. Linagliptin may inhibit inflammation through multiple mechanisms, centered around the TLR-4-mediated pathway.
Abbreviations
LPS, lipopolysaccharide; TLR, Toll-like receptor; LBP, LPS-binding protein; FBS, fetal bovine serum; DPP-4, dipeptidyl peptidase-4; PMA, phorbol 12-myristate 13-acetate; RPMI 1640, Roswell Park Memorial Institute medium; IL, interleukin; RAGE, receptor of advanced glycation end products.
Data Sharing Statement
All data generated or analyzed during this study are included in this published article.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
Additional information
Funding
References
- Nakamura Y, Hasegawa H, Tsuji M, et al. Linagliptin inhibits lipopolysaccharide-stimulated interleukin-6 production, intranuclear p65 expression, and p38 mitogen-activated protein kinase phosphorylation in human umbilical vein endothelial cells. Ren Replace The. 2016;2:17. doi:10.1186/s41100-016-0030-6
- Nakamura Y, Inagaki M, Tsuji M, et al. Linagliptin has wide-ranging anti-inflammatory points of action in human umbilical vein endothelial cells. Jpn Clin Med. 2016;7:27–32. doi:10.4137/JCM.S39317
- Yamadera S, Nakamura Y, Inagaki M, et al. Linagliptin inhibits lipopolysaccharide induced inflammation in human U937 monocytes. Inflamm Regen. 2018;38:13. doi:10.1186/s41232-018-0071-z
- Sato N, Nakamura Y, Yamadera S, et al. Linagliptin inhibits lipopolysaccharide-induced inflammation concentration-dependently and-independently. J Inflamm Res. 2019;12:285–291. doi:10.2147/JIR.S221761
- Wright SD, Ramos RA, Tobias PS, et al. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990;249:1431–1433.
- Nishijima M, Hara-Kuge S, Takasuka N, et al. Identification of a biochemical lesion, and characteristic response to lipopolysaccharide (LPS) of a cultured macrophage-like cell mutant with defective LPSbinding. J Biochem. 1994;116:1082–1087. doi:10.1093/oxfordjournals.jbchem.a124631
- Weinstein SL, June CH, DeFranco AL. Lipopolysaccharide-induced protein tyrosine phosphorylation in human macrophages is mediated by CD14. J Immunol. 1993;151:3829–3838.
- Yamamoto Y, Harashima A, Saito H, et al. Septic shock is associated with receptor for advanced glycation end products (RAGE) ligation of LPS. J Immunol. 2011;186:3248–3257. doi:10.4049/jimmunol.1002253
- Jerala R. Structural biology of the LPS recognition. Int J Med Microbiol. 2007;297:353–363. doi:10.1016/j.ijmm.2007.04.001
- Zweigner J, Schumann RR, Weber JR. The role of lipopolysaccharide-binding protein in modulating the innate immune response. Microbes Infect. 2006;8:946–952. doi:10.1016/j.micinf.2005.10.006
- Stenvinkel P, Heimbürger O, Lindholm B, et al. Are there two types of malnutrition in chronic renal failure? Evidence for relationships between malnutrition, inflammation and atherosclerosis (MIA syndrome). Nephrol Dial Transplant. 2000;15:953–960. doi:10.1093/ndt/15.7.953
- Jiao H, Zhang Y, Yan Z, et al. Caveolin-1 Tyr14 phosphorylation induces interaction with TLR4 in endothelial cells and mediates MyD88-dependent signaling and sepsis-induced lung inflammation. J Immunol. 2013;191:6191–6199. doi:10.4049/jimmunol.1300873
- Hiromura M, Nohtomi K, Mori Y, et al. Caveolin-1, a binding protein of CD26, is essential for the anti-inflammatory effects of dipeptidyl peptidase-4 inhibitors on human and mouse macrophages. Biochem Biophys Res Commun. 2018;495:223–229. doi:10.1016/j.bbrc.2017.11.016
- Boehringer Ingelheim Pharmaceuticals, Inc. Tradjenta (linagliptin) tablets. Available from: http://bidocs.boehringer-ingelheim.com/BIWebAccess/ViewServlet.ser?docBase=renetnt&folderPath=/Prescribing+Information/PIs/Tradjenta/Tradjenta.pdf. Accessed May 1, 2021.
- Tachado SD, Li X, Bole M, et al. MyD88-dependent TLR4 signaling is selectively impaired in alveolar macrophages from asymptomatic HIV+ persons. Blood. 2010;115:3606–3615. doi:10.1182/blood-2009-10-250787
