Abstract
Background
The LuoBiTong (LBT) capsule, a novel traditional Chinese medicine formulation, is currently in Phase III clinical trials. Preliminary preclinical and Phase II clinical studies suggest its efficacy and safety in treating rheumatoid arthritis (RA). However, the underlying mechanisms of its action remain to be elucidated.This research aims to explore the effects and mechanisms of LBT in conjunction with a maintenance dose of methotrexate (M-MTX) on RA.
Methods
A Collagen-Induced Arthritis (CIA) mouse model was used to evaluate the anti-RA effects of LBT combined with M-MTX. Assessments included foot swelling, arthritis scoring, serum inflammatory factor analysis, and histopathological examination of the foot. These effects were compared with those of high-dose MTX (H-MTX). Network pharmacology was employed to construct a compound-target network for RA, based on drug composition, to predict its potential mechanism of action. Flow cytometry, Western Blot, and immunohistochemical analyses in animal models identified multiple inflammatory pathways targeted by LBT to augment the anti-RA effects of MTX.
Results
The study revealed that LBT combined with M-MTX significantly alleviated CIA-induced arthritis without adverse effects. The combination of LBT and M-MTX showed similar or superior efficacy in regulating macrophage polarization, NF-κB, MAPK signaling pathways, and in the suppression of TH-17 expression in proinflammatory cells. These findings suggest that LBT may exert a multi-pathway therapeutic effect in RA treatment. The predicted pharmacological targets and mechanisms align well with this hypothesis.
Conclusion
LBT, when combined with MTX, enhances the anti-RA effect by targeting multiple inflammatory pathways, demonstrating significant therapeutic potential.
Introduction
Rheumatoid Arthritis(RA), one of the most frequently encountered rheumatologic conditions in clinical settings,Citation1 is a prevalent inflammatory autoimmune disease, affecting approximately 0.5–1% of the global population.Citation2 RA is characterized by symmetrical synovitis, which leads to the gradual degradation of articular cartilage and surfaces.Citation3,Citation4 The primary clinical manifestations of RA are pain, stiffness, and joint swelling, which can ultimately lead to deformity and functional impairment as the disease progresses.Citation5,Citation6 Additionally, RA is frequently associated with systemic complications, such as cardiovascular disease, neurological disease, and osteoporosis.Citation7–9
The pathogenesis of RA is a complex and multifactorial phenomenon that involves interactions between genetic, environmental, immunological, and other factors.Citation10 Although the accurate pathogenesis is still elusive, RA symptomatically is a chronic inflammatory disease that causes damage to the joints and other organs.Citation11,Citation12 The inflammation is mediated by various immune cells and molecules, such as T cells, B cells, macrophages, cytokines, chemokines, and autoantibodies.Citation13–16 These factors can activate the NF-κB signaling pathway, which regulates the expression of genes involved in inflammation, cell survival, proliferation, and differentiation. Recent research has demonstrated that the development and progression of rheumatoid arthritis are influenced by multiple cytokines produced by helper T cell-17 (Th17), notably Interleukin-17A (IL-17A) and Interleukin-17F (IL-17F).Citation17,Citation18 Therefore, the management of rheumatoid arthritis currently relies on disease-modifying antirheumatic drugs (DMARDs) as soon as possible after the diagnosis.Citation19–21 They work by suppressing or modifying the immune system to reduce pain, swelling, stiffness, and prevent or slow down joint damage and disability. For instance, Methotrexate (MTX), a first-line DMARD, is considered a safe and effective drug for most patients with RA when used appropriately.Citation22 However, methotrexate also has some limitations and drawbacks in some patients,Citation23,Citation24 such as (a) Methotrexate has a narrow therapeutic window, which means that there is a fine balance between its efficacy and toxicity, and requires regular monitoring of blood counts, liver function, and kidney function.; (b) Methotrexate has a slow onset of action, which means that it may take several weeks or months to achieve its full therapeutic effect.Citation25,Citation26 Therefore, Methotrexate can also be combined with other drugs or modalities to enhance its efficacy and reduce its toxicity.Citation27,Citation28
Additionally, combined therapy is generally considered to be more beneficial than single-agent therapy in the treatment of RA. The main reasons can be attributed to the following: RA is a complex disease with multiple inflammatory pathways involved; Combined therapy can be more effective in controlling symptoms; Combining medications can allow for lower doses of each individual medication. Traditional Chinese medicine (TCM) has emerged as a promising therapeutic alternative.Citation29,Citation30 Combining Traditional Chinese Medicine (TCM) with small molecular medicines (SMMs) is an emerging approach with significant potential for treating various diseases.Citation31 For instance, the therapeutic regime of Tripterygium wilfordii Hook F (TwHF) combined with MTX is more effective than MTX or TwHF alone in the treatment of RA.Citation32,Citation33 Therefore, the Integrated TCM-SMM for RA treatment is promising and deserves attention. However, despite its therapeutic potential, TwHF comes with several safety concerns that require careful consideration before use.Citation34 Luobitong capsules, developed by Yiling Pharmaceutical Co., Ltd., are currently undergoing phase III clinical trials. Preclinical and phase II clinical data suggest that the drug has a therapeutic effect on RA with good safety. Luobitong capsules are composed of extracts from a variety of botanicals, including Astragali Radix (Huang Qi), Gentianae Macrophyllae Radix (Qin Jiao), Stephaniae Tetrandrae Radix (Fang Ji), Radix Aconiti Praeparata (Hei Shun Pian), Polygoni Cuspidati Rhizoma Et Radix (Hu Zhang), Spatholobi Caulis (Ji Xue Teng), Clematidis Radix Et Rhizoma (Wei Ling Xian), Sinapis Semen (Jie Zi), Paeoniae Radix Alba (Bai Shao), Rehmanniae Radix (Di Huang), Angelicae Sinensis Radix (Dang Gui), Myrrha (Mo Yao), Cyperi Rhizoma (Xiang Fu), Cinnamomi Ramulus (Gui Zhi), and Cyathulae Radix (Chuan Niu Xi). LuoBiTong capsules have anti-inflammatory, analgesic, and immunomodulatory effects.
In this study, to gain more insight into the therapeutic targets and pathways, we employed a “disease-drug” network pharmacological analysis to delve into the therapeutic efficacy of LBT and unravel the potential of LBT in anti-inflammation.Citation35,Citation36 Subsequently, synthetic effect of combined LBT and the maintenance dose of MTX (M-MTX) was evaluated in a CIA mouse model.
