Abstract
Purpose
C-reactive protein (CRP) level and platelet (PLT) count have been demonstrated to be independent risk factor for neonatal sepsis. However, no data is currently available in regarding the association between CRP-to-PLT ratio (CPR) and neonatal sepsis.
Methods
A total of 1048 neonates with suspected sepsis were enrolled in this study. Complete clinical and laboratory data were collected. CPR was calculated as CRP (mg/L)/PLT (107 cells/L). Multivariate logistic regression analysis was performed to identify the potential independent risk factors of neonatal sepsis. Receiver operating characteristic (ROC) curve analysis was used to evaluate the prediction accuracy of CPR in predicting neonatal sepsis.
Results
Neonates with sepsis had a higher CPR. CPR also showed a gradual increase in the infection, mild sepsis and severe sepsis groups. Multivariate analysis revealed that CPR was a significant independent predictor of the presence of neonatal sepsis (odds ratio [OR], 1.015; 95% confidence interval [CI], 1.008–1.022, P < 0.001) and severe sepsis (OR, 1.002; 95% CI, 1.000–1.003, P = 0.007). ROC curve revealed showed that CPR had a well-discriminatory power in predicting sepsis (area under curve [AUC], 0.68; 95% CI, 0.65–0.72, P < 0.001) and severe sepsis (AUC, 0.68; 95% CI, 0.65–0.72, P < 0.001).
Conclusion
The present study demonstrated that a higher CPR is an independent predictor of the presence and severity of neonatal sepsis.
Instruction
Neonatal sepsis remains a serious and life-threatening disease in infants worldwide, despite the improvements in neonatology. Previously published data revealed that neonatal sepsis constituted 15.2% of deaths in the neonatal period worldwide,Citation1 and is a major cause of infant mortality.Citation2,Citation3 The gold-standard for diagnosis of neonatal sepsis is blood culture. However, blood culture poses several challenges, such as a long laboratory turnaround time, an inadequate volume of blood and pre-hospital antimicrobial therapy.Citation4,Citation5 In addition, the clinical signs of neonatal sepsis are multiple and non-specific, including bradycardia, temperature instability, diminished spontaneous activity and respiratory distress.Citation6 Therefore, identifying rapid, sensitive, and specific new biomarkers is critical.
C-reactive protein (CRP) is an acute-phase protein produced by the liver and closely associated with systemic inflammatory status.Citation7 In 1988, Povoa et alCitation8 reported for the first time that CRP was an indicator of sepsis. Subsequently, multiple studies confirmed that CRP was an important predictor and risk factor for sepsis.Citation9,Citation10 CRP evaluation was also one of the most investigated and used laboratory tests for diagnosing neonatal sepsis, and higher CRP levels were associated with an increased risk of sepsis.Citation11 However, CRP was not efficiently validated as a screening biomarker.Citation12 Platelets (PLTs) are circulating blood cells, which play an important role in haemostasis and coagulation. PLTs can release inflammatory cytokines, interact with endothelial cells and contribute to the formation of a microthrombus, eventually leading to multiple organ failure.Citation13 Studies have demonstrated that PLTs are involved in the pathogenesis of sepsis and contribute to its complications.Citation14,Citation15 Clinical studies have reported that patients with severe sepsis have a lower PLT count,Citation16 which is associated with enhanced mortality and a more disturbed host response.Citation17 The CRP-to-PLT ratio (CPR), based on CRP levels and PLT counts, indicates not only the inflammation but also the coagulation status. However, no data is currently available regarding the association of CPR with the neonatal sepsis. Therefore, this study aimed to assess the relationship between CPR and neonatal sepsis.
Materials and Methods
Study Design and Patient Population
From January 2016 to February 2020, a total 1098 consecutive neonates with suspected sepsis in Henan Children’s Hospital (Zhengzhou, China) were enrolled in this study. The inclusion criteria were described as follows: (1) neonates with suspected sepsis and (2) neonates aged 1–28 days. Neonates with the following conditions were excluded from this study: (1) with haematological system diseases, malignancies or major congenital malformations and (2) incomplete clinical and laboratory data at admission. The study protocol complied with the Declaration of Helsinki and was approved by the ethics review board of the hospital. Informed consent was not required because the data were anonymised.
