Abstract
Purpose
With the rapid development of immunotherapy, cancer treatment has entered a new phase. Medical imaging, as a primary diagnostic method, is closely related to cancer immunotherapy. However, until now, there has been no systematic bibliometric analysis of the state of this field. Therefore, the main purpose of this article is to clarify the past research trajectory, summarize current research hotspots, reveal dynamic scientific developments, and explore future research directions.
Patients and Methods
A comprehensive search was conducted on the Web of Science Core Collection (WoSCC) database to identify publications related to immunotherapy specifically for the medical imaging of carcinoma. The search spanned the period from the year 2003 to 2023. Several analytical tools were employed. These included CiteSpace (6.2.4), and the Microsoft Office Excel (2016).
Results
By searching the database, a total of 704 English articles published between 2003 and 2023 were obtained. We have observed a rapid increase in the number of publications since 2018. The two most active countries are the United States (n=265) and China (n=170). Pittock, Sean J and Abu-sbeih, Hamzah are very concerned about the relationship between cancer immunotherapy and medical images and have published more academic papers (n = 5; n = 4). Among the top 10 co-cited authors, Topalian Sl (n=43) cited ranked first, followed by Graus F (n=40) cited. According to clustering, timeline, and burst word analysis, the results show that the current research focus is on “MRI”, “deep learning”, “tumor microenvironment” and so on.
Conclusion
Medical imaging and cancer immunotherapy are hot topics. The United States is the country with the most publications and the greatest influence in this field, followed by China. “MRI”, “PET/PET-CT”, “deep learning”, “immune-related adverse events” and “tumor microenvironment” are currently hot research topics and potential targets.
Introduction
Cancer Cancer is the second leading cause of human mortality. Data from 2020 indicates that there were 180 million cancer patients, and it is projected that global cancer incidence will continue to rise from 2020 to 2040. By 2040, it is estimated that there will be approximately 280 million new cancer cases annually, representing a 55% increase compared to 2020.Citation1 In 2011, the United States FDA approved the first immune checkpoint inhibitor, Ipilimumab, for second-line treatment of advanced melanoma, marking the beginning of a new era in cancer immunotherapy.Citation2 Immunotherapy is altering the treatment paradigm for cancer, ushering in a new phase of development. However, while immunotherapy enhances the immune system’s capacity to target tumor cells, it may also inadvertently damage other organs and tissues, increasing the risk of patients developing autoimmune diseases and causing side effects distinct from traditional cancer treatment methods. Moreover, unlike traditional cancer treatment modalities, patients undergoing immunotherapy may experience pseudo-progress ion or hyper-progression,Citation3 necessitating timely assessment and treatment modifications. Additionally, despite immunotherapy becoming a key component of cancer treatment, only 27.3% of individuals experience clinical benefits,Citation4 and 40% to 70% of the population develops acquired resistance.Citation5–7 Therefore, the need for supplementary tools to predict, assess, and monitor the efficacy and adverse reactions of immunotherapy is evident.
Medical imaging is the visual representation process of the structure and function of various tissues and organs in the human body, used for clinical purposes and medical scientific exploration.Citation8 The relationship between tumor treatment effectiveness and medical imaging is closely intertwined. The continually evolving field of immunotherapy further highlights the critical issue of imaging assessment while also presenting new opportunities for the development of medical imaging. Currently, the efficacy and adverse events of tumor immunotherapy can be preliminarily assessed through imaging studies.Citation9–11 It is also possible to establish image-based biomarkersCitation12,Citation13 or efficacy prediction models combined with clinical characteristics to screen the appropriate population for immunotherapy and implement personalized treatment.Citation14,Citation15 Clinically, it is routine to use various medical image-based efficacy evaluation standards for solid tumors to assess treatment efficacy.Citation16–18 However, there are still several shortcomings in the context of immunotherapy-related measurements, including a lack of standardized measurement criteria, limited predictive capability for adverse events and treatment efficacy, and an unclear diagnosis of progression patterns.Citation19–21 Given these limitations, we aim to summarize the current state of knowledge to further high-quality research, improving the evaluation capabilities of immunotherapy, and enhancing its overall effectiveness.
Over the past two decades, a substantial number of articles on cancer immunotherapy and medical imaging have been published in academic journals. However, there has been no systematic analysis of this data. Bibliometrics is a method for exploring research hotspots in specific fields.Citation22 In recent years, numerous bibliometric studies have been published in journals across various domains.Citation23,Citation24 The purpose of this article is to shed light on the research hotspots and new directions that have emerged in this field over the past two decades.
Materials and Methods
Data Collection
The reasons for selecting the WoSCC database are as follows:1) WoSCC is one of the commonly used databases in bibliometric analysis. It provides a rich source of literature, enabling researchers to conduct comprehensive literature searches and analyses; 2) WoSCC includes high-quality journals and comprehensive citation records, ensuring the credibility and accuracy of the research. This database offers abundant citation information, which aids in tracking the citation of literature and assessing the impact of research.Citation25,Citation26 To mitigate any potential bias introduced by database updates, we conducted literature searches and data export using the WoSCC on February 28th, 2024. The search terms were #1TS= (Immunotherapy) OR (immunotherapeutic) OR (immunotherapies) OR (immunization therapy) OR (immune therapy) OR (immunity treatment); #2TS= (cancer) OR (carcinoma) OR (tumor oncology) OR (neoplasms) OR (carcinomatosis) OR (tumor); #3TS= (medical imaging) OR (diagnostic imaging) OR (radiography). The time span was from January 1, 2003, to December 31, 2023, the language was set to English, and the article type was selected as “article”. Finally, 704 articles were included in the study. Detailed steps can be found in
Statistical Analysis
CiteSpace is a scientific literature analysis tool jointly developed by Dr. Chaomei Chen and the WISE laboratory. This software can visualize the relationships between literature in the form of a scientific knowledge map. It can not only help to clarify the past research trajectory, current research status, and present hot topics in a specific field but also reveal the future directions of that field.Citation25
We utilized CiteSpace (6.2.4) to analyze the collected literature. The analyzed data included authors and cited authors, institutions, countries/regions, cited journals, keywords, and references. The period was set from January 1, 2003, to December 31, 2023, with a yearly time slice. The parameters used were a K-value of 35, a link retention factor (LRF) of 3, look-back years (LBY) of 8, e-value (e) of 2.0, and time-link strength (cosine) with a range within the time slice. The selection criteria included the g-index (k = 25) and minimum duration (MD = 1). A log-likelihood ratio was employed as the clustering algorithm, and all clusters were annotated with keywords. Microsoft Office Excel (2016) was used to record data and analyze trends, as well as to simulate and predict future outcomes.
Results
Annual Growth Trends in Publication Volume
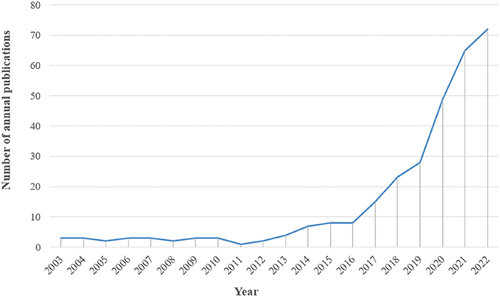
After searching the WoSCC database, a total of 704 medical imaging articles related to cancer immunotherapy, published between 2003 and 2023, were obtained. As shown in , from 2003 to 2011, the annual publication count was relatively low, indicating that research was in its early stages. From 2011 to 2018, there was a steady increase in annual publications, indicating a mid-stage development. Since 2018, there has been a rapid growth in annual publication numbers, reaching a peak in 2023. Between 2018 and 2023, there were a total of 524 articles on the topic of medical imaging in cancer immunotherapy, accounting for 74.4% of the past twenty years. These findings suggest that with the continuous development of cancer immunotherapy and its close connection to clinical practice, this topic has gained widespread attention in the medical field and has entered a rapid growth phase.