Methods
Materials
Universal SP kit, SP-9000, Zhongshan Jinqiao Biological Engineering Co., LTD., Beijing, China
Mouse IL-6 ELISA Kit (enzyme-linked immunosorbent assay), EK206, United Biotechnology Co., LTD., Hangzhou, China
Mouse IL-17A ELISA Kit (enzyme-linked immunosorbent assay), EK217, United Biotechnology Co., LTD., Hangzhou, China
Anti-IL-17A, ab79056, Abcam (Shanghai) Trading Co., LTD., Shanghai, China
Anti-IL-6, ab290735, Abcam (Shanghai) Trading Co., LTD., Shanghai, China
Anti-p38 MAPK, 9216s, Cell Signaling Technology company, USA
Anti--SAPK/JNK, 9255s, Cell Signaling Technology company, USA
Anti-TNF alpha, ab183218, Abcam (Shanghai) Trading Co., LTD., Shanghai, China
Anti-NF-kB p65, ab32536, Abcam (Shanghai) Trading Co., LTD., Shanghai, China
Methotrexate, 036210701, 2.5mg/tablet, Shanghai medicine sym pharma co., LTD., Shanghai,
LuoBiTong, A2103001,0.44g/tablet (Equivalent to 1.42g of crude drugs) shijiazhuang ridge pharmaceutical co., LTD., Shijiazhuang, China
Animal Handling
Eight-week-old SPF-grade DBA/1J mice (n=70, body weight 20±2 g) were purchased from Jiangsu Jicui Pharmachem Biotech Co. and its molecular companies. Seventy mice were placed in a light-dark cycle at 23±3°C and 50±10% humidity for 12 h. Food and water were available ad libitum. mice were acclimatized to these living conditions one week before the experiment. All animal experiments were performed in strict accordance with the guidelines approved by the Ethical Committee of Animal Experiments of Jiangsu Jichi Pharmaceutical Kang Biotechnology Co., LTD.
Drugs Preparation
Calculation of LBT Formulation Concentration
The low and high doses correspond to 1.42 and 5.66 g of crude drug/kg, respectively. The volume of the administered animal is 10 mL/kg. Therefore, the calculation of the formulation concentration of the drug is 0.142 g of crude drug/mL and 0.566 g of crude drug/mL, respectively. Each tablet of LBT contains 1.42 g of crude drug. The drug is formulated according to the specifications of the whole tablets, which are listed as follows:
| (1) | LBT Low Dose: Prepare at a concentration of 0.142 g of crude drug/mL, where 1 tablet of LBT is equivalent to 1.42 g of crude drug. Place the tablet in a mortar and gently grind it into a fine powder. Wet the powder with a small amount of 0.5% CMC-Na grinding, add solvent for grinding suspension, and adjust the volume to 10 mL. | ||||
| (2) | LBT High Dose: Prepare at a concentration of 0.566 g of crude drug/mL, where 4 tablets of LBT are equivalent to 5.68 g of crude drug. Place the tablets in a mortar and gently grind them into a fine powder. Wet the powder with a small amount of 0.5% CMC-Na grinding, add solvent for grinding and mixing, and adjust the volume to 10 mL. | ||||
MTX Formulation Method, Configured to Whole Tablet Size (2.5 Mg/Tablet)
(1) MTX Alone Group (high-dose MTX group, H-MTX): Prepare at a concentration of 0.14 mg/mL, where 1 tablet is taken and ground into powder. Moisten the powder with a small amount of 0.5% CMC-Na, grind it, suspend it with solvent, and adjust the volume to 18 mL.
(2) MTX Co-administration Group(Maintenance dose MTX group, M-MTX): Prepare at a concentration of 0.05 mg/mL, where 1 tablet is taken and ground into powder. Moisten the powder with a small amount of 0.5% CMC-Na grinding, grind it, suspend it with solvent, and adjust the volume to 50 mL.
Establishment of Collagen-Induced Arthritis Model
Ten mice were randomly selected as a Control+vehicle group, and another 60 were subjected to CIA modeling. The RA model was constructed by subcutaneous immunization of the mice at the tail root using type II bovine collagen in combination with complete Fuchsin’s adjuvant as well as incomplete Fuchsin’s adjuvant, respectively. Specifically, 50 μL of type II bovine collagen emulsion formulated with complete Freund’s adjuvant was injected subcutaneously into the tail root of each mouse after acclimatization feeding as the first immunization. Three weeks after the first immunization, a second immunization was performed using incomplete Fuchs’ adjuvant formulated type II bovine collagen emulsion.
Grouping and Treatment
Control+Vehicle group (0.5% sodium carboxymethyl cellulose) (n=10).
After the second immunization, 60 CIA model mice were randomly divided into four groups according to their body weight: CIA+Vehicle group (0.5% sodium carboxymethyl cellulose) (n=15); CIA + MTX (1.4 mg/kg) (n=15); CIA + MTX (0.5 mg/kg) +LBT-L(1.42g crude drugs/kg) (n=15); The CIA + MTX (0.5 mg/kg) + LBT - H (5.66 g crude drugs/kg) (n=15). All animals were administered with the corresponding agents through oral gavage. Methotrexate was administered every 2 days for a total of 14 doses. LBT was administered once daily for a total of 28 doses. Body weight was measured three times a week. Animals were sacrificed on day 51 after the first immunization.
Arthritis Score
Body weight and arthritis scores were measured weekly after the first immunization.
Body weight and arthritis scores were measured twice weekly after the second immunization.
A score of 0 represented no obvious symptoms in the limbs of the mice. A score of 1 represents slight swelling of the lesser metatarsal joints; A score of 2 represents swelling of the metatarsal joints and toes; A score of 3 represents paw swelling below the ankle; A score of 4 represents total paw swelling including within the ankle joints.
The score for each mouse was the sum of the limb scores and the severity of arthritis was assessed by visual inspection by three independent observers. Observers were unaware of the presence of the treatment group.
Serum and Tissue Collection
After CIA was established, the experimental endpoints were amenable to mice after 4 weeks of administration by drug grouping. Blood was collected from the heart and a portion of the blood was anticoagulated and then split red and used for FACS to detect macrophage percentage. The other portion of blood was separated from serum and used for ELISA assay. Blood was taken and then executed, and the skin, fascia and muscles around the ankle joint were excised. The sole of the right foot (together with the bone) was fixed in 4% paraformaldehyde for 72 hours. The left paw was frozen instantly in liquid nitrogen.
Histological Examination
Upon completion of the experiment, the soles of the mice’s feet were removed, fixed with 4% paraformaldehyde, and decalcified with 10% EDTA for histopathological analysis. Afterwards, the specimens were embedded in paraffin, sectioned into 5 mm thick sections, and stained with Hematoxylin and Eosin (H&E) and Safranin O-Fast Green.
Enzyme-Linked Immunosorbent Assay (ELISA)
Serum levels of IL-6 and IL-17A were measured by ELISA. The blood samples collected were 10 in Control+Vehicle group, and 15 in each of the other four groups.
The procedure was performed as directed by the ELISA kit for the detection of the serum inflammatory factors IL-6 and IL-17A. The absorbance of the samples at 450 nm was determined and the OD values were recorded for data processing and analysis.
