Abstract
Objective
The objective of this study was to investigate the efficacy and safety profile of levetiracetam as add-on therapy in patients with refractory epilepsy.
Methods
Web of Science, MEDLINE (Ovid and PubMed), Cochrane Library, EMBASE, and Google Scholar were systematically searched to identify potential eligible randomized controlled trials by two reviewers independently. Pooled estimates of risk ratios (RRs) for 50%, 75%, and 100% reduction from baseline were calculated using the fixed-effect model or random-effect model. Quality of included studies was assessed with the Cochrane Collaboration’s Risk of Bias tool. Serious adverse events and withdrawals induced by interventions and the most common side effects were analyzed.
Results
Seventeen trials with a total of 3,205 participants were included in this meta-analysis, including 14 trials for adulthood and three trials for children. Pooled estimates suggested that levetiracetam was an effective anti-epileptic drug at 1,000–3,000 mg/day (RR =2.00 for 1,000 mg/day, RR =2.68 for 2,000 mg/day, RR =2.18 for 3,000 mg/day) for adults and 60 mg/kg/day (RR =2.00) for children compared to placebo in terms of 50% reduction from baseline. Likewise, as for seizure freedom rate, levetiracetam had an advantage over placebo at 1,000–3,000 mg/day (RR =5.84 for 1,000 mg/day, RR =4.55 for 2,000 mg/day, RR =4.57 for 3,000 mg/day, respectively) for adults and 60 mg/kg/day (RR =4.52) for children. Regarding safety profile, patients treated with levetiracetam had significantly higher occurrence than placebo for somnolence, asthenia, dizziness, infection, nasopharyngitis, anxiety, and irritability; however, most studies reported that these adverse events were mild and transient.
Conclusion
Levetiracetam is an effective anti-epileptic drug for both adults and children with generalized or partial-onset refractory seizures at 1,000–3,000 or 60 mg/kg/day, with a favorable adverse event profile.
Introduction
Epilepsy is a serious neurological disorder, with the prevalence of ~0.5%–1% in developed countries,Citation1 and it rises up to 7.4% in developing countries because of inferior health care and a higher proportion of children.Citation2,Citation3 Maintaining seizure freedom by using a tolerated anti-epileptic drug (AED) schedule is the goal of epilepsy treatment. However, of the 50 million people who suffer epilepsy, nearly one third were treated with available AEDs but due to lack of favorable effect, they still have onsets; this is regarded as “drug-resistant” or “refractory.”Citation4 Besides, side effects induced by AEDs leading to failed adequate seizure control account for 20%–30% of the patients.Citation5
As a broad-spectrum AED, levetiracetam ((S)-α-ethyl-2-oxo-1-pyrrolidine acetamide) has unique mechanisms of action that differs from other AEDs. A study published recently revealed that through binding, levetiracetam modulates the activity of synaptic vesicle protein 2A in brain neurons to maintain a normal level, and as a result seizures reduce.Citation6 Compared to other AEDs, levetiracetam has a favorable pharmacokinetic profile in both adulthood and childhood. After oral administration, levetiracetam will be rapidly and almost 100% absorbed in a few hours; the peak serum concentration is achieved iñ1 hour (0.6–1.3 hours). Mean half-life of levetiracetam is about 6–8 hours in young adulthood and increases to 10–11 hours in elderly patients, and within 24–48 hours, the dose-proportional pharmacokinetic maintains a steady state serum level. One favorable characteristic of levetiracetam is that to date, there are few reports concerning pharmacokinetic drug interactions with levetiracetam in adults and children alike.Citation7,Citation8 In comparison with adulthood, body clearance of levetiracetam in childhood is higher of about 30%–40%, and therefore, the recommended dose for children is about 130%–140% of that of adulthood, equivalent to 20–60 mg/kg/day, but should be adjusted according to body weight.Citation9 Moreover, studies revealed that the pharmacokinetic profile of single-dose levetiracetam in children aged 2–46 months is similar to those aged >4 years, suggesting that levetiracetam could be used in infants and young children.Citation10 Since introduced in the market in 2000, levetiracetam has become first-line and one of the most commonly prescribed AED and is recommended as add-on agent for partial seizures, benign childhood epilepsy with centrotemporal spikes, and myoclonic epilepsy.Citation11 This meta-analysis aimed to investigate the effects of levetiracetam as adjunctive therapy for patients suffering from refractory generalized or partial-onset epilepsy.