Analysis of Countries and Institutions
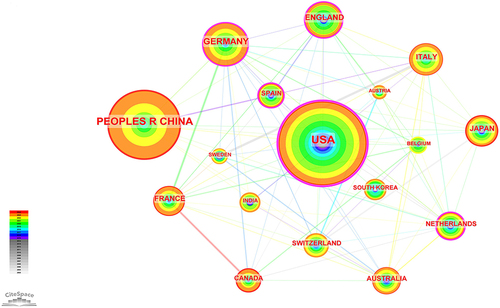
These publications originate from 58 different countries and 412 institutions. From the visual map (), it is evident that the United States not only has the highest cumulative publication count but also the highest annual publication count in the past year. Furthermore, from , it can be observed that the top ten countries are primarily distributed in Europe (n=6) and Asia (n=2). Among these countries, the United States has the highest publication count (n=265), followed by China (n=170). It’s worth noting that there is significant collaboration among different countries. For example, the United States is the most collaborative country, and China actively collaborates with multiple countries.
Table 1 Top10 Productive Countries in Cancer Immunotherapy and Medical Imaging
The top ten institutions are located in three countries, with the majority of them being in the United States (). The top three institutions in terms of publishing related papers are Harvard University (n=33), the University of Texas System (n=27), and Mayo Clinic (n=27). In CiteSpace, nodes with centrality exceeding 0.1 are considered key nodes.Citation27 In this study, two institutions with centrality exceeding 0.1 are Harvard University (USA, 0.1) and University of California System (USA, 0.1), indicating that these two institutions occupy central positions in the collaborative network. The data shows that while the United States maintains long-term dominance in terms of annual publications when combining both country and institution-level annual publication counts, there is still significant collaboration among institutions.
Table 2 Top10 Productive Institutions in Cancer Immunotherapy and Medical Imaging
Authors and Co-Cited Authors
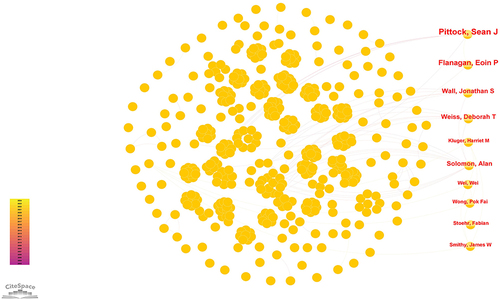
If multiple authors are cited in one or more papers at the same time, it is called a co-citation relationship between multiple authors.Citation23 A total of 884 researchers have been involved in studies related to cancer immunotherapy medical imaging. displays the top ten authorCitation28–37 with the highest publication volume and the top ten most co-cited authorsCitation38–47 in the research field. shows the co-occurrence of some authors. Among these, the top three authors with the highest number of publications are Pittock, Sean J (n=5), Abu-sbeih, Hamzah (n=4), and Flanagan, Eoin P (n=4). In the top 10 co-cited authors, Topalian Sl (n=43) ranks first, followed by Graus F (n=40) and Dalmau J (n=38). It’s worth noting that both the authors and co-cited authors have relatively low centrality (≤0.01), indicating a lack of influential authors in this field.
Table 3 Top10 Authors and Co-Cited Authors in Cancer Immunotherapy and Medical Imaging
References and Co-Cited Journals
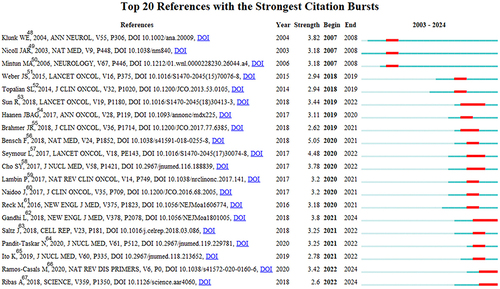
The most frequently cited references are often considered foundational to research in a specific field.Citation26 In our study, CiteSpace identified the top 20 burst-reference publications,Citation48–67 as shown in . The burst references appeared as early as 2007 and as late as 2022. The top burst reference with the highest intensity (5.05) is titled “89Zr-atezolizumab imaging as a non-invasive approach to assess clinical response to PD-L1 blockade in cancer”, written by Bensch et alCitation56, and it had a burst period from 2020 to 2021. The second highest burst reference (intensity=4.48) is ”iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics”, authored by Seymour et alCitation57 and published in The Lancet Oncology, with a burst period from 2020 to 2022. Overall, the burst intensity of these 20 references ranges from 2.62 to 5.05, with a duration of 2 to 4 years.
Figure 5 Top 20 references with the strongest citation bursts in Cancer Immunotherapy and Medical Imaging.
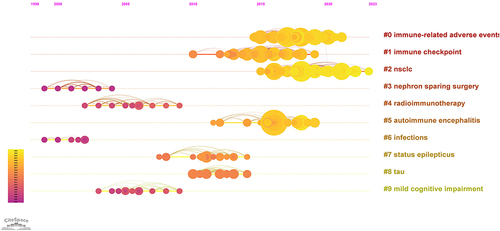
The timeline of references usually reflects research hotspots.Citation24 We systematically analyzed the temporal development of references (). From the perspective of references, the current research hotspots are primarily focused on #0 immune-related adverse events and #2 nsclc.
Knowledge distribution in a certain research field can be obtained through co-citation analysis of journals.Citation23 The top ten co-cited journals are presented in , with the top three co-cited journals being The New England Journal of Medicine (n=315), Journal of Clinical Oncology (n=232), and Clinical Cancer Research (n=202). Both topics have accumulated more than 200 published articles in the field.
Table 4 Top10 Co-Cited Journals in Cancer Immunotherapy and Medical Imaging
Keywords Analysis
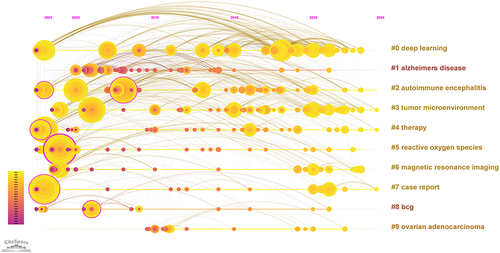
Keywords typically reflect the main topics and research content of articles.Citation27 By analyzing keyword co-occurrence, we can quickly grasp the focus and trends in a specific research field. We have listed the top 10 keywords by frequency in , and the co-occurrence of keywords is shown in , with “immunotherapy” being the most frequently mentioned keyword. In addition to “immunotherapy”, keywords that appear more than 50 times include “cancer” and “therapy”. Based on keyword co-occurrence, we identified 26 clusters and selected the top ten clusters, as shown in . Clusters with a module value (Q value) greater than 0.3 are considered significant, and clusters with an average silhouette value (S) greater than 0.7 are considered convincing.Citation68 Therefore, we can infer that our analysis results are both significant and convincing. Based on the analysis of keyword clustering () and timeline chart (), current research hotspots are primarily dominated by clusters related to #0 deep learning, #3 tumor microenvironment, and #6 MRI.