Clinical Definition
According to the recommendations of the International Pediatric Sepsis Consensus,Citation18 neonatal sepsis is defined as suspected or proven infection accompanied by≥ 2 systemic inflammatory response syndrome (SIRS) criteria, one being an abnormal body temperature or leukocyte count. The criteria for SIRS are as follows: (1) body temperature > 38.5°C or < 36°C; (2) mean heart rate > 2 SD above normal for age in the absence of external stimuli or unexplained persistent elevation in children aged < 1 year old or mean heart rate < 10th percentile for age, or unexplained persistent depression over a 0.5hr; (3) mean respiratory rate of > 2 SD above normal for age or in the presence of mechanical ventilation; and (4) abnormal leukocyte count or >10% immature neutrophils. Severe sepsis is defined as sepsis accompanied by one of the following: cardiovascular organ dysfunction or acute respiratory distress syndrome or ≥ 2 organ dysfunctions. Detailed information can be obtained from the International Pediatric Sepsis Consensus.Citation18
Data Collection and Biochemical Analyses
All clinical and laboratory data were obtained from medical records, including age; gender; weight; temperature; respiratory rate; heart rate; systolic blood pressure; diastolic blood pressure and the levels of procalcitonin (PCT), CRP, white blood cells (WBCs), neutrophils, PLTs, aspartate aminotransferase (AST), alanine aminotransferase (ALT), blood urea nitrogen (BUN), creatinine (CREA) and uric acid (UA). The detection methods of these laboratory indices are described in our previous published study.Citation19 CRP level < 0.8 mg/L or PCT level > 100 ng/mL or < 0.02 ng/mL were considered as 0.7 mg/L, 101 ng/mL and 0.01 ng/mL, respectively. CPR was calculated as CRP/PLT *103.
Statistical Analysis
Continuous data were expressed as the mean ± standard deviation (SD) or medians (interquartile range) and analysed using independent Student’s t-tests, one-way analysis of variance (ANOVA) or Mann–Whitney U-test, according to their distribution. Categorical variables were presented as percentages (n, %) and analysed using the chi-square or Fisher exact tests. The Pearson or Spearman correlation analysis was performed to determine the correlation between two continuous variables. Univariate and multivariate logistic regression analyses were performed to evaluate whether CPR was an independent risk factor for the presence and severity of neonatal sepsis. Variables with a P value < 0.05 in the univariate logistic analysis were included in the multiple regression analysis. The prediction accuracy was evaluated using the area under the receiver operating characteristic (ROC) curves. All statistical analyses were performed using SPSS 22.0 (SPSS Inc., Chicago, Illinois, USA). A two-sided P value < 0.05 was considered statistically significant.
Results
Patient Characteristics
A total of 1098 neonates with suspected sepsis who met the inclusion criteria were enrolled in this study. Of the 1098 neonates, 599 were clinically diagnosed with sepsis; of which, 256 and 343 were diagnosed with mild sepsis and severe sepsis, respectively. The remaining 449 neonates without sepsis were classified as the infection group. Clinical characteristics and laboratory data of patients are provided in . Compared with neonates in the infection group, neonates with sepsis were older; had higher body temperature, respiratory rate and heart rate; and had higher levels of PCT, CRP, neutrophils, ALT, BUN, UA and CPR (P < 0.001). However, the levels of PLT and CREA were significantly decreased (P < 0.05) in neonates with sepsis. Based on the severity of sepsis, the neonates were divided into the following two groups: mild sepsis group and severe sepsis group. Further analysis showed that only PCT levels, CRP levels and CPR showed a significant gradual increase in the infection, mild sepsis and severe sepsis groups (P < 0.05).
Table 1 Basic Characteristics of Study Subjects
Association of CPR with Neonatal Sepsis
According to the CPR tertiles, we classified the neonates into three groups. As shown in , neonates in tertile 3 had higher level of PCT levels and neutrophils counts. The prevalence of sepsis increased significantly from 44.6% in tertile 1 to 81.7% in tertile 3 (P < 0.001), whereas the infection group was more likely to be in CPR tertile 1 and tertile 2. Further analysis revealed that the prevalence of severe sepsis was significantly higher in CPR tertile 3 than that in tertile 1 and tertile 2 (P < 0.05).
Table 2 The Presence and Severity of Neonatal Sepsis Based on CPR Tertiles
Predictive Value of CPR for Neonatal Sepsis
Univariate and multivariable binary logistic regression analyses were performed to evaluate the role of CPR in the diagnosis of neonatal sepsis (). After adjusting age; temperature; heart rate; respiratory rate; weight; neutrophil counts and the levels of PCT, ALB, ALP, AST, ALT, UREA and UA, CPR was proved to be an independent predictor of neonatal sepsis (odds ratio [OR], 1.015, 95% confidence interval [CI], 1.008–1.022, P < 0.001). Furthermore, our data also revealed that the tertiles of CPR were independently associated with an increased prevalence of neonatal sepsis. In addition, our data also confirmed that CPR and CPR tertiles were independent predictor of severe sepsis.