Materials and methods
This meta-analysis was performed and reported in accordance with the PRISMA.Citation12 Responder rate (50% reduction from baseline) and seizure freedom (100% reduction from baseline) were the primary outcomes for this meta-analysis. Serious adverse events (SAEs) during treatment induced by interventions and premature termination related to interventions were regarded as the secondary outcomes.
Search strategy and selection criteria
For this systematic review and meta-analysis, following databases were searched from inception up to May 31, 2018: EMBASE, MEDLINE, Web of Science, Cochrane Library PubMed, and Google Scholar, as well as Chinese National Knowledge Infrastructure and Wanfang Data databases. A combination of relevant keywords, abbreviations, and synonyms for levetiracetam and refractory partial epilepsy are as follows: (levetiracetam) and ([refractory] or [uncontrolled] or [drug-resistant]) and ([onset*] or [seizure*] or [epilepsy]). Database search was supplemented by manual screening of the references of relevant articles and reviews, and there was no restriction on publication language. Two reviewers (Chen and Bian) assessed the eligible articles independently, disagreements were resolved via discussion and, if necessary, arbitrated by the third reviewer (Zhang).
Inclusion criteria
Studies were included only if they meet all the following criteria: 1) involved refractory epilepsy, regardless of age and gender, 2) must be randomized controlled trials (RCTs) that involved levetiracetam, 3) reported at least one efficacy of responder or seize freedom rate, and 4) detailed adverse events (AEs) including dropouts owing to AEs and SAEs were reported.
Exclusion criteria
Studies were excluded if they meet any of the following criteria: 1) non-RCT studies such as retrospective and observational studies, 2) compared to other AEDs rather than placebo, 3) not for refractory epilepsy but other diseases such as migraine or autism, 4) not reported detailed efficacy of responder and/or seizure freedom and adverse profile, and 5) conference abstracts, guidelines, editorials, letters, and reviews.
Data extraction and quality evaluation
To standardize the data extraction process, we developed a data collection form with Excel (Office 2013; Microsoft Corporation, Redmond, WA, USA), and following data were extracted from each study: 1) study and demographic characteristics: first author, year of publication, country, sample size, patient age, and ratio of male/female and 2) clinical characteristics: dosage, follow-up period, responder and seizure freedom number, total number of AEs, premature termination owing to AEs, SAEs, and specific side effects reported by more than three trials. Risk of bias for included studies was evaluated with the Cochrane Collaboration’s Risk of Bias tool, which covered seven aspects of random sequence generation, allocation concealment, blinding of outcome participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias. Each RCT was regarded as high, low, or unclear risk of bias for these aspects. Quality assessment was performed by two reviewers (Chen and Bian) independently; in case of disagreements, the third reviewer (Zhang) was consulted.
Data synthesis and analysis
Outcomes were reported as risk ratio (RR) with 95% CI, with fixed-effect model if there was no significant heterogeneity identified;Citation13 otherwise the random-effect model was used to calculate.Citation14 Heterogeneity across studies was assessed by Cochran’s Q test and measured with inconsistency index (I2), value of which was interpreted as follows: 1) 0%–40% was considered as not important, 2) 30%–60% was considered as moderate, 3) 50%–90% was considered as substantial, and 4) 75%–100% was regarded as considerable.Citation15 A funnel plot was presented to visually evaluate the publication bias, quantified by Egger regression and Begg–Mazumdar test.Citation16,Citation17 All randomized participants were analyzed based on intention-to-treat patient population, namely in the treatment group they had been allocated, irrespective of the treatment that they actually received. Participants randomized but excluded from analysis were assumed non-responders. If the difference in dosage between studies was slight, then for sake of convenient calculation, we categorize them in the same group; in this case, we would make a specific declaration. Subgroup analyses based on dosage and age were performed as well. A P-value <0.05 was regarded as statistically significant. Pooled estimates of RRs and corresponding 95% CIs of 50%, 75%, and 100% of seizure reduction from baseline, along with SAEs and dropout due to interventions, were presented in the forest plots. Another measurement for epilepsy treatment is quality of life (QoL), but to date widely accepted instruments to assess it are still lacking; therefore, we did not combine the results of QoL. All analyses were performed with the STATA 14.1 (Stata Corporation, College Station, TX, USA) and the “metan” module of it.