Table 5 Top ten Keywords in Cancer Immunotherapy and Medical Imaging
Figure 7 (A) A visual map for CiteSpace network among keywords. (B) The cluster of keywords in the studies of Cancer Immunotherapy and Medical Imaging. (C) Top 20 keywords with the strongest citation bursts in Cancer Immunotherapy and Medical Imaging.
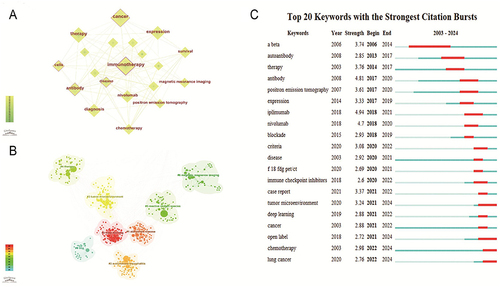
Keyword bursts are considered indicators of evolving frontiers or emerging topics in a specific research area over time.Citation68 In , we summarize the top 20 keywords based on their burst strength. In the diagram, “Year” represents the year when the keyword first appeared, while “Begin” and “End” indicate the years when the keyword began and ended its burst. The figure reflects research frontiers in different time periods. The keyword “ipilimumab” has the highest burst strength, with a burst strength of 4.94. Early bursts (2006–2017) include keywords like “therapy”, and “autoantibody”. Mid-term bursts (2017–2020) feature keywords such as “pet”, “blockade”, and “nivolumab”. In recent years (2020–2024), “Tumor microenvironment”, “deep learning”, and “lung cancer” have become the focus of current research attention. In the field of imaging, “pet-ct/pet” is the strongest and earliest prominent term.
Discussion
In today’s interconnected era across different academic disciplines, characterized by rapidly evolving knowledge, staying informed about the directions and trends in one’s target field is of paramount importance for researchers. To examine and describe the hotspots in the intersection of cancer immunotherapy and medical imaging, we utilized CiteSpace to identify emerging keywords. As illustrated in and , the evolution of emerging keywords over the past two decades reflects the ongoing progress in research related to the integration of immunotherapy and medical imaging. Combining keyword and highlighted word analysis, keywords such as“ MRI”, “deep learning”, and “tumor microenvironment” have become hot topics in the research field in recent years. Among the developments in imaging over the past 20 years, “PET, PET-CT” are undoubtedly among the most prominent keywords.
MRI has become an indispensable examination method in the process of tumor diagnosis and treatment due to its significant advantages of high soft tissue resolution and multi-parametric imaging. Vicentin et al conducted an in-depth study on the inter-observer reliability of the evaluation criteria for solid tumor response in patients with hepatocellular carcinoma receiving neoadjuvant therapy before liver transplantation and found that the inter-observer reliability showed different levels ranging from moderate to almost perfect in the evaluation of tumor number, size, and transplantation criteria, with the inter-observer reliability of MRI before neoadjuvant therapy being particularly prominent.Citation69 According to Response Evaluation Criteria in Solid Tumors (RECIST 1.1), MRI images can accurately reflect the therapeutic effect of solid tumors by comparing the size of target lesions before and after treatment due to their objectivity and reproducibility.Citation21 In addition to the Recist 1.1 standard, new imaging response standards, such as immune-related RECIST (irRECIST), immune-related response criteria (irRC), and immune-modified RECIST (imRECIST), are being implemented in various trials.Citation16–18 These standards not only enrich the evaluation system of tumor efficacy but also provide strong support for individualized treatment plans of tumor. At the same time, MRI can provide parameters such as signal characteristics and enhancement degree as biomarkers,Citation70,Citation71 and combined with the advantages of MRI functional imaging,Citation72 provide important reference for clinical diagnosis and treatment. With the rapid development of artificial intelligence and big data technology, these biomarkers can be further expanded to more complex radiomics and other advanced features. For example, based on the integrated imaging omics model of T2WI and ADC images, Xue et alCitation73 successfully constructed a non-invasive tool for predicting preoperative immune scores in rectal cancer, which is of great significance for evaluating patient prognosis and guiding individualized immunotherapy. Similarly, Gong et al also established an imaging omics model based on MRI, which has the potential to predict PD-1 expression in preoperative hepatocellular carcinoma and is expected to become an imaging biomarker for PD-1 therapy.Citation74 In addition, using special nanomaterials to label immune cells, with the help of MRI technology, we can effectively track and monitor the distribution, migration, and activity of immune cells in the body. This not only enables real-time understanding of the dynamics of immune cells but also provides powerful guidance for adjusting and optimizing immunotherapy regimens. Tremblay et al used MRI technology to quantitatively track cytotoxic T lymphocytes (CTLs) and myeloid cells (MLCs) labeled with superparamagnetic iron oxide nanoparticles (SPIO) in vitro.Citation75 The results showed that these quantitative molecular MRI techniques can be extended to the study of various cancers and immunotherapy combinations, thereby deepening the understanding of longitudinal immunological changes and their mechanisms. Dekaban et al reviewed the use of MRI technology to track the migration of dendritic cells (DCs) labeled with iron oxide nanoparticles in vitro.Citation76 By quantitatively tracking DC migration, we can deeply explore the influence of different maturation stages on migration efficiency, and thus judge the vaccine immunogenicity and the effect of tumor immunotherapy. Wu et al improved the efficacy of DC vaccine therapy for cancer by using N-alkyl-PEI2k-LAC/SPIO nanocomposite materials and monitored the homing of the vaccine in vivo using MRI technology.Citation77 The experimental results showed that this improved DC vaccine performed well in suppressing tumors, providing new hope and possibilities for cancer treatment.