Constructing the Network Pharmacology of Diseases and Drugs
We explore the molecular mechanism of LBT for the treatment of RA using a network pharmacological approach combining chemical and therapeutic properties. Using “rheumatoid arthritis” as the search term, the RA gene was retrieved from six sources, including DisGeNET,Citation37 Open Target Platform,Citation38 MalaCards,Citation39 CTD,Citation40 GeneCards and text mining.Citation41 Using The Database for Annotation, Visualization and Integrated Discovery (DAVID) 2021 (https://david.ncifcrf.gov/) for standardization gene name.Citation42 To ensure the reliability of the data, only genes that appear in more than three databases are retained as the core gene set for RA. The gene sets related to RA and the potential target of LBT were analyzed to find the functional target of LBT for preventing and treating RA. Then the target data were submitted to STRING Version 12.0 (https://string-db.org/) protein interaction background network for the construction of PPI network (confidence 0.7).Citation43 The obtained PPI network is visualized in Cytoscape v3.9.0.Citation44 The comprehensive target spectrum of LBT is essential for the study of its substantive basis and mechanism of action in the treatment of RA. We collected targets from DrugBank,Citation45 TTD,Citation46 ChEMBL,Citation47 PubChem and standardized their name through UniProt.Citation48–50 In order to further explore LBT key active compounds in their treatment of RA and targets, we collected the literature of targets and therapeutic targets from PHARMACODIA database (https://www.pharmacodia.com/). To explain the mechanisms of LBT against RA from a systematic perspective, we performed gene ontological (GO) and KEGG pathway enrichment analyses using Metascape (https://metascape.org) and ClueGO plugin in Cytoscape.Citation51
Flow Cytometry Analysis of Blood Cells
Macrophage proportions were detected by FACS and stained with various anti-mouse antibodies coupled with different fluorescent markers. Indicators included CD45, CD11b, F4/80, MHC II, and CD206.After gating the live cells according to the scatter characteristics according to the manufacturer’s protocols, acquisition and analysis were performed using BD Aril3 and FlowJO software.
Western Blot Analysis
Paw tissue specimens were ground into powder and used for Western blot analysis. After lysis and centrifugation, sample supernatants were quantified using the BCA protein assay kit. The protein samples were then separated by 10% sodium polyacrylamide gel electrophoresis (SDS-PAGE). These proteins were transferred to PVDF membranes and blocked with 5% skim milk to prevent non-specific binding. Membranes were then incubated with appropriate anti-IL-6 (1:1000), TNF-α(1:1000), IL-17A (1:1000), P-NFκB p65 (1:1000), P-p38 (1:1000), P-SAPK/JNK(1:1000), and GAPDH The primary antibody (Abcam) was incubated overnight at 4◦C. After incubation, membranes were washed with PBST and then incubated with appropriate peroxidase-labeled secondary antibodies. Mean area density was expressed for all protein blots relative to GAPDH expression. Results for all target bands were performed at least 3 times.
Immunohistochemistry
For immunohistochemical (IHC) staining, tissue sections were rehydrated and microwave radiation antigen repair method was used. Specifically, the slices were exposed to 10 mM citrate buffer in a microwave oven for 3 minutes and then left for 1 minute. Low fire for 3 min, let stand for 1 min, and this operation was repeated three times. The plates were allowed to drop to room temperature, and flushed 3 times with PBS buffer. An appropriate amount of endogenous peroxidase blocker was added, and the cells were incubated at room temperature for 10 minutes, followed by three flushes with PBS buffer. An appropriate amount of normal goat serum working solution for blocking was added and incubated at 37 ° C for 15 min. Rabbit anti-IL-17A antibody (1:200 concentration) was added to the serum and incubated at 4 ° C overnight. The next day, the cells were flushed with PBS buffer three times. Biotin-labeled goat anti-mouse IgG was added dropfold and incubated at 37 ° C for 15 min followed by three rinses with PBS buffer. The cells were incubated with the working solution of horseradish enzyme-labeled streptavidin for 15 min at 37 ° C and then rinsed with PBS buffer three times. Sections were incubated with DAB chromogenic reagent (Zhongshan Jinqiao Biological Engineering Co., LTD.) for 5 min at room temperature. These sections were then viewed with a light microscope.
Statistical Analysis
The experimental data are expressed as Mean ± SEM. Independent sample T-Test was used for comparison between Two groups of samples, One-way ANOVA test or two-way ANOVA was used for comparison between multiple groups. The data analysis and mapping software was Graphpad prism. p < 0.05 was considered as significant difference.
Results
LBT Alleviates Arthritis Symptoms in CIA Mouse Models
The experiment was terminated on the 51st day after the construction of the CIA model, as shown in . Each mouse was weighed three times a week for 49 days during the experiment (). Compared with CIA+Vehicle group, body weight in CIA+Vehicle group decreased from day 23 to 49. Compared with CIA+Vehicle, the weight of mice after drug treatment increased, but the difference was not statistically significant (p>0.05).
Figure 1 LBT alleviated the signs and symptoms of CIA in mice. (A) Flow chart of animal experiments. (B) Changes in body weight of mice. (C) Effect of LBT and MTX on arthritis score. (D) Effects of LBT and MTX on claw swelling score. # P < 0.05, compare with Control +Vehicle; ## P < 0.01, compare with Control +Vehicle; ### P < 0.001, compare with Control +Vehicle; * P < 0.05, compare with CIA+Vehicle; ** P < 0.01, compare with CIA+Vehicle; *** P < 0.001, compare with CIA+Vehicle.
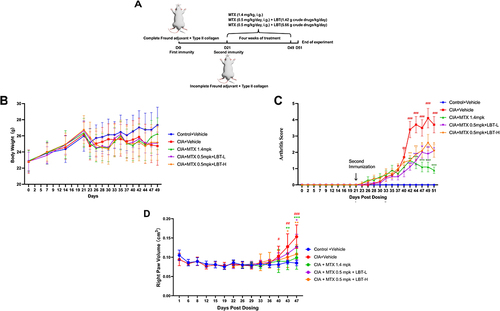
To assess the severity of arthritis in CIA mice, arthritis scores were measured from day 0 to day 51 (). In the CIA+Vehicle group, arthritis scores increased significantly from day 28 to day 51. Compared with CIA+Vehicle mice, both MTX and MTX combined with LBT reduced the severity of arthritis in CIA mice.
Interestingly, we found the same trend in paw volume scores (). Compared with the Control +Vehicle group, we found that the degree of toe swelling in the CIA+Vehicle group was statistically significant on the 40th day after modeling (P<0.05). At the end of the experiment, the volume was significantly higher than that in the Control +Vehicle group (P<0.001). Again, the medication group alleviated this phenomenon. CIA+MTX 1.4mpk group, CIA+MTX 0.5mp+ LBT-L group and CIA+MTX 0.5mp+ LBT-H group reduced the paw swelling to different degrees (P<0.05, P<0.01, P<0.001).
MTX and M-MTX Combined with LBT Can Alleviate Collagen-Induced Arthritis Inflammation and Cartilage Damage
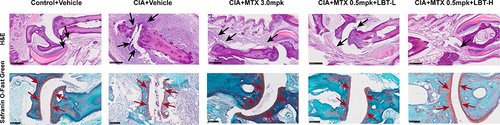
To investigate the anti-inflammatory effects of LBT on RA in vivo, we conducted histological analyses on CIA DBA/1J mice (). H&E staining revealed prominent histomorphological changes in the arthritis of the CIA model group, characterized by extensive infiltration of inflammatory cells and fibrosis in the interdigital joints and surrounding tissues of the mouse paws. Additionally, joint synovial hyperplasia and erosion of the joint cartilage were observed. In comparison to the CIA+Vehicle group, the high dose of MTX (H-MTX) group, as well as the high and low dose LBT combined with M-MTX groups, exhibited significant improvement. These treatment groups showed reduced infiltration of inflammatory cells, slight synovial membrane hyperplasia, and no evident bone destruction. Analysis of Safranin O-Fast Green staining revealed that the articular cartilage matrix between the hind paws and toes of the CIA+Vehicle group was visibly diminished, with multiple cracks present. However, treatment with H-MTX or M-MTX combined with LBT resulted in reduced cartilage injury in CIA mice, with no significant loss of cartilage matrix observed. This was evident from the smooth surface of the articular cartilage and the absence of cartilage fractures.