Results
Literature search
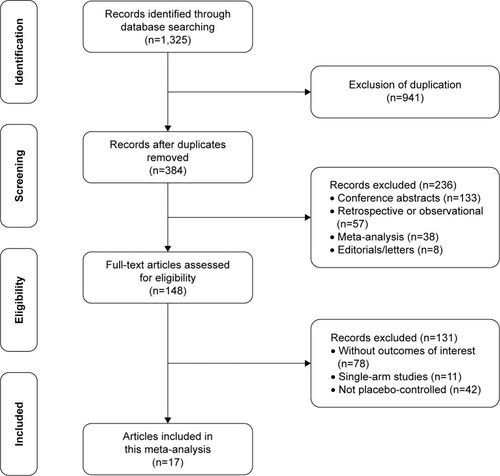
describes an overview of the study selection process. The initial systematic search yielded 1,325 results, of which 975 were removed for duplicates. Of the remaining 384 articles, 138 were excluded for conference abstracts, 57 for retrospective or observational studies, and 38 for systematic reviews or meta-analyses. Full-text screening was performed by two reviewers independently and manually, then 128 articles were ruled out for following reasons: 75 studies did not report sufficient data for efficacy or AEs; 11 studies were single-arm trials, and 42 studies were compared to other AEDs such as oxcarbazepine, sulthiame, or carbamazepine rather than placebo. Eventually, a total of 17 RCTs with 3,205 participants were included in the current meta-analysis.Citation2,Citation5,Citation10,Citation18–Citation30
Study characteristics
Details of demographic and clinical characteristics for 17 RCTs are summarized in . Sample size for these trials ranged from 24 to 351. Fourteen trials involved adult patientsCitation2,Citation5,Citation18–Citation25,Citation27,Citation31 and three involved children,Citation10,Citation26,Citation28 with age ranging from 1 month to 69 years. Regarding the 14 trials involving adults, the most administered dosages were 1,000, 2,000, and 3,000 mg/day. However, in the trial of Inoue et al, single-arm participants were administered at 500 mg/day.Citation27 Another exception was in the trial of Betts et al, in which dosage reached 4,000 mg/day.Citation5 Of the three trials involving children, two used the maximum dosage of 60 mg/kg/day,Citation26,Citation28 the remaining one used slightly less, at a maximum of 50 mg/kg/day.Citation27 In nearly all the included trials, levetiracetam was administered orally twice-daily; the only exception was trial of Peltola et al, in which levetiracetam was administered 1,000 mg/day once daily.Citation18 Most of the trials lasted at least 16 weeks; however, the trial of Piña-Garza et al only lasted 7 days, which may bring about potential risk of bias for outcomes.Citation10 Of the 17 RCTs, 15 involved patients with refractory partial-onset seizures, whereas the two others were designed to assess the efficacy for patients with uncontrolled idiopathic generalized epilepsy.Citation29,Citation30
Table 1 Demographic characteristics of included trials
Quality assessment of included studies
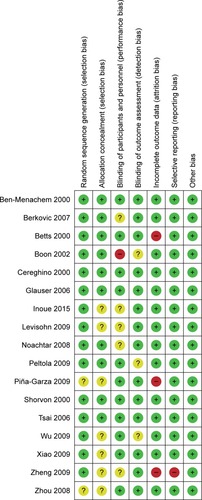
Details of risk of bias for each RCT are presented in . Ten trials were considered as low risk of bias, because sequence generation and allocation method were described.Citation2,Citation5,Citation18–Citation21,Citation25,Citation26,Citation29,Citation30 The remaining seven trials were regarded as risk of selection bias, mainly because insufficient information for random list generation and allocation concealment were not reported.Citation10,Citation22–Citation24,Citation27,Citation28,Citation31 All trials were reported to be double-blind trials; however, six trials did not describe the details of approaches applied to blind participants and personnel, then regarded as unclear for risk of bias.Citation19,Citation27–Citation31 Most of the trials were viewed as low risk of bias concerning incomplete outcome data biases; nevertheless, three trials were considered as high risk of bias, for the number of patients reported after treatment was not consistent with the initial number.Citation5,Citation10,Citation31 In general, the quality assessment for all included RCTs was not very high.