Moreover, with the widespread application of Artificial Intelligence (AI) in the field of medicine, it has become a hot topic in such literature. AI is generally defined as a set of advanced computational algorithms that aid in performing tasks requiring human intelligence, including language interpretation, visual perception, decision-making, and precise reasoning and learning from vast amounts of data.Citation13,Citation78 In the realm of medical imaging, AI consists of two main components: radiomics and deep learning. It is now widely employed across various medical fields, including medical imaging,Citation14 nuclear medicine,Citation79 musculoskeletal systems,Citation80 and ophthalmology,Citation81 among others. Radiomics involves the collection, extraction, quantification, and analysis of medical images through image segmentation and feature extraction.Citation82 It serves as a non-invasive biomarker for assessing the response to cancer immunotherapy.Citation83 Tunali’s study has utilized radiomics and clinical data to build predictive models for identifying non-small cell lung cancer (NSCLC) patients who do not respond markedly to immunotherapy.Citation15 Granata et al have used radiomics features extracted from CT scans to differentiate beneficiaries of immunotherapy among lung adenocarcinoma patients.Citation84 These features also serve as non-invasive markers for tumor recurrence prediction, facilitating patient stratification for personalized management.Citation85 Deep learning, on the other hand, is gaining widespread attention due to its superior capabilities in handling massive data when compared to traditional methods.Citation86 Tian et al conducted a retrospective analysis of 939 NSCLC patients’ PD-L1 (Programmed Cell Death Protein 1) dataset, using a CT-based deep learning model to predict PD-L1 expression in NSCLC patients and infer clinical outcomes.Citation87 Similarly, He et al collected CT images from 327 patients with tumor mutational burden (TMB) features. By combining deep learning and imaging, they developed a non-invasive imaging biomarker capable of distinguishing between high TMB and low TMB patients and successfully predicting the outcomes of NSCLC patients undergoing immunotherapy.Citation88
Furthermore, in recent years, researchers have increasingly focused on the cellular and molecular level, introducing the concept of the tumor microenvironment (TME).Citation89 The immune infiltration within the TME has been proven to play a crucial role in tumor development and impact the clinical outcomes of cancer patients.Citation90 A recent study collected CT imaging data from 2686 gastric cancer patients, revealing that radiological models can accurately predict TME. Importantly, it was found to be an independent prognostic factor surpassing clinical and pathological variables. Moreover, imaging features can identify which patients will benefit from treatment and enhance the predictive ability of immunotherapy responses.Citation91 Sun et al integrated multiple machine learning algorithms based on CT image features to construct non-invasive imaging omics biomarkers that can predict the immune microenvironment of the lymphoid and myeloid lineage, respectively.Citation92 The results were effective in predicting the prognosis of gastric cancer patients and were validated in a prospective observation cohort. This study has opened up a new non-invasive imaging diagnostic method for the immune microenvironment and immune therapy response of gastric cancer, which is expected to provide a new paradigm for the exploration of intelligent and precise diagnosis and treatment of gastric cancer in clinical practice. Wen et al constructed a model to predict the expression level of CD8+T cells in patients with esophageal squamous cell carcinoma by combining the imageomics characteristics and clinical factors, and this model increased the AUC of CD8+T cells to 0.832.Citation93
As important imaging technologies, PET and PET/CT also play a pivotal role in the field of immunotherapy. Through PET examination, it is possible to assess the metabolic activity of tumors and understand the biological characteristics of tumor cell proliferation, metabolism, etc. PET/CT is an organic combination of PET and CT imaging diagnosis techniques, which realizes the perfect fusion of morphological and functional metabolic imaging, and can reveal metabolic changes that cannot be captured by traditional imaging. Therefore, PET/CT can observe the early metabolic changes of primary tumors and can monitor the immune response of tumors earlier and more sensitively than conventional imaging based on morphology. It plays an important role in the diagnosis of irAEs. In the study by Iravani et al, 80% of suspected irAEs detected on PET/CT were clinically confirmed, and 7% of irAEs were detected by PET/CT before clinical symptoms appeared.Citation94 In addition, unlike traditional treatment methods, immunotherapy aims to kill tumor cells by activating immune cells and enhancing immune infiltration.Citation95 However, this mechanism can lead to unconventional response patterns, such as false progressions.Citation96 PET/CT can make up for the deficiency of traditional CT morphological indicators to a certain extent by using early metabolic indicators of tumors, and more accurately identifying unconventional response patterns. Akhoundova et al used F-FET PET to correctly identify 9 out of 11 patients with 81.8% false progression.Citation97 Furthermore, labeled tracers are injected into patients, which reach cells expressing PD-1/L1 through blood circulation and specifically bind to PD-1/L1, enabling whole-body imaging of PD-1/L1. Compared to invasive tissue biopsies, this non-invasive prediction method is more acceptable to patients and provides valuable information for comprehensive assessment of the condition and formulation of treatment plans. For instance, Niemeijer et al found that SUV peak is associated with tumor PD-L1 expression, providing the possibility of non-invasive quantification of PD-1/PD-L1 expression for future immunotherapy research.Citation98 Meanwhile, novel immune PET tracer techniques such as those developed by NatarajanCitation99 and JungCitation100 can non-invasively assess PD-1 expression in tumor-infiltrating lymphocytes. The Chatterjee team has developed a highly sensitive peptide-based PET imaging agent that can quickly and non-invasively detect PD-L1 in tumors.Citation101 These techniques provide new perspectives and tools for the research and application of tumor immunotherapy. In summary, PET imaging has been initially applied to various aspects of tumor diagnosis, staging, evaluation of treatment effects, and prognosis prediction. In the future, more specific PET tracers will serve to accurately stratify patient populations.
Furthermore, to further investigate and describe the hotspots between cancer immunotherapy and medical imaging, we utilized CiteSpace to analyze co-cited references. As shown in current research is concentrated in the areas of “Immune-Related Adverse Events (#0)“, and ”Non-Small Cell Lung Cancer (#2)”.
While ICIs have significantly improved the prognosis of cancer patients, they can also lead to immune-related adverse events (irAEs).Citation19 Most irAEs are mild and manageable, but some can be life-threatening. Wang et al summarized 613 fatal irAE cases and found that fatal irAEs typically occur early after the initiation of combination therapy, with myocarditis having the highest fatality rate.Citation20 Therefore, it is crucial to accurately identify irAEs and provide timely follow-up treatment. Medical imaging modalities such as CT and MRI play a significant role in assessing adverse events in various systems caused by immune therapy.Citation102 Researchers have developed CT radiomics and machine learning models to differentiate immune-related pneumonitis from radiation pneumonitis in lung cancer patients.Citation103 Kurokawa et al retrospectively included 20 melanoma patients, and an MRI revealed that immune-related hypophysitis appeared as low signal intensity on T2-weighted images, with predominant enhancement in the pituitary gland.Citation104 While medical imaging can help identify irAEs, the imaging findings are often nonspecific and need to be interpreted in conjunction with clinical information to explain post-treatment changes and support treatment decisions.Citation105 However, diagnosing rare irAEs remains challenging. In the future, specific contrast agents can be developed to enhance diagnostic performance. Multicenter studies with larger sample sizes can be conducted to establish predictive models. Furthermore, advancing a multidisciplinary collaborative diagnostic and treatment approach, involving multiple departments is essential. This approach should focus on researching the mechanisms, diagnostics, and treatment methods of irAEs, implementing stratified management for high-risk individuals, and improving treatment effectiveness and patients’ quality of life.
In the field of immunotherapy, NSCLC has become a focal point, acting as a bridge between imaging and cancer immunotherapy.Citation106 Several aspects have matured, such as establishing non-invasive biomarkers using imaging, identifying irAEs through clinical manifestations and imaging diagnostic techniques, assessing the efficacy of immunotherapy based on solid tumor assessment criteria, and using imaging radiomics combined with machine learning models for predicting the efficacy of immunotherapy in NSCLC.Citation83,Citation102,Citation107 Given the rapid development of immunotherapy, our analysis, along with keyword clustering and emerging trends, indicates that future attention may gradually shift from lung cancer to other types of cancer such as liver cancer, breast cancer, etc. These tumors have historically been defined as immunologically “cold” tumors. However, recent studies have shown that certain patients, including those with advanced liver cancer (treated with the combination of hepatic arterial infusion chemotherapy and anti-PD-1 immunotherapy),Citation108 triple-negative breast cancer (responding well to a combination of immunotherapy and chemotherapy),Citation109 and some microsatellite stable (MSS) colorectal cancer patients (benefiting from immune-based combination therapy), exhibit favorable treatment responses.Citation110 Therefore, in a subset of patients with shorter survival, lower incidence, and more complex immune mechanisms, there is still a research gap in the selection of immunotherapy approaches, efficacy assessment, post-treatment recurrence monitoring, etc. This necessitates further research development using medical imaging and emerging imaging technologies to visualize immunotherapy-sensitive biomarkers and focus on treatment-responsive populations, thus supporting cancer immunotherapy to reach its maximum potential.