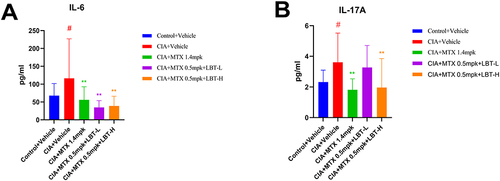
LBT Effectively Suppressed the Production of Inflammatory Factors in the Serum
To assess the impact of LBT on inflammatory factors, we conducted enzyme-linked immunosorbent assay (ELISA) tests to measure the levels of IL-6 and IL-17A in the serum. The concentrations of IL-6 and IL-17A in the serum of CIA+Vehicle group mice were significantly elevated compared to the control+Vehicle group (P < 0.05) ( and ). However, the H-MTX group and the M-MTX combined with LBT treatment group significantly reduced the serum levels of these inflammatory cytokines. Notably, the combined treatment with a high dose of LBT exhibited the most pronounced effect.
Figure 3 The Effects of Methotrexate or Methotrexate Combined with LBT on Serum Inflammation in DBA/1J Mice with Collagen-Induced Arthritis (Control+Vehicle group n = 10, other groups n = 15). (A) Serum IL-6 Levels; (B) Serum IL-17 Levels. ** P < 0.01, compare with CIA+Vehicle. # P < 0.05, compare with Control +Vehicle.
Establishment of Network Pharmacology and Target Analysis of LBT and RA
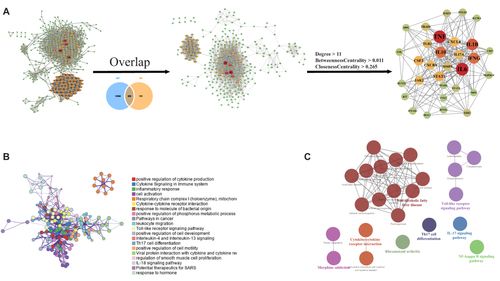
In view of the efficacy of LBT, to further understand its mechanism of action, we constructed a network pharmacology of rheumatoid arthritis and LBT. Based on the pathogenic genes reported in the RA literature and the therapeutic effect targets of approved drugs, the PPI molecular network of RA-specific pathogenesis was constructed and mechanism were explored. There are 309 targets that can be regulated by LBT, and 31 key targets were selected through network parameters, such as IL6, TNF, IL1B, IL10, STAT1, CXCL8, JAK2, IL17A, TLR2, MMP9, etc. (threshold: degree> 11, between centrality> 0.011, closeness centrality> 0.265) (). In addition, key biological processes regulated by LBT include inflammatory response, cytokine-mediated signaling pathway, leukocyte migration, etc. (). IL-17 signaling pathway, Th17 cell differentiation, Toll-like receptor signaling pathway, NF-kappa B signaling pathway, and Rheumatoid arthritis, etc. are the potential key signaling pathways regulated by LBT exerting RA effects ().
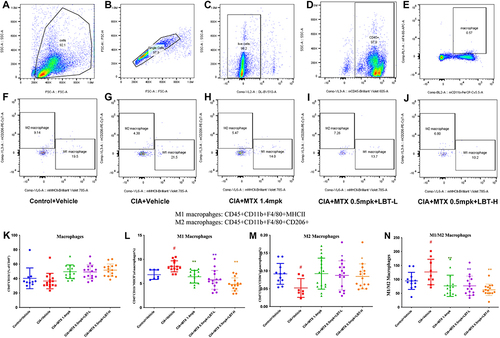
Effect of LBT Combined with MTX Treatment on Macrophages
Based on the network pharmacology results, we found that LBT targets multiple inflammatory pathways, so we first performed flow cytometry analysis of macrophages in serum. Macrophages were screened from serum samples of each group, following the general procedure shown (). Under inflammatory conditions, macrophages undergo activation and differentiation into two distinct subtypes: classically activated macrophages (M1) and alternatively activated macrophages (M2). Thus, further differentiation between M1/M2 macrophages was conducted in each group (), and macrophages in each group were quantified (). Our findings revealed a dysregulated expression pattern of macrophage subtypes in the CIA+Vehicle group, characterized by a high proportion of M1 macrophages () and a low proportion of M2 macrophages (), leading to an imbalanced M1/M2 ratio (). However, treatment with H-MTX or M-MTX combined with LBT resulted in a shift in macrophage polarization, with an increase in the proportion of M2 macrophages, which possess anti-inflammatory properties, and a suppression of M1 macrophages, which have pro-inflammatory characteristics.
Figure 5 Effect of LBT combined with MTX treatment on macrophages. (A) Flow Cytometry Sample Charts Depicting All Cells Detected in a Single Sample; (B) Diagram of a Single Cell in a Single Sample; (C) Diagram of Living Cells in a Single Sample; (D) Examples of White Blood Cells in a Single Sample; (E) Samples of Macrophages in a Single Sample; (F) Macrophage Polarization Levels in a Single Sample of the Control + Vehicle Group; (G) Macrophage Polarization Levels in a Single Sample of the CIA + Vehicle Group; (H) Macrophage Polarization Levels in the CIA + MTX 1.4mpk Group; (I) Macrophage Polarization Levels in the CIA + MTX 0.5mpk + LBT-L Group; (J) Macrophage Polarization Levels in the CIA + MTX 0.5mpk + LBT-L Group; (K) Macrophage Levels in the Blood of Each Group; (L) The Number of M1 Macrophages in Each Group; (M) The Number of M2 Macrophages in Each Group; (N) The M1/M2 Ratio of Macrophages in Each Group. #P < 0.05, compare with Control +Vehicle; * P < 0.05, compare with CIA+Vehicle; ** P < 0.01, compare with CIA+Vehicle.
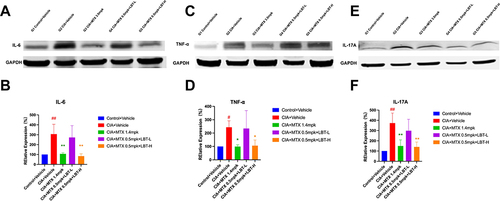
LBT Combined with M-MTX Inhibited the Expression of IL-6, TNF-α and IL-17A Proteins
To investigate the impact on local inflammation, we conducted Western Blot analysis on paw tissue samples, focusing on the corresponding inflammatory factors. As depicted in , the expression levels of IL-6, TNF-α, and IL-17A were significantly lower in the Control + Vehicle group compared to the CIA + Vehicle group. Remarkably, the CIA + MTX 1.4 mpk group and the CIA + MTX 0.5 mpk + LBT-H group exhibited a substantial downregulation of IL-6 and IL-17A expressions (p < 0.01). Additionally, TNF-α showed a moderate decrease (p < 0.05). These observations highlight the significant reduction in IL-6, IL-17A, and TNF-α levels in CIA mice following treatment with MTX 0.5 mpk + LBT-H, achieving a therapeutic efficacy comparable to that of high-dose MTX. However, it is important to note that the regulatory effect on inflammatory factors in the paw tissues of the CIA + MTX 0.5 mpk + LBT-L group was suboptimal.