50% reduction from baseline
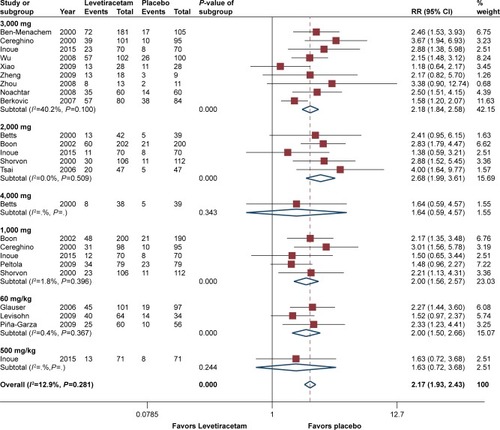
50% reduction from baseline was reported by all RCTs. Pooled estimates suggested that patients treated with levetiracetam had substantial higher responder rate than those with placebo (RR =2.17, 95% CI 1.93–2.43, P<0.05), and heterogeneity test showed that there was no significant different (I2=12.9%, P=0.28). Subgroup analysis based on dosage showed that pooled estimates from five trials at 2,000 mg/day possessed the optimal efficacy of the responders (RR =2.68, 95% CI 1.99–3.61),Citation2,Citation5,Citation19,Citation21,Citation27 and for other dosages of 1,000, 3,000, and 60 mg/kg/day, they had comparable efficacy (RR =2.00 with 95% CI 1.56–2.57; RR =2.18 with 95% CI 1.84–2.58; and RR =2.00 with 95% CI 1.50–2.67, respectively). Moreover, results suggested that regarding these four dosages, levetiracetam had a considerable advantage over placebo (P<0.05). One trial involved a dosage of 500 mg/day and one involved 4,000 mg/day, and the results suggested that the efficacy was not as good as the other dosages (RR =1.63, 95% CI 0.72–3.68, P=0.24 and RR =1.64, 95% CI 0.59–4.57, P=0.34). Subgroup analysis based on age (<16 years vs >16 years) showed that adult patients treated with levetiracetam had a slightly better efficacy of responder rate than children (RR =2.08, 95% CI 1.83–2.34 and RR =1.94, 95% CI 1.46–2.57). Subgroup analysis according to epilepsy type (generalized vs partial) showed that levetiracetam had a better efficacy of responder rate in patients with partial epilepsy (for partial-onset, RR =2.14, and for generalized epilepsy, RR =1.75). However, there was no statistically significant difference between them (P=0.14). presents the details of responder rate based on dosage.
Seizure freedom from baseline
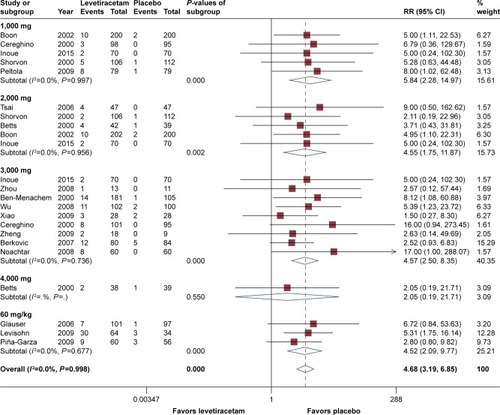
All RCTs reported details regarding seizure freedom within the treatment period, and pooled estimates demonstrated that levetiracetam behaved considerably better than placebo overall (RR =4.68, 95% CI 3.19–6.85). According to subgroup analysis, dosage of 1,000 mg/day had the best efficacy compared to placebo (RR =5.84, 95% CI 2.28–14.97, P<0.05), followed by the dosages of 2,000, 3,000, and 60 mg/kg/day with minute difference among these three doses (RR =4.55, 95% CI 1.75–11.87; RR =4.57, 95% CI 2.50–8.35; and RR =4.52, 95% CI 2.09–9.77, respectively). For all of the four dosages, levetiracetam behaved substantially better than placebo (P<0.05). However, on the other hand, at a dose of 4,000 mg/day, a RR value of 2.05 (95% CI 0.19–21.71) suggested that the efficacy was not substantial (P=0.55). Subgroup analysis showed that there was no statistically significant difference between groups of age, with RR =4.14 for adulthood (95% CI 2.65–6.48) and RR =4.31 for children (95% CI 1.99–9.32). Heterogeneity test suggested that there was no significant difference (P=0.93) across trials. Analysis according to epilepsy type suggested that compared to refractory generalized epilepsy, refractory partial-onset seizures had a better seizure freedom rate (RR =3.11 vs RR =4.44); however, the difference was not significant (P=0.5). shows the details of seizure freedom from baseline compared to placebo.