This study employed a bibliometric analysis approach to comprehensively examine the global trends and current status of cancer immunotherapy medical imaging over the past two decades. We conducted searches on WoSCC, downloaded all relevant data on the same day, and subsequently identified data related to countries/regions, organizations, authors, cited journals, and research focal points. This provided an all-encompassing overview of the state of development and trending topics within this field.
However, it is important to note that there are certain inevitable limitations to our study. First, we exclusively analyzed English-language data from WoSCC, excluding data from other crucial databases such as PubMed and data in languages other than English. Second, all data sets were identified by computer-based systems (eg, WoSCC) rather than manual selection, which may introduce potential biases, considering the various forms of medical imaging. Therefore, it is necessary to continue updating our study to keep up with evolving research outcomes. Finally, due to the incomplete dataset this year, data published in 2024 were not included in our analysis, which means that our research results are only relevant to the research period from 2003 to 2023. Therefore, it is necessary to continue to update to keep pace with changes in new research results.
Despite these limitations, we believe that our study can still provide insights into the research trends and hot topics within the field of cancer immunotherapy and medical imaging.
Conclusion
In the current bibliometric study, we observed a consistent growth in the quantity of medical imaging research related to cancer immunotherapy since 2003. The United States holds a prominent position in this field, and Pittock, Sean J identified as the most prolific author in this domain.
Presently, researchers are increasingly focusing on extracting radiomics features from images and employing artificial intelligence algorithms to establish models for predicting patient treatment outcomes. Future research trends may shift gradually from lung cancer to other diseases like liver cancer. Researchers will place greater emphasis on treatment management models and treatment-related adverse events. This will involve enhancing the connection between medical imaging and clinical data and developing models for reasonable predictions of treatment efficacy, relapse, and other relevant aspects.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgments
This work was supported by Harbin Medical University Cancer Hospital Haiyan Funds (JJQN202107), Harbin Medical University Cancer Hospital Haiyan Funds (No. JJZD2022-12), Natural Science Foundation of Heilongjiang Provincial Government (LH2022H067), 2023 Heilongjiang provincial colleges and universities basic research expenses research project.
References
- Worldwide cancer incidence statistics. Cancer Research UK. Available from: https://www.cancerresearchuk.org/health-professional/cancer-statistics/worldwide-cancer/incidence. Accessed October 18, 2023.
- Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. doi:10.1056/NEJMoa1003466
- Frelaut M, du Rusquec P, de Moura A, Le Tourneau C, Borcoman E. Pseudoprogression and hyperprogression as new forms of response to immunotherapy. BioDrugs Clin Immunother Biopharm Gene Ther. 2020;34(4):463–476. doi:10.1007/s40259-020-00425-y
- Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894–1905. doi:10.1056/NEJMoa1915745
- Schoenfeld AJ, Rizvi HA, Memon D, et al. Systemic and Oligo-Acquired Resistance to PD-(L)1 blockade in lung cancer. Clin Cancer Res off J Am Assoc Cancer Res. 2022;28(17):3797–3803. doi:10.1158/1078-0432.CCR-22-0657
- Schoenfeld AJ, Hellmann MD. Acquired resistance to immune checkpoint inhibitors. Cancer Cell. 2020;37(4):443–455. doi:10.1016/j.ccell.2020.03.017
- O’Donnell JS, Long GV, Scolyer RA, Teng MWL, Smyth MJ. Resistance to PD1/PDL1 checkpoint inhibition. Cancer Treat Rev. 2017;52:71–81. doi:10.1016/j.ctrv.2016.11.007
- Hussain S, Mubeen I, Ullah N, et al. Modern diagnostic imaging technique applications and risk factors in the medical field: a review. BioMed Res Int. 2022;2022:5164970. doi:10.1155/2022/5164970
- Berz AM, Boughdad S, Vietti-Violi N, et al. Imaging assessment of toxicity related to immune checkpoint inhibitors. Front Immunol. 2023;14:1133207. doi:10.3389/fimmu.2023.1133207
- García-Figueiras R, Baleato-González S, Luna A, et al. Assessing immunotherapy with functional and molecular imaging and radiomics. Radiogr Rev Publ Radiol Soc N Am Inc. 2020;40(7):1987–2010. doi:10.1148/rg.2020200070
- Hughes DJ, Subesinghe M, Taylor B, et al. 18F FDG PET/CT and novel molecular imaging for directing immunotherapy in cancer. Radiology. 2022;304(2):246–264. doi:10.1148/radiol.212481
- Wang X, Yang X, Wang J, et al. Metabolic tumor volume measured by 18F-FDG PET/CT is associated with the survival of unresectable hepatocellular carcinoma treated with PD-1/PD-L1 inhibitors plus molecular targeted agents. J Hepatocell Carcinoma. 2023;10:587–598. doi:10.2147/JHC.S401647
- Yang Y, Zhao Y, Liu X, Huang J. Artificial intelligence for prediction of response to cancer immunotherapy. Semin Cancer Biol. 2022;87:137–147. doi:10.1016/j.semcancer.2022.11.008
- Hamet P, Tremblay J. Artificial intelligence in medicine. Metabolism. 2017;69S:S36–S40. doi:10.1016/j.metabol.2017.01.011
- Zhang R, Wang C, Cui K, et al. Prognostic role of computed tomography textural features in early-stage non-small cell lung cancer patients receiving stereotactic body radiotherapy. Cancer Manag Res. 2019;11:9921–9930. doi:10.2147/CMAR.S220587
- Nishino M, Giobbie-Hurder A, Gargano M, Suda M, Ramaiya NH, Hodi FS. Developing a common language for tumor response to immunotherapy: immune-related response criteria using unidimensional measurements. Clin Cancer Res off J Am Assoc Cancer Res. 2013;19(14):3936–3943. doi:10.1158/1078-0432.CCR-13-0895
- Olson DJ, Eroglu Z, Brockstein B, et al. Pembrolizumab plus ipilimumab following anti-PD-1/L1 failure in melanoma. J Clin Oncol off J Am Soc Clin Oncol. 2021;39(24):2647–2655. doi:10.1200/JCO.21.00079