Figure 6 Effects of MTX combined with LBT on inflammation-related markers (n = 3). (A, C, E) representative Western blot bands of IL-6, TNF-α and IL-17A. (B, D, F) Quantitative analysis of the relative expression levels of IL-6, TNF-α and IL-17A. # p < 0.05, compared with Control +Vehicle; ## p < 0.01, compared with Control +Vehicle; * P < 0.05, compare with CIA+ Vehicle; ** P < 0.01, compare with CIA+ Vehicle.
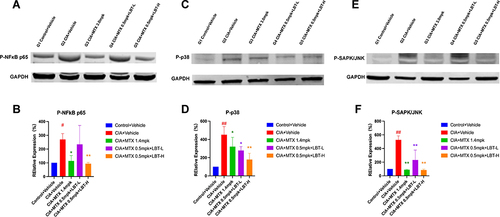
LBT Combined with M-MTX Regulates IL-17A-Related Signaling Pathways
In light of the aforementioned experimental findings, we were particularly intrigued by the expression of IL-17A, as recent studies have implicated its pivotal role in the pathogenesis of RA.Citation52–54 Consequently, we proceeded to investigate the pharmacological mechanism underlying the downstream signaling pathway of IL-17. To this end, we employed Western Blot analysis to assess the levels of downstream inflammation-related proteins, namely P-NFκB p65, P-p38, and P-SAPK/JNK. As depicted in and , the expression of P-NFκB p65 in the paw tissue of CIA + Vehicle mice was significantly upregulated compared to the Control + Vehicle group (p < 0.05). However, treatment with MTX 1.4 mpk or MTX 0.5 mpk + LBT-H effectively attenuated the levels of P-NFκB p65 in the paw tissue. Moreover, the expression levels of P-p38 and P-SAPK/JNK were notably elevated in the paw tissue of CIA + Vehicle mice relative to the Control + Vehicle group (). Remarkably, treatment with MTX or MTX combined with LBT resulted in a significant reduction in the expression of P-p38 and P-SAPK/JNK in the paw tissue of CIA mice (). Collectively, these findings strongly suggest that LBT exerts regulatory effects on the IL-17 inflammatory signaling pathway. Additionally, it is worth noting that while low-dose LBT combined with MTX also exhibited an effect, it was not as pronounced as that observed with high-dose LBT.
Figure 7 The impact of MTX or MTX combine LBT treatment on IL-17 signaling (n = 3). (A, C, E) Representative Western blot band. (B, D, F) Quantification of the relative expression of P-NFκB p65, P-p38 and P-SAPK/JNK. # p < 0.05, compared with Control +Vehicle; ## p < 0.01, compared with Control + Vehicle; * P < 0.05, compare with CIA+ Vehicle; ** P < 0.01, compare with CIA+ Vehicle.
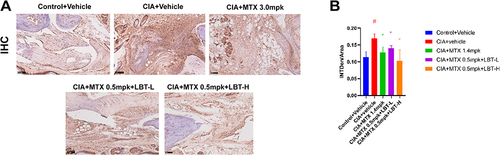
LBT Regulates the Production of IL17-Related Inflammatory Factors by Inhibiting the Proliferation of TH17 Cells
Based on the aforementioned findings, it has become evident that the primary mechanism underlying LBT therapy in CIA mice involves the regulation of IL-17 and its downstream inflammatory signaling pathways. Extensive research has demonstrated that IL-17 is primarily secreted by TH17 cells.Citation55,Citation56 Furthermore, the proliferation of TH17 cells has been identified as a key pathogenic factor in RA.Citation57,Citation58 In light of these observations, we conducted immunohistochemistry analysis to assess the expression of TH17 cells (). Our results revealed a significant increase in the expression of TH17 cells in the model group, particularly around the joints, compared to the Control + Vehicle group (). However, this increase was mitigated by LBT intervention. Consequently, we can infer that LBT primarily exerts its effects by inhibiting TH17 cell proliferation, thereby reducing the levels of inflammatory factors such as IL-17 and its downstream inflammatory pathways.
Discussion
In this study, we demonstrated that combined therapy with varying doses of LBT and M-MTX is effective in treating RA. Significantly, high-dose LBT combined with M-MTX matched the efficacy of the H-MTX dose. This combination notably reduced hallmark symptoms of CIA in mice, including synovial hyperplasia, paw swelling, arthritis scores, and cartilage degradation, and also alleviated local and systemic inflammation. Pharmacological network analysis and in vivo validation revealed that LBT’s effect in RA might be due to modulation of multiple inflammatory pathways, including the inhibition of Th17 cell proliferation and related signaling pathways.
While MTX remains a mainstay in treating inflammatory arthritis due to the lack of definitive cures,Citation59 its side effect profile, heavily influenced by dosage and regimen, remains a concern. Highlighting this dose-dependent variability, we employed a murine model of CIA to explore the efficacy of a novel combination therapy: LBT with a low MTX maintenance dose (0.5mg/kg every other day, mirroring the clinically recommended 7.5mg/week). Notably, despite this significantly lower MTX dose compared to the monotherapy group receiving the high dose (20mg/week, equivalent to 1.4mg/kg every other day), this LBT-MTX combination demonstrated comparable, if not superior, efficacy in certain parameters, particularly reducing arthritis activity and paw swelling (). These findings suggest that LBT-MTX combination therapy could not only significantly enhance the therapeutic effectiveness of MTX but also hold promise for mitigating safety concerns associated with long-term use of high-dose MTX.
The intricate nature of Traditional Chinese Medicine (TCM) compounds often involves targeting multiple components and pathways, making their therapeutic mechanisms challenging to elucidate.Citation60 To address this complexity, we employed network pharmacology analysis, a method merging systems biology and network theory,Citation61,Citation62 to elucidate the mechanisms of LBT in RA treatment. This approach excels at revealing intricate drug-disease-pathway interactions, particularly in the context of TCM.Citation63 Our analysis revealed that LBT directly targets key inflammatory molecules like IL-6, TNF-α, and IL-17A (), suggesting its potent anti-inflammatory effects. Abundant in the synovial fluid and tissue of RA patients, M1 macrophages, or classically activated macrophages, play a crucial role in RA pathogenesis.Citation64,Citation65 They are major producers of pro-inflammatory cytokines, including IL-6 and TNF-α, which drive crucial disease processes.Citation66,Citation67 These macrophage-derived cytokines not only contribute to pannus formation but also stimulate osteoclastogenesis, leading to bone erosion, a hallmark of RA severity.Citation68,Citation69 Additionally, emerging evidence highlights the key role of Th17 cells in RA pathogenesis.Citation70 Their elevated presence in RA patients and their ability to secrete pro-inflammatory cytokines like IL-17A further illustrate their pathogenic contribution.Citation71–73 Furthermore, clinical studies report high levels of IL-17A in RA patients, and anti-IL-17 therapy has demonstrated efficacy in reducing arthritis severity in animal models.Citation74,Citation75 These findings collectively support the potential of LBT in modulating both M1 macrophage and Th17 cell signaling pathways, thereby targeting critical pathogenic factors in RA.