75% reduction from baseline
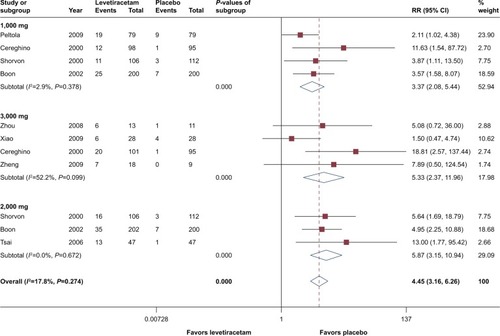
Besides responder and seizure freedom rates, eight trials reported >75% reduction from baseline,Citation2,Citation18–Citation23,Citation31 and all of them involved patients of adulthood, with dosage of 1,000, 2,000, and 3,000 mg/day. Overall pooled estimates showed that for 75% reduction from baseline, levetiracetam had a substantial advantage over placebo (RR =4.45, 95% CI 3.16– 6.26, P<0.05). Subgroup analysis based on dosage showed that 2,000 and 3,000 mg had comparable efficacy, calculated RRs were 5.87 (95% CI 3.15–10.94) and 5.33 (95% CI 2.37–6.26), respectively. However, heterogeneity test in 3,000 mg group showed higher inconsistency (I2=52.2%, P=0.10) than 2,000 mg/day group (I2=0.0%, P=0.67), but it did not reach statistical significance. At a dosage of 1,000 mg/day, even though levetiracetam performed inferior to 2,000 and 3,000 mg/day, it is still significantly better than placebo (RR =3.37, 95% CI 2.08–5.44, P<0.05). Details are presented in .
SAE and side effects leading to withdrawal
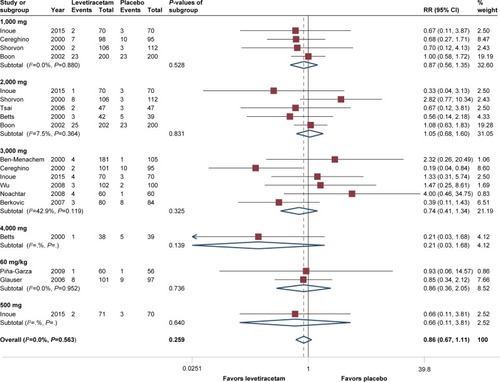
Almost all studies reported SAEs and withdrawals induced by interventions. As for SAEs, regardless of age and dosage, pooled estimates of RR =0.87 (95% CI 0.67–1.11, P=0.37) suggested that there was no statistically significant difference between levetiracetam and placebo, and heterogeneity test of P=0.56 showed there was no significant heterogeneity was observed across included trials. Our subgroup analysis suggested that there was no statistically significant difference among dosages excepted at 4,000 mg/day, in which RR =0.21 (95% CI 0.03–1.68, P=0.14). Subgroup analysis based on age showed no statistically significant difference between children (RR =0.86, 95% CI 0.36–2.05) and adults (RR =0.89, 95% CI 0.66–1.15), with P=0.95. Analysis according to epilepsy type showed that for partial-onset, RR =0.90 (95% CI 0.68–1.17), and for generalized epilepsy, RR =0.72 (95% CI 0.24–2.19), and difference between them was not significant (P=0.71). Details of SAEs are demonstrated in .