- Hodi FS, Ballinger M, Lyons B, et al. Immune-Modified Response Evaluation Criteria In Solid Tumors (imRECIST): refining guidelines to assess the clinical benefit of cancer immunotherapy. J Clin Oncol off J Am Soc Clin Oncol. 2018;36(9):850–858. doi:10.1200/JCO.2017.75.1644
- Chhabra N, Kennedy J. A review of cancer immunotherapy toxicity: immune checkpoint inhibitors. J Med Toxicol off J Am Coll Med Toxicol. 2021;17(4):411–424. doi:10.1007/s13181-021-00833-8
- Wang DY, Salem JE, Cohen JV, et al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol. 2018;4(12):1721–1728. doi:10.1001/jamaoncol.2018.3923
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer Oxf Engl 1990. 2009;45(2):228–247. doi:10.1016/j.ejca.2008.10.026
- Jin J, Wan Y, Shu Q, Liu J, Lai D. Knowledge mapping and research trends of IL-33 from 2004 to 2022: a bibliometric analysis. Front Immunol. 2023;14:1158323. doi:10.3389/fimmu.2023.1158323
- Liu S, Sun YP, Gao XL, Sui Y. Knowledge domain and emerging trends in Alzheimer’s disease: a scientometric review based on CiteSpace analysis. Neural Regen Res. 2019;14(9):1643–1650. doi:10.4103/1673-5374.255995
- Lai P, Xu S, Xue JH, Zhang HZ, Zhong YM, Liao YL. Current hotspot and study trend of innate immunity in COVID-19: a bibliometric analysis from 2020 to 2022. Front Immunol. 2023;14:1135334. doi:10.3389/fimmu.2023.1135334
- Liao J, Yu X, Chen J, et al. Knowledge mapping of autophagy in osteoarthritis from 2004 to 2022: a bibliometric analysis. Front Immunol. 2023;14:1063018. doi:10.3389/fimmu.2023.1063018
- Shen H, Wang L, Zhang Y, Huang G, Liu B. Knowledge mapping of image-guided tumor ablation and immunity: a bibliometric analysis. Front Immunol. 2023;14:1073681. doi:10.3389/fimmu.2023.1073681
- Chen C, Hu Z, Liu S, Tseng H. Emerging trends in regenerative medicine: a scientometric analysis in CiteSpace. Expert Opin Biol Ther. 2012;12(5):593–608. doi:10.1517/14712598.2012.674507
- Geschwind MD, Tan KM, Lennon VA, et al. Voltage-gated potassium channel autoimmunity mimicking creutzfeldt-jakob disease. Arch Neurol. 2008;65(10):1341–1346. doi:10.1001/archneur.65.10.1341
- Tian Y, Abu-Sbeih H, Wang Y. Immune Checkpoint Inhibitors-Induced Hepatitis. Adv Exp Med Biol. 2018;995:159–164. doi:10.1007/978-3-030-02505-2_8
- Flanagan EP, Keegan BM. Paraneoplastic myelopathy. Neurol Clin. 2013;31(1):307–318. doi:10.1016/j.ncl.2012.09.001
- O’Nuallain B, Hrncic R, Wall JS, Weiss DT, Solomon A. Diagnostic and therapeutic potential of amyloid-reactive IgG antibodies contained in human sera. J Immunol Baltim Md 1950. 2006;176(11):7071–7078. doi:10.4049/jimmunol.176.11.7071
- Zhang H, Zhao H, Huang Y, Sun G, Zhang Y. Microenvironment-activatable cascaded responsive carbonized polymer dots as a theranostic platform for precise rapamycin delivery to potentiate the synergy of chemotherapy and γδ T cells-mediated immunotherapy against tumor. Appl Mater Today. 2022;26:101364. doi:10.1016/j.apmt.2022.101364
- Sigurdsson EM. Tau immunotherapy and imaging. Neurodegener Dis. 2014;13(2–3):103–106. doi:10.1159/000354491
- Js W, Sj K, P M, et al. 2006; Radioimaging of light chain amyloid with a fibril-reactive monoclonal antibody. J Nucl Med off Publ Soc Nucl Med. 47(12):2016–2024.
- Pan Y, Volkmer JP, Mach KE, et al. Endoscopic molecular imaging of human bladder cancer using a CD47 antibody. Sci Transl Med. 2014;6(260):260ra148. doi:10.1126/scitranslmed.3009457
- McArthur GA, Mohr P, Ascierto PA, et al. Health care resource utilization and associated costs among metastatic cutaneous melanoma patients treated with Ipilimumab (INTUITION Study). oncologist. 2017;22(8):951–962. doi:10.1634/theoncologist.2016-0272
- Sheng R, Sun W, Huang X, et al. Apparent diffusion coefficient MRI shows association with early progression of unresectable intrahepatic cholangiocarcinoma with combined targeted-immunotherapy. J Magn Reson Imaging JMRI. 2023;57(1):275–284. doi:10.1002/jmri.28214
- G C, R A, Dp C, et al. Non-small-cell lung cancer. Nat Rev Dis Primer. 2015:1. doi:10.1038/nrdp.2015.9
- La Puma D, Llufriu S, Sepúlveda M, et al. Long-term follow-up of immunotherapy-unresponsive recurrent tumefactive demyelination. J Neurol Sci. 2015;352(1–2). doi:10.1016/j.jns.2015.03.038
- Dalmau J. The case for autoimmune neurology. Neurol Neuroimmunol Neuroinflammation. 2017;4(4):e373. doi:10.1212/NXI.0000000000000373
- Jamal R, Goodwin RA, Tu D, Walsh W, Lacombe D, Eisenhauer EA. Performance of multinomial designs in comparison with response-based designs in non-randomized Phase II trials of targeted cancer agents. Ann Oncol off J Eur Soc Med Oncol. 2013;24(7):1936–1942. doi:10.1093/annonc/mdt122
- Gauci ML, Lanoy E, Champiat S, et al. Long-term survival in patients responding to anti-PD-1/PD-L1 therapy and disease outcome upon treatment discontinuation. Clin Cancer Res off J Am Assoc Cancer Res. 2019;25(3):946–956. doi:10.1158/1078-0432.CCR-18-0793
- N J, W X, Km W, et al. Pneumonitis in patients treated with anti-programmed death-1/programmed death ligand 1 therapy. J Clin Oncol off J Am Soc Clin Oncol. 2017;35(7). doi:10.1200/JCO.2016.68.2005
- Krajewski KM, Nishino M, Ramaiya NH, Choueiri TK. RECIST 1.1 compared with RECIST 1.0 in patients with advanced renal cell carcinoma receiving vascular endothelial growth factor-targeted therapy. AJR Am J Roentgenol. 2015;204(3):W282–W288. doi:10.2214/AJR.14.13236
- Chang LS, Barroso-Sousa R, Tolaney SM, Hodi FS, Kaiser UB, Min L. Endocrine toxicity of cancer immunotherapy targeting immune checkpoints. Endocr Rev. 2019;40(1):17–65. doi:10.1210/er.2018-00006
- Ds S, R A. The evolution of checkpoint blockade as a cancer therapy: what’s here, what’s next? Curr Opin Immunol. 2015;33. doi:10.1016/j.coi.2015.01.006
- Naidoo J, Zhang J, Lipson EJ, et al. A multidisciplinary toxicity team for cancer immunotherapy-related adverse events. J Natl Compr Cancer Netw JNCCN. 2019;17(6):712–720. doi:10.6004/jnccn.2018.7268
- Klunk WE, Engler H, Nordberg A, et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann Neurol. 2004;55(3):306–319. doi:10.1002/ana.20009
- Nicoll JAR, Wilkinson D, Holmes C, Steart P, Markham H, Weller RO. Neuropathology of human Alzheimer disease after immunization with amyloid-β peptide: a case report. Nat Med. 2003;9(4):448–452. doi:10.1038/nm840