Table 3 Regression Analysis to Assess the Presence of Neonatal Sepsis and Severe Sepsis Based on CPR Tertiles
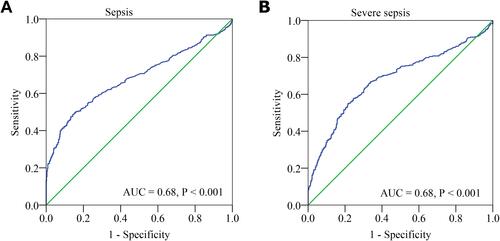
Diagnostic Performance of the CPR
ROC curve analysis was performed to evaluate the predictive value of CPR for sepsis. As shown in , the area under the ROC curves (AUC) showed a well discriminatory power of CPR (AUC = 0.68, 95% CI, 0.65–0.72, P < 0.001) in predicting neonatal sepsis. The optimal diagnostic cut-off point was 5.41 mg/106 cells, with 49% sensitivity and 86% specificity. In addition, we also analyzed the role of CPR in predicting severe sepsis. The AUC value for CPR in predicting severe sepsis was 0.68 (95% CI, 0.64–0.72, P < 0.001) (). The optimal diagnostic cut-off point was 6.10 mg/106 cells (with a sensitivity of 55% and specificity of 78%).
Discussion
Neonatal sepsis is a serious life-threatening disease. Although the current diagnosis and treatment technologies have made significant progress, the diagnosis of neonatal sepsis still faces many challenges. For example, blood culture, the gold standard for diagnosis of neonatal sepsis, has a long laboratory turnaround time, which may cause a delay in diagnosis and the effective treatment.Citation20 In addition, owing to the use of antibiotics before hospitalisation and an inadequate volume of blood, blood culture yields a low positive rate.Citation20 Moreover, the clinical signs of neonatal sepsis are multiple and non-specific.Citation6 Therefore, circulating blood biomarkers that may be useful in the early diagnosis of neonatal sepsis are under investigation.Citation21
Sepsis is characterized by SIRS caused by pathogens infection, and biomarkers of inflammation play an important role in the diagnosis of neonatal sepsis.Citation22,Citation23 CRP is a well-known and commonly used marker, which was closely associated with inflammation-related diseases.Citation7 Various studies have demonstrated the CRP is an important prognostic factor for neonatal sepsis.Citation24–Citation27 However, CRP exhibited a low specificity owing to its physiological increase after birth or the presence of non-infection-related conditions.Citation11,Citation28 PLTs are anucleate cells that play an important role in modulating haemostasis and developing thrombosis.Citation29,Citation30 Increasing evidences has revealed that PLTs played a critical role in inflammation and immune responses.Citation31,Citation32 PLTs can interacts with other leukocytes by expressing and secreting adhesion molecules and immune modulators, which further enhances inflammation.Citation33–Citation36 In addition to the hyperinflammation caused by PLTs, they can also catalyse the development of disseminated intravascular coagulation and microthrombosis, leading to sepsis complications and organ dysfunction.Citation13 Clinical studies have demonstrated that a low PLT count is a well-known biomarker for disease severity of sepsisCitation33,Citation37 and an independent prognostic predictor of 1-year overall survival of patients with sepsis.Citation38
CPR, as an emerging risk factor, can reflect both inflammation and coagulation status. However, there are no published studies regarding the relationship between CPR and neonatal sepsis. In this study, for the first time, we investigated the predictive role of CPR in neonatal sepsis based on a relatively large sample size. Our data revealed that the level of CPR was higher in neonates with sepsis and increased with the disease severity of sepsis. The neonates were divided into three groups based on the CPR tertiles. Further analysis revealed that the prevalence of sepsis was significantly higher in CPR tertile 3 (up to 81.7%), than in CPR tertile 1 and tertile 2. Multivariate analysis revealed that the CPR was an independent predictor of the presence and severity of neonatal sepsis. The ROC curve analysis revealed that the CPR had a well discriminatory power in predicting sepsis and severe sepsis.
Limitations to the study include the fact that this is a retrospective single-center study, which may lead to some information and selection biases. Second, the diagnosis of neonatal sepsis was based on clinical features and was not confirmed by positive blood culture. Therefore, the incidence rate of sepsis may be underestimated or overestimated. Third, CPR was only measured at admission; serial CPR evaluation may be useful to further explore the dynamic correlation between CPR and neonatal sepsis. Lastly, all of the enrolled patients were neonates with suspected sepsis. Therefore, the findings of this study may not be applicable to other populations.