To ascertain if the observed synergistic effect stemmed from inflammation suppression, we examined the impact of LBT in combination with M-MTX on M1 macrophage polarization. Flow cytometry analysis showed that this combination therapy led to a decrease in pro-inflammatory M1 macrophages and an increase in anti-inflammatory M2 macrophages, effectively altering the M1/M2 ratio. Notably, LBT combined with M-MTX outperformed the H-MTX in modulating macrophage polarization (). Immunohistochemical analysis showed that the combination of LBT and M-MTX more effectively diminished Th17 cell expression compared to the H-MTX (). Additionally, we assessed the levels of inflammatory cytokines, including IL-17 and IL-6, in the serum and paw tissues. The results demonstrated that high-dose LBT with M-MTX was as effective as H-MTX in diminishing inflammatory cytokine expression (). A subsequent focus on IL-17’s downstream signaling pathways, such as NF-κB, MAPK, ERK, and JNK, revealed that the LBT and M-MTX combination notably inhibited these inflammation-related pathways, showing greater efficacy than H-MTX alone (). All these results indicated that high-dose LBT in combination with M-MTX exhibits a clear advantage in anti-inflammatory effects compared to M-MTX.
Conclusions
In conclusion, our findings demonstrate that LBT significantly enhances the anti-inflammatory effects of MTX at a lower dose, potentially offering a safer and more effective treatment option for RA patients. This synergy likely involves targeting multiple pathways, including modulation of macrophage polarization, Th17 cell activation, and downstream inflammatory signaling.
Abbreviations
LBT, LuoBiTong; MTX, Methotrexate; RA, Rheumatoid arthritis; DMARDs disease-modifying anti-rheumatic drugs; TCM, Traditional Chinese medicine; TwHF, Tripterygium wilfordii Hook F; SMMs, small molecular medicines; H&E, hematoxylin-eosin staining; ELISA, enzyme-linked immunosorbent assays; Th17, helper T cell-17; IL-17A, Interleukin-17A; IL-17F, Interleukin-17F; IL-6, Interleukin 6; TNF-α, tumor necrosis factor-alpha; NFκB, nuclear factor kappa B; IHC, immunohistochemical.
Ethics Statements
This study was approved by the Ethics Committee of Jiangsu Jicuiyaokang Institutional Animal Care and Use Committee, and all animals received humanitarian care. The institution is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC International) and holds a license for animal use.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work. All authors read and approved the final manuscript.
Disclosure
The authors report no conflicts of interest in this work.
Additional information
Funding
References
- Tobón GJ, Youinou P, Saraux A. The environment, geo-epidemiology, and autoimmune disease: rheumatoid arthritis. J Autoimmun. 2010;35(1):10–14. doi:10.1016/j.jaut.2009.12.009
- Balanescu AR, Citera G, Pascual-Ramos V, et al. Infections in patients with rheumatoid arthritis receiving tofacitinib versus tumour necrosis factor inhibitors: results from the open-label, randomised controlled ORAL Surveillance trial. Ann Rheum Dis. 2022;81(11):1491–1503. doi:10.1136/ard-2022-222405
- Tang Y, Zhang Y, Li L, Xie Z, Wen C, Huang L. Kunxian Capsule for Rheumatoid Arthritis: inhibition of Inflammatory Network and Reducing Adverse Reactions Through Drug Matching. Front Pharmacol. 2020;11:485. doi:10.3389/fphar.2020.00485
- Zhu L, Wang J, Wei T, et al. Effects of Naringenin on inflammation in complete freund’s adjuvant-induced arthritis by regulating Bax/Bcl-2 balance. Inflammation. 2015;38(1):245–251. doi:10.1007/s10753-014-0027-7
- Hu C, Qian L, Miao Y, et al. Antigen-presenting effects of effector memory Vγ9Vδ2 T cells in rheumatoid arthritis. Cell Mol Immunol. 2012;9(3):245–254. doi:10.1038/cmi.2011.50
- Liu W, Zhang Y, Zhu W, et al. Sinomenine Inhibits the Progression of Rheumatoid Arthritis by Regulating the Secretion of Inflammatory Cytokines and Monocyte/Macrophage Subsets. Front Immunol. 2018;9:2228. doi:10.3389/fimmu.2018.02228
- Abtahi S, Driessen J, Burden AM, et al. Low-dose oral glucocorticoid therapy and risk of osteoporotic fractures in patients with rheumatoid arthritis: a cohort study using the Clinical Practice Research Datalink. Rheumatology (Oxford). 2022;61(4):1448–1458. doi:10.1093/rheumatology/keab548
- Morris G, Berk M, Walder K, Maes M. Central pathways causing fatigue in neuro-inflammatory and autoimmune illnesses. BMC Med. 2015;13:28. doi:10.1186/s12916-014-0259-2
- Roubille C, Richer V, Starnino T, et al. The effects of tumour necrosis factor inhibitors, methotrexate, non-steroidal anti-inflammatory drugs and corticosteroids on cardiovascular events in rheumatoid arthritis, psoriasis and psoriatic arthritis: a systematic review and meta-analysis. Ann Rheum Dis. 2015;74(3):480–489. doi:10.1136/annrheumdis-2014-206624
- Lee DM, Weinblatt ME. Rheumatoid arthritis. Lancet. 2001;358(9285):903–911. doi:10.1016/S0140-6736(01)06075-5
- Dougados M, Soubrier M, Antunez A, et al. Prevalence of comorbidities in rheumatoid arthritis and evaluation of their monitoring: results of an international, cross-sectional study (COMORA). Ann Rheum Dis. 2014;73(1):62–68. doi:10.1136/annrheumdis-2013-204223
- Peng X, Wang Q, Li W, et al. Comprehensive overview of microRNA function in rheumatoid arthritis. Bone Res. 2023;11(1):8. doi:10.1038/s41413-023-00244-1
- Roberts CA, Dickinson AK, Taams LS. The Interplay Between Monocytes/Macrophages and CD4(+) T Cell Subsets in Rheumatoid Arthritis. Front Immunol. 2015;6:571. doi:10.3389/fimmu.2015.00571
- Shi X, Song P, Tao S, Zhang X, Chu CQ. Silencing RORγt in Human CD4(+) T cells with CD30 aptamer-RORγt shRNA Chimera. Sci Rep. 2019;9(1):10375. doi:10.1038/s41598-019-46855-9
- Tu J, Huang W, Zhang W, Mei J, Zhu C. A Tale of Two Immune Cells in Rheumatoid Arthritis: the Crosstalk Between Macrophages and T Cells in the Synovium. Front Immunol. 2021;12:655477. doi:10.3389/fimmu.2021.655477