- Mintun MA, LaRossa GN, Sheline YI, et al. [11C]PIB in a nondemented population. Neurology. 2006;67(3):446–452. doi:10.1212/01.wnl.0000228230.26044.a4
- Weber JS, D’Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, Phase 3 trial. Lancet Oncol. 2015;16(4):375–384. doi:10.1016/S1470-2045(15)70076-8
- Topalian SL, Sznol M, McDermott DF, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32(10):1020–1030. doi:10.1200/JCO.2013.53.0105
- Sun R, Limkin EJ, Vakalopoulou M, et al. A radiomics approach to assess tumour-infiltrating CD8 cells and response to anti-PD-1 or anti-PD-L1 immunotherapy: an imaging biomarker, retrospective multicohort study. Lancet Oncol. 2018;19(9):1180–1191. doi:10.1016/S1470-2045(18)30413-3
- Haanen JB, Carbonnel F, Robert C, et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Ann Oncol. 2017;28:iv119–iv142. doi:10.1093/annonc/mdx225
- Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2018;36(17):1714–1768. doi:10.1200/JCO.2017.77.6385
- Bensch F, van der Veen EL, Lub-de Hooge MN, et al. 89Zr-atezolizumab imaging as a non-invasive approach to assess clinical response to PD-L1 blockade in cancer. Nat Med. 2018;24(12):1852–1858. doi:10.1038/s41591-018-0255-8
- Seymour L, Bogaerts J, Perrone A, et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017;18(3):e143–e152. doi:10.1016/S1470-2045(17)30074-8
- Cho SY, Lipson EJ, Im HJ, et al. Prediction of response to immune checkpoint inhibitor therapy using early-time-point 18F-FDG PET/CT imaging in patients with advanced melanoma. J Nucl Med. 2017;58(9):1421–1428. doi:10.2967/jnumed.116.188839
- Lambin P, Leijenaar RTH, Deist TM, et al. Radiomics: the bridge between medical imaging and personalized medicine. Nat Rev Clin Oncol. 2017;14(12):749–762. doi:10.1038/nrclinonc.2017.141
- Naidoo J, Wang X, Woo KM, et al. Pneumonitis in patients treated with anti–programmed death-1/programmed death Ligand 1 therapy. J Clin Oncol. 2016. doi:10.1200/JCO.2016.68.2005
- Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1–positive non–small-cell lung cancer. N Engl J Med. 2016;375(19):1823–1833. doi:10.1056/NEJMoa1606774
- Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non–small-cell lung cancer. N Engl J Med. 2018;378(22):2078–2092. doi:10.1056/NEJMoa1801005
- Saltz J, Gupta R, Hou L, et al. Spatial organization and molecular correlation of tumor-infiltrating lymphocytes using deep learning on pathology images. Cell Rep. 2018;23(1):181–193.e7. doi:10.1016/j.celrep.2018.03.086
- Pandit-Taskar N, Postow MA, Hellmann MD, et al. First-in-humans imaging with 89Zr-Df-IAB22M2C anti-CD8 minibody in patients with solid malignancies: preliminary pharmacokinetics, biodistribution, and lesion targeting. J Nucl Med. 2020;61(4):512–519. doi:10.2967/jnumed.119.229781
- Ito K, Teng R, Schöder H, et al. 18F-FDG PET/CT for monitoring of ipilimumab therapy in patients with metastatic melanoma. J Nucl Med. 2019;60(3):335–341. doi:10.2967/jnumed.118.213652
- Ramos-Casals M, Brahmer JR, Callahan MK, et al. Immune-related adverse events of checkpoint inhibitors. Nat Rev Dis Primer. 2020;6(1):1–21. doi:10.1038/s41572-020-0160-6
- Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018;359(6382):1350–1355. doi:10.1126/science.aar4060
- Shi Y, Luo J, Wang X, et al. Emerging trends on the correlation between neurotransmitters and tumor progression in the last 20 years: a bibliometric analysis via CiteSpace. Front Oncol. 2022;12:800499. doi:10.3389/fonc.2022.800499
- Vicentin I, Mosconi C, Garanzini E, et al. Inter-center agreement of mRECIST in transplanted patients for hepatocellular carcinoma. Eur Radiol. 2021;31(12):8903–8912. doi:10.1007/s00330-021-08088-1
- Aoki T, Nishida N, Ueshima K, et al. Higher enhancement intrahepatic nodules on the hepatobiliary phase of Gd-EOB-DTPA-enhanced MRI as a poor responsive marker of anti-PD-1/PD-L1 monotherapy for unresectable hepatocellular carcinoma. Liver Cancer. 2021;10(6):615–628. doi:10.1159/000518048
- Sasaki R, Nagata K, Fukushima M, et al. Evaluating the role of hepatobiliary phase of gadoxetic acid-enhanced magnetic resonance imaging in predicting treatment impact of lenvatinib and atezolizumab plus bevacizumab on unresectable hepatocellular carcinoma. Cancers. 2022;14(3):827. doi:10.3390/cancers14030827
- van der Hulst HJ, Vos JL, Tissier R, et al. Quantitative diffusion-weighted imaging analyses to predict response to neoadjuvant immunotherapy in patients with locally advanced head and neck carcinoma. Cancers. 2022;14(24):6235. doi:10.3390/cancers14246235
- Xue K, Liu L, Liu Y, Guo Y, Zhu Y, Zhang M. Radiomics model based on multi-sequence MR images for predicting preoperative immunoscore in rectal cancer. Radiol Med. 2022;127(7):702–713. doi:10.1007/s11547-022-01507-3
- Gong XQ, Liu N, Tao YY, et al. Radiomics models based on multisequence MRI for predicting PD-1/PD-L1 expression in hepatocellular carcinoma. Sci Rep. 2023;13(1):7710. doi:10.1038/s41598-023-34763-y
- Tremblay ML, O’Brien-Moran Z, Rioux JA, et al. Quantitative MRI cell tracking of immune cell recruitment to tumors and draining lymph nodes in response to anti-PD-1 and a DPX-based immunotherapy. Oncoimmunology. 2020;9(1):1851539. doi:10.1080/2162402X.2020.1851539
- Dekaban GA, Hamilton AM, Fink CA, et al. Tracking and evaluation of dendritic cell migration by cellular magnetic resonance imaging. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2013;5(5):469–483. doi:10.1002/wnan.1227
- Wu C, Zhu W, Jin R, Ai H, Xu Y. The MRI-Visible nanocomposite facilitates the delivery and tracking of siRNA loaded DC vaccine in the breast cancer model. Front Oncol. 2020;10:621642. doi:10.3389/fonc.2020.621642
- Dercle L, Sun S, Seban RD, et al. Emerging and evolving concepts in cancer immunotherapy imaging. Radiology. 2023;306(1):32–46. doi:10.1148/radiol.210518
- Nensa F, Demircioglu A, Rischpler C. Artificial intelligence in nuclear medicine. J Nucl Med off Publ Soc Nucl Med. 2019;60(Suppl 2):29S–37S. doi:10.2967/jnumed.118.220590
- D’Angelo T, Caudo D, Blandino A, et al. Artificial intelligence, machine learning and deep learning in musculoskeletal imaging: current applications. J Clin Ultrasound. 2022;50(9):1414–1431. doi:10.1002/jcu.23321