Conclusions
In conclusion, our study demonstrated that CPR was higher in neonates with sepsis and increased with the disease severity. After adjusting for other variables, CPR was independently associated with the presence and severity of neonatal sepsis. Our findings highlight the potential clinical value of CPR in predicting the risk of neonatal sepsis.
Ethics Approval and Consent to Participate
The study was conducted according to the guidelines of the Declaration of Helsinki and was approved by the Hospital Ethics Review Board of the Henan Children’s Hospital. We confirmed that all the data were anonymized and maintained with confidentiality; therefore, the requirement for informed consent was waived because of the retrospective nature of the present study.
Disclosure
The authors report no conflicts of interest in this work.
Additional information
Funding
References
- Liu L, Oza S, Hogan D, et al. Global, regional, and national causes of child mortality in 2000-13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet. 2015;385(9966):430–440. doi:10.1016/s0140-6736(14)61698-6
- Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2095–2128. doi:10.1016/S0140-6736(12)61728-0
- Wang H, Liddell CA, Coates MM, et al. Global, regional, and national levels of neonatal, infant, and under-5 mortality during 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384(9947):957–979. doi:10.1016/S0140-6736(14)60497-9
- Scheer CS, Fuchs C, Gründling M, et al. Impact of antibiotic administration on blood culture positivity at the beginning of sepsis: a prospective clinical cohort study. Clin Microbiol Infect. 2019;25(3):326–331. doi:10.1016/j.cmi.2018.05.016
- Lamy B, Dargère S, Arendrup MC, Parienti JJ, Tattevin P. How to optimize the use of blood cultures for the diagnosis of bloodstream infections? A state-of-the art. Front Microbiol. 2016;7:697. doi:10.3389/fmicb.2016.00697
- Shane AL, Sánchez PJ, Stoll BJ. Neonatal sepsis. Lancet. 2017;390(10104):1770–1780. doi:10.1016/s0140-6736(17)31002-4
- Sproston NR, Ashworth JJ. Role of C-reactive protein at sites of inflammation and infection. Front Immunol. 2018;9:754. doi:10.3389/fimmu.2018.00754
- Povoa P, Almeida E, Moreira P, et al. C-reactive protein as an indicator of sepsis. Intensive Care Med. 1998;24(10):1052–1056. doi:10.1007/s001340050715
- Stocker M, van Herk W, El Helou S, et al. C-reactive protein, procalcitonin, and white blood count to rule out neonatal early-onset sepsis within 36 hours: a secondary analysis of the neonatal procalcitonin intervention study. Clin Infect Dis. 2020. doi:10.1093/cid/ciaa876
- Wang HE, Shapiro NI, Safford MM, et al. High-sensitivity C-reactive protein and risk of sepsis. PLoS One. 2013;8(7):e69232. doi:10.1371/journal.pone.0069232
- Hofer N, Zacharias E, Muller W, Resch B. An update on the use of C-reactive protein in early-onset neonatal sepsis: current insights and new tasks. Neonatology. 2012;102(1):25–36. doi:10.1159/000336629
- Khan F. C-reactive protein as a screening biomarker in neonatal sepsis. J Coll Physicians Surg Pak. 2019;29(10):951–953. doi:10.29271/jcpsp.2019.10.951
- de Stoppelaar SF, van’t Veer C, van der Poll T. The role of platelets in sepsis. Thromb Haemost. 2014;112(4):666–677. doi:10.1160/TH14-02-0126
- Greco E, Lupia E, Bosco O, Vizio B, Montrucchio G. Platelets and multi-organ failure in sepsis. Int J Mol Sci. 2017;18(10):2200. doi:10.3390/ijms18102200
- Russwurm S, Vickers J, Meier-Hellmann A, et al. Platelet and leukocyte activation correlate with the severity of septic organ dysfunction. Shock. 2002;17(4):263–268. doi:10.1097/00024382-200204000-00004
- Guclu E, Durmaz Y, Karabay O. Effect of severe sepsis on platelet count and their indices. Afr Health Sci. 2013;13(2):333–338. doi:10.4314/ahs.v13i2.19