- Yoon SS, Moon EY. B Cell Adhesion to Fibroblast-Like Synoviocytes Is Up-Regulated by Tumor Necrosis Factor-Alpha via Expression of Human Vascular Cell Adhesion Molecule-1 Mediated by B Cell-Activating Factor. Int J Mol Sci. 2021;22(13):7166. doi:10.3390/ijms22137166
- Fu Y, Chryssafidis AL, Browne JA, O’Sullivan J, McGettigan PA, Mulcahy G. Transcriptomic Study on Ovine Immune Responses to Fasciola hepatica Infection. PLoS Negl Trop Dis. 2016;10(9):e0005015. doi:10.1371/journal.pntd.0005015
- Li X, Lei Y, Gao Z, et al. Effect of IL-34 on T helper 17 cell proliferation and IL-17 secretion by peripheral blood mononuclear cells from rheumatoid arthritis patients. Sci Rep. 2020;10(1):22239. doi:10.1038/s41598-020-79312-z
- Joseph RM, Ray DW, Keevil B, van Staa TP, Dixon WG. Low salivary cortisol levels in patients with rheumatoid arthritis exposed to oral glucocorticoids: a cross-sectional study set within UK electronic health records. RMD Open. 2018;4(2):e000700. doi:10.1136/rmdopen-2018-000700
- Li J, Bao J, Zeng J, Yan A, Zhao C, Shu Q. Iguratimod: a valuable remedy from the Asia Pacific region for ameliorating autoimmune diseases and protecting bone physiology. Bone Res. 2019;7:27. doi:10.1038/s41413-019-0067-6
- Singh JA, Hossain A, Mudano AS, et al. Biologics or tofacitinib for people with rheumatoid arthritis naive to methotrexate: a systematic review and network meta-analysis. Cochrane Database Syst Rev. 2017;5(5):CD012657. doi:10.1002/14651858.CD012657
- Arbab D, König DP. Atraumatic Femoral Head Necrosis in Adults. Dtsch Arztebl Int. 2016;113(3):31–38. doi:10.3238/arztebl.2016.0031
- Bellinger DL, Wood C, Wergedal JE, Lorton D. Driving β2- While Suppressing α-Adrenergic Receptor Activity Suppresses Joint Pathology in Inflammatory Arthritis. Front Immunol. 2021;12:628065. doi:10.3389/fimmu.2021.628065
- Pham DT, Thao N, Thuy B, Tran V, Nguyen T, Nguyen N. Silk fibroin hydrogel containing Sesbania sesban L. extract for rheumatoid arthritis treatment. Drug Deliv. 2022;29(1):882–888. doi:10.1080/10717544.2022.2050848
- Davies H, Olson L, Gibson P. Methotrexate as a steroid sparing agent for asthma in adults. Cochrane Database Syst Rev. 2000;1998(2):CD000391. doi:10.1002/14651858.CD000391
- Vanni K, Lyu H, Solomon DH. Cytopenias among patients with rheumatic diseases using methotrexate: a meta-analysis of randomized controlled clinical trials. Rheumatology (Oxford). 2020;59(4):709–717. doi:10.1093/rheumatology/kez343
- Emery P, Burmester GR, Bykerk VP, et al. Evaluating drug-free remission with Abatacept in early rheumatoid arthritis: results from the phase 3b, multicentre, randomised, active-controlled AVERT study of 24 months, with a 12-month, double-blind treatment period. Ann Rheum Dis. 2015;74(1):19–26. doi:10.1136/annrheumdis-2014-206106
- Schaff LR, Lobbous M, Carlow D, et al. Routine use of low-dose glucarpidase following high-dose methotrexate in adult patients with CNS lymphoma: an open-label, multi-center Phase I study. BMC Cancer. 2022;22(1):60. doi:10.1186/s12885-021-09164-x
- Zhai KF, Duan H, Chen Y, et al. Apoptosis effects of imperatorin on synoviocytes in rheumatoid arthritis through mitochondrial/caspase-mediated pathways. Food Funct. 2018;9(4):2070–2079. doi:10.1039/c7fo01748k
- Zhai KF, Duan H, Khan GJ, et al. Salicin from Alangium chinense Ameliorates Rheumatoid Arthritis by Modulating the Nrf2-HO-1-ROS Pathways. J Agric Food Chem. 2018;66(24):6073–6082. doi:10.1021/acs.jafc.8b02241
- Ma Z, Fan Y, Wu Y, et al. Traditional Chinese medicine-combination therapies utilizing nanotechnology-based targeted delivery systems: a new strategy for antitumor treatment. Int J Nanomed. 2019;14:2029–2053. doi:10.2147/IJN.S197889
- Luo Y, Hou X, Xi A, Luo M, Wang K, Xu Z. Tripterygium wilfordii Hook F combination therapy with methotrexate for rheumatoid arthritis: an updated meta-analysis. J Ethnopharmacol. 2023;307:116211. doi:10.1016/j.jep.2023.116211
- Zhou YZ, Zhao LD, Chen H, et al. Comparison of the impact of Tripterygium wilfordii Hook F and Methotrexate treatment on radiological progression in active rheumatoid arthritis: 2-year follow up of a randomized, non-blinded, controlled study. Arthritis Res Ther. 2018;20(1):70. doi:10.1186/s13075-018-1563-6
- Li M, Luo Q, Chen X, et al. Screening of major hepatotoxic components of Tripterygium wilfordii based on hepatotoxic injury patterns. BMC Complement Med Ther. 2023;23(1):9. doi:10.1186/s12906-023-03836-w
- Wei X, Zhou R, Chen Y, et al. Systemic pharmacological verification of Baixianfeng decoction regulating TNF-PI3K-Akt-NF-κB pathway in treating rheumatoid arthritis. Bioorg Chem. 2022;119:105519. doi:10.1016/j.bioorg.2021.105519
- Yuan H, Ma Q, Cui H, et al. How Can Synergism of Traditional Medicines Benefit from Network Pharmacology. Molecules. 2017;22(7):1135. doi:10.3390/molecules22071135
- Piñero J, Ramírez-Anguita JM, Saüch-Pitarch J, et al. The DisGeNET knowledge platform for disease genomics: 2019 update. Nucleic Acids Res. 2020;48(D1):D845–D855. doi:10.1093/nar/gkz1021
- Carvalho-Silva D, Pierleoni A, Pignatelli M, et al. Open Targets Platform: new developments and updates two years on. Nucleic Acids Res. 2019;47(D1):D1056–D1065. doi:10.1093/nar/gky1133
- Rappaport N, Twik M, Plaschkes I, et al. MalaCards: an amalgamated human disease compendium with diverse clinical and genetic annotation and structured search. Nucleic Acids Res. 2017;45(D1):D877–D887. doi:10.1093/nar/gkw1012
- Davis AP, Grondin CJ, Johnson RJ, et al. The Comparative Toxicogenomics Database: update 2019. Nucleic Acids Res. 2019;47(D1):D948–D954. doi:10.1093/nar/gky868
- Safran M, Dalah I, Alexander J, et al. GeneCards Version 3: the human gene integrator. Database (Oxford). 2010;2010:baq020. doi:10.1093/database/baq020
- Dennis G, Sherman BT, Hosack DA, et al. DAVID: database for Annotation, Visualization, and Integrated Discovery. Genome Biol. 2003;4(5).