- Mishra K, Leng T. Artificial intelligence and ophthalmic surgery. Curr Opin Ophthalmol. 2021;32(5):425–430. doi:10.1097/ICU.0000000000000788
- Lambin P, Rios-Velazquez E, Leijenaar R, et al. Radiomics: extracting more information from medical images using advanced feature analysis. Eur J Cancer Oxf Engl 1990. 2012;48(4):441–446. doi:10.1016/j.ejca.2011.11.036
- Trebeschi S, Drago SG, Birkbak NJ, et al. Predicting response to cancer immunotherapy using noninvasive radiomic biomarkers. Ann Oncol off J Eur Soc Med Oncol. 2019;30(6):998–1004. doi:10.1093/annonc/mdz108
- Granata V, Fusco R, Costa M, et al. Preliminary report on computed tomography radiomics features as biomarkers to immunotherapy selection in lung adenocarcinoma patients. Cancers. 2021;13(16):3992. doi:10.3390/cancers13163992
- Hectors SJ, Lewis S, Besa C, et al. MRI radiomics features predict immuno-oncological characteristics of hepatocellular carcinoma. Eur Radiol. 2020;30(7):3759–3769. doi:10.1007/s00330-020-06675-2
- Zhou LQ, Wang JY, Yu SY, et al. Artificial intelligence in medical imaging of the liver. World J Gastroenterol. 2019;25(6):672–682. doi:10.3748/wjg.v25.i6.672
- Tian P, He B, Mu W, et al. Assessing PD-L1 expression in non-small cell lung cancer and predicting responses to immune checkpoint inhibitors using deep learning on computed tomography images. Theranostics. 2021;11(5):2098–2107. doi:10.7150/thno.48027
- He B, Dong D, She Y, et al. Predicting response to immunotherapy in advanced non-small-cell lung cancer using tumor mutational burden radiomic biomarker. J Immunother Cancer. 2020;8(2):e000550. doi:10.1136/jitc-2020-000550
- Bilotta MT, Antignani A, Fitzgerald DJ. Managing the TME to improve the efficacy of cancer therapy. Front Immunol. 2022;13:954992. doi:10.3389/fimmu.2022.954992
- Zhang Y, Zhang Z. The history and advances in cancer immunotherapy: understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell Mol Immunol. 2020;17(8):807–821. doi:10.1038/s41423-020-0488-6
- Jiang Y, Zhou K, Sun Z, et al. Non-invasive tumor microenvironment evaluation and treatment response prediction in gastric cancer using deep learning radiomics. Cell Rep Med. 2023;4(8):101146. doi:10.1016/j.xcrm.2023.101146
- Sun Z, Zhang T, Ahmad MU, et al. Comprehensive assessment of immune context and immunotherapy response via noninvasive imaging in gastric cancer. J Clin Invest. 2024;134(6):e175834. doi:10.1172/JCI175834
- Wen Q, Yang Z, Zhu J, et al. Pretreatment CT-based radiomics signature as a potential imaging biomarker for predicting the expression of PD-L1 and CD8+TILs in ESCC. Oncol Targets Ther. 2020;13:12003–12013. doi:10.2147/OTT.S261068
- Iravani A, Osman MM, Weppler AM, et al. FDG PET/CT for tumoral and systemic immune response monitoring of advanced melanoma during first-line combination ipilimumab and nivolumab treatment. Eur J Nucl Med Mol Imaging. 2020;47(12):2776–2786. doi:10.1007/s00259-020-04815-w
- Billan S, Kaidar-Person O, Gil Z. Treatment after progression in the era of immunotherapy. Lancet Oncol. 2020;21(10):e463–e476. doi:10.1016/S1470-2045(20)30328-4
- Xiang Y, Tang W, Wang J, Wang Z, Bi N. Pseudoprogression of thoracic tumor after radiotherapy in the era of immunotherapy: a case series. Front Oncol. 2023;13:1021253. doi:10.3389/fonc.2023.1021253
- Akhoundova D, Hiltbrunner S, Mader C, et al. 18F-FET PET for diagnosis of pseudoprogression of brain metastases in patients with non-small cell lung cancer. Clin Nucl Med. 2020;45(2):113–117. doi:10.1097/RLU.0000000000002890
- Niemeijer AN, Leung D, Huisman MC, et al. Whole body PD-1 and PD-L1 positron emission tomography in patients with non-small-cell lung cancer. Nat Commun. 2018;9(1):4664. doi:10.1038/s41467-018-07131-y
- Natarajan A, Mayer AT, Xu L, Reeves RE, Gano J, Gambhir SS. Novel radiotracer for ImmunoPET imaging of PD-1 checkpoint expression on tumor infiltrating lymphocytes. Bioconjug Chem. 2015;26(10):2062–2069. doi:10.1021/acs.bioconjchem.5b00318
- Jung K-H, Lee JH, Kim M, Cho YS, Lee K-H, Azhdarinia A. 89 Zr immuno-PET imaging of tumor PD-1 reveals that PMA upregulates lymphoma PD-1 through NFκB and JNK signaling. Mol Imaging. 2022;2022:5916692. doi:10.1155/2022/5916692
- Chatterjee S, Lesniak WG, Nimmagadda S. Noninvasive imaging of immune checkpoint ligand PD-L1 in tumors and metastases for guiding immunotherapy. Mol Imaging. 2017;16:1536012117718459. doi:10.1177/1536012117718459
- Bai R, Chen N, Li L, et al. Mechanisms of Cancer Resistance to Immunotherapy. Front Oncol. 2020;10:1290. doi:10.3389/fonc.2020.01290
- Cheng J, Pan Y, Huang W, et al. Differentiation between immune checkpoint inhibitor-related and radiation pneumonitis in lung cancer by CT radiomics and machine learning. Med Phys. 2022;49(3):1547–1558. doi:10.1002/mp.15451
- Kurokawa R, Ota Y, Gonoi W, et al. MRI findings of immune checkpoint inhibitor-induced hypophysitis: possible association with fibrosis. AJNR Am J Neuroradiol. 2020;41(9):1683–1689. doi:10.3174/ajnr.A6692
- Schierz JH, Sarikaya I, Wollina U, Unger L, Sarikaya A. Immune checkpoint inhibitor-related adverse effects and 18F-FDG PET/CT findings. J Nucl Med Technol. 2021;49(4):324–329. doi:10.2967/jnmt.121.262151
- Wang M, Herbst RS, Boshoff C. Toward personalized treatment approaches for non-small-cell lung cancer. Nat Med. 2021;27(8):1345–1356. doi:10.1038/s41591-021-01450-2
- Almansour H, Afat S, Serna-Higuita LM, et al. Early tumor size reduction of at least 10% at the first follow-up computed tomography can predict survival in the setting of advanced melanoma and immunotherapy. Acad Radiol. 2022;29(4):514–522. doi:10.1016/j.acra.2021.04.015
- Mei J, Li SH, Li QJ, et al. Anti-PD-1 immunotherapy improves the efficacy of hepatic artery infusion chemotherapy in advanced hepatocellular carcinoma. J Hepatocell Carcinoma. 2021;8:167–176. doi:10.2147/JHC.S298538
- Schmid P, Rugo HS, Adams S, et al. Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): updated efficacy results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2020;21(1):44–59. doi:10.1016/S1470-2045(19)30689-8
- Baraibar I, Mirallas O, Saoudi N, et al. Combined treatment with immunotherapy-based strategies for MSS metastatic colorectal cancer. Cancers. 2021;13(24):6311. doi:10.3390/cancers13246311