- Claushuis TA, van Vught LA, Scicluna BP, et al. Thrombocytopenia is associated with a dysregulated host response in critically ill sepsis patients. Blood. 2016;127(24):3062–3072. doi:10.1182/blood-2015-11-680744
- Goldstein B, Giroir B, Randolph A. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med. 2005;6(1):2–8. doi:10.1097/01.Pcc.0000149131.72248.E6
- Li T, Dong G, Zhang M, et al. Association of neutrophil-lymphocyte ratio and the presence of neonatal sepsis. J Immunol Res. 2020;2020:7650713. doi:10.1155/2020/7650713
- Iroh Tam PY, Bendel CM. Diagnostics for neonatal sepsis: current approaches and future directions. Pediatr Res. 2017;82(4):574–583. doi:10.1038/pr.2017.134
- Sharma D, Farahbakhsh N, Shastri S, Sharma P. Biomarkers for diagnosis of neonatal sepsis: a literature review. J Matern Fetal Neonatal Med. 2018;31(12):1646–1659. doi:10.1080/14767058.2017.1322060
- Opal SM, Wittebole X. Biomarkers of Infection and Sepsis. Crit Care Clin. 2020;36(1):11–22. doi:10.1016/j.ccc.2019.08.002
- Li T, Zhang Z, Li X, et al. Neutrophil extracellular traps: signaling properties and disease relevance. Mediators Inflamm. 2020;2020:9254087. doi:10.1155/2020/9254087
- Ruan L, Chen GY, Liu Z, et al. The combination of procalcitonin and C-reactive protein or presepsin alone improves the accuracy of diagnosis of neonatal sepsis: a meta-analysis and systematic review. Crit Care. 2018;22(1):316. doi:10.1186/s13054-018-2236-1
- Omran A, Maaroof A, Saleh MH, Abdelwahab A. Salivary C-reactive protein, mean platelet volume and neutrophil lymphocyte ratio as diagnostic markers for neonatal sepsis. J Pediatr. 2018;94(1):82–87. doi:10.1016/j.jped.2017.03.006
- Liu Y, Zhao L, Wu Z. Accuracy of C-reactive protein test for neonatal septicemia: a diagnostic meta-analysis. Med Sci Monit. 2019;25:4076–4081. doi:10.12659/MSM.916968
- Li T, Li X, Wei Y, et al. Predictive value of C-reactive protein-to-albumin ratio for neonatal sepsis. J Inflamm Res. 2021;14:3207–3215. doi:10.2147/JIR.S321074
- Eschborn S, Weitkamp JH. Procalcitonin versus C-reactive protein: review of kinetics and performance for diagnosis of neonatal sepsis. J Perinatol. 2019;39(7):893–903. doi:10.1038/s41372-019-0363-4
- Gremmel T, Frelinger AL, Michelson AD. Platelet physiology. Semin Thromb Hemost. 2016;42(3):191–204. doi:10.1055/s-0035-1564835
- Hvas AM. Platelet function in thrombosis and hemostasis. Semin Thromb Hemost. 2016;42(3):183–184. doi:10.1055/s-0036-1572329
- Koupenova M, Clancy L, Corkrey HA, Freedman JE. Circulating platelets as mediators of immunity, inflammation, and thrombosis. Circ Res. 2018;122(2):337–351. doi:10.1161/CIRCRESAHA.117.310795
- Thomas MR, Storey RF. The role of platelets in inflammation. Thromb Haemost. 2015;114(3):449–458. doi:10.1160/TH14-12-1067
- Morrell CN, Aggrey AA, Chapman LM, Modjeski KL. Emerging roles for platelets as immune and inflammatory cells. Blood. 2014;123(18):2759–2767. doi:10.1182/blood-2013-11-462432
- Elzey BD, Tian J, Jensen RJ, et al. Platelet-mediated modulation of adaptive immunity. A communication link between innate and adaptive immune compartments. Immunity. 2003;19(1):9–19. doi:10.1016/s1074-7613(03)00177-8
- Semple JW, Italiano JE, Freedman J. Platelets and the immune continuum. Nat Rev Immunol. 2011;11(4):264–274. doi:10.1038/nri2956
- Sonmez O, Sonmez M. Role of platelets in immune system and inflammation. Porto Biomed J. 2017;2(6):311–314. doi:10.1016/j.pbj.2017.05.005
- Thiery-Antier N, Binquet C, Vinault S, et al. Is thrombocytopenia an early prognostic marker in septic shock? Crit Care Med. 2016;44(4):764–772. doi:10.1097/CCM.0000000000001520
- Zhao L, Zhao L, Wang YY, et al. Platelets as a prognostic marker for sepsis: a cohort study from the MIMIC-III database. Medicine. 2020;99(45):e23151. doi:10.1097/MD.0000000000023151