- Szklarczyk D, Morris JH, Cook H, et al. The STRING database in 2017: quality-controlled protein-protein association networks, made broadly accessible. Nucleic Acids Res. 2017;45(D1):D362–D368. doi:10.1093/nar/gkw937
- Shannon P, Markiel A, Ozier O, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13(11):2498–2504. doi:10.1101/gr.1239303
- Wishart DS, Feunang YD, Guo AC, et al. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 2018;46(D1):D1074–D1082. doi:10.1093/nar/gkx1037
- Wang Y, Zhang S, Li F, et al. Therapeutic target database 2020: enriched resource for facilitating research and early development of targeted therapeutics. Nucleic Acids Res. 2020;48(D1):D1031–D1041. doi:10.1093/nar/gkz981
- Gaulton A, Hersey A, Nowotka M, et al. The ChEMBL database in 2017. Nucleic Acids Res. 2017;45(D1):D945–D954. doi:10.1093/nar/gkw1074
- UniProt Consortium. UniProt: a worldwide hub of protein knowledge. Nucleic Acids Res. 2019;47(D1):D506–D515. doi:10.1093/nar/gky1049
- Davis AP, Wiegers TC, Johnson RJ, Sciaky D, Wiegers J, Mattingly CJ. Comparative Toxicogenomics Database (CTD): update 2023. Nucleic Acids Res. 2023;51(D1):D1257–D1262. doi:10.1093/nar/gkac833
- Kim S, Thiessen PA, Bolton EE, et al. PubChem Substance and Compound databases. Nucleic Acids Res. 2016;44(D1):D1202–1213. doi:10.1093/nar/gkv951
- Bindea G, Mlecnik B, Hackl H, et al. ClueGO: a Cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics. 2009;25(8):1091–1093. doi:10.1093/bioinformatics/btp101
- Kotake S, Udagawa N, Takahashi N, et al. IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J Clin Invest. 1999;103(9):1345–1352. doi:10.1172/JCI5703
- Taams LS. Interleukin-17 in rheumatoid arthritis: trials and tribulations. J Exp Med. 2020;217(3):e20192048. doi:10.1084/jem.20192048
- van den Berg WB, Miossec P. IL-17 as a future therapeutic target for rheumatoid arthritis. Nat Rev Rheumatol. 2009;5(10):549–553. doi:10.1038/nrrheum.2009.179
- Cua DJ, Tato CM. Innate IL-17-producing cells: the sentinels of the immune system. Nat Rev Immunol. 2010;10(7):479–489. doi:10.1038/nri2800
- McGeachy MJ, Cua DJ, Gaffen SL. The IL-17 Family of Cytokines in Health and Disease. Immunity. 2019;50(4):892–906. doi:10.1016/j.immuni.2019.03.021
- Lin J, Tang J, Lin J, et al. YY1 regulation by miR-124-3p promotes Th17 cell pathogenicity through interaction with T-bet in rheumatoid arthritis. JCI Insight. 2021;6(22):e149985. doi:10.1172/jci.insight.149985
- Roeleveld DM, van Nieuwenhuijze AE, van den Berg WB, Koenders MI. The Th17 pathway as a therapeutic target in rheumatoid arthritis and other autoimmune and inflammatory disorders. BioDrugs. 2013;27(5):439–452. doi:10.1007/s40259-013-0035-4
- Kumar LD, Karthik R, Gayathri N, Sivasudha T. Advancement in contemporary diagnostic and therapeutic approaches for rheumatoid arthritis. Biomed Pharmacother. 2016;79:52–61. doi:10.1016/j.biopha.2016.02.001
- Jiang H, Li M, Du K, et al. Traditional Chinese Medicine for adjuvant treatment of breast cancer: taohong Siwu Decoction. Chin Med. 2021;16(1):129. doi:10.1186/s13020-021-00539-7
- Hou J, Chen W, Lu H, et al. Exploring the Therapeutic Mechanism of Desmodium styracifolium on Oxalate Crystal-Induced Kidney Injuries Using Comprehensive Approaches Based on Proteomics and Network Pharmacology. Front Pharmacol. 2018;9:620. doi:10.3389/fphar.2018.00620
- Kong X, Liu C, Lu P, et al. Combination of UPLC-Q-TOF/MS and Network Pharmacology to Reveal the Mechanism of Qizhen Decoction in the Treatment of Colon Cancer. ACS Omega. 2021;6(22):14341–14360. doi:10.1021/acsomega.1c01183
- Guo B, Zhao C, Zhang C, et al. Elucidation of the anti-inflammatory mechanism of Er Miao San by integrative approach of network pharmacology and experimental verification. Pharmacol Res. 2022;175:106000. doi:10.1016/j.phrs.2021.106000
- Han C, Yang Y, Sheng Y, et al. Glaucocalyxin B inhibits cartilage inflammatory injury in rheumatoid arthritis by regulating M1 polarization of synovial macrophages through NF-κB pathway. Aging (Albany NY). 2021;13(18):22544–22555. doi:10.18632/aging.203567
- Kanagawa H, Masuyama R, Morita M, et al. Methotrexate inhibits osteoclastogenesis by decreasing RANKL-induced calcium influx into osteoclast progenitors. J Bone Miner Metab. 2016;34(5):526–531. doi:10.1007/s00774-015-0702-2
- Nistala K, Adams S, Cambrook H, et al. Th17 plasticity in human autoimmune arthritis is driven by the inflammatory environment. Proc Natl Acad Sci U S A. 2010;107(33):14751–14756. doi:10.1073/pnas.1003852107
- Tardito S, Martinelli G, Soldano S, et al. Macrophage M1/M2 polarization and rheumatoid arthritis: a systematic review. Autoimmun Rev. 2019;18(11):102397. doi:10.1016/j.autrev.2019.102397
- Fang Q, Zhou C, Nandakumar KS. Molecular and Cellular Pathways Contributing to Joint Damage in Rheumatoid Arthritis. Mediators Inflamm. 2020;2020:3830212. doi:10.1155/2020/3830212
- Saxena Y, Routh S, Mukhopadhaya A. Immunoporosis: role of Innate Immune Cells in Osteoporosis. Front Immunol. 2021;12:687037. doi:10.3389/fimmu.2021.687037
- Qiuping L, Pan P, Zhenzhen L, Zhen Z, Xuezhu Z, Shuting L. Acupuncture regulates the Th17/Treg balance and improves cognitive deficits in a rat model of vascular dementia. Heliyon. 2023;9(2):e13346. doi:10.1016/j.heliyon.2023.e13346
- Hashimoto M. Th17 in Animal Models of Rheumatoid Arthritis. J Clin Med. 2017;6(7):73. doi:10.3390/jcm6070073
- Park H, Li Z, Yang XO, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6(11):1133–1141. doi:10.1038/ni1261
- Wu W, Huang J, Duan B, et al. Th17-stimulating protein vaccines confer protection against Pseudomonas aeruginosa pneumonia. Am J Respir Crit Care Med. 2012;186(5):420–427. doi:10.1164/rccm.201202-0182OC
- Deligne C, Casulli S, Pigenet A, et al. Differential expression of interleukin-17 and interleukin-22 in inflamed and non-inflamed synovium from osteoarthritis patients. Osteoarthritis Cartilage. 2015;23(11):1843–1852. doi:10.1016/j.joca.2014.12.007
- Pan F, Xiang H, Yan J, et al. Dendritic Cells from Rheumatoid Arthritis Patient Peripheral Blood Induce Th17 Cell Differentiation via miR-363/Integrin αv/TGF-β Axis. Scand J Immunol. 2017;85(6):441–449. doi:10.1111/sji.12550