Figure 6 Forest plot of serious adverse events, levetiracetam vs placebo.
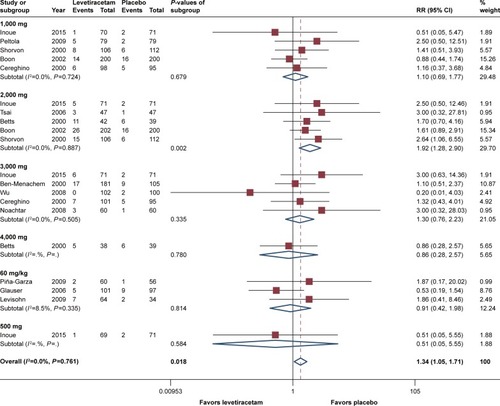
Regarding premature withdrawal, the situation was a little different. The pooled estimates of RR =1.34 (95% CI 1.05–1.71, P=0.02) indicated that discontinuation occurred in patients with levetiracetam and was substantially more common than placebo. Subgroup analysis showed dosages of 1,000, 3,000, and 60 mg/kg/day had comparable RRs, and there was no statistically significant difference between levetiracetam and placebo (). However, for the dosage of 2,000 mg/day, calculated RR reached 1.92 (95% CI 1.28–2.90), and a P-value of 0.002 showed that withdrawal was significantly more common in patients treated with levetiracetam. Since 2,000 mg/day was the significant factor affected the heterogeneity, it could also explain the discrepancy between children and adults, and the subgroup analysis according to age show that RR for children was 0.89 (95% CI 0.39–2.00, P=0.77), and for adults, it was 1.39 (95% CI 1.07–1.81, P=0.01).
Most common AEs
Eleven AEs were reported by more than four studies, as demonstrated in . The most common side effect was somnolence, reported by 13 studies, including all the three trials related to children; RR =1.67 (95% CI 1.37–2.04) and P<0.05 suggested that the occurrence of this side effect was significantly higher in patients treated with levetiracetam. Subgroup analysis based on dosage showed that incidences in dosages of 1,000, 2,000, and 60 mg/kg/day were significantly more common in levetiracetam (P<0.05). With regard to age, the analysis suggested that there was no statistically significant difference (P=0.31) between children and adults for this AE, even though children had a higher occurrence of AE than adults (RR =2.11 vs RR =1.54). Asthenia (fatigue) was also more frequent in patients with levetiracetam, and calculated RR =1.38 (95% CI 1.05–1.81, P=0.02) suggested that the statistical difference between levetiracetam and placebo was significant. Subgroup analysis according to dosage suggested that 2,000 mg/day was the most effective dose (RR =1.80); however, the occurrence of AE in levetiracetam group was not significantly higher than in placebo group (P=0.05). Analysis based on age showed that this AE was more common in children (RR =1.74) than in adulthood (RR =1.41), but the differences between them did not reach statistical significance (P=0.05). Another side effect that was widely reported was dizziness (RR =1.50, 95% CI 1.13–2.00, P<0.05). Subgroup analysis suggested that dosage of 1,000 and 2,000 mg/day had the highest occurrence (RR =1.72 and 1.66, respectively); however, none of them reached statistically significant difference (P=0.09 and P=0.10, respectively) compared to placebo. With respect to infection, pooled estimates of RR was 1.56 (95% CI 1.16–2.10, P<0.05) which suggested that there was significant difference between levetiracetam and placebo, and the results from subgroup analysis showed that this side effect was more common for 1,000 mg/day (RR =1.94, P<0.05) and 3,000 mg/day (RR =2.05, P<0.05). Nasopharyngitis was also a widely reported AE, by nine trials, and RR values through dosages ranged from 1.07 (2,000 mg/day) to 1.62 (3,000 mg/day). Though there was no single dosage substantially higher than placebo, pooled estimates of RR =1.37 (95% CI 1.07–1.77, P<0.05) suggested that occurrence of AE in levetiracetam group was significantly more common. Another AE of nausea was described by seven trials; nevertheless, pooled estimates of RR =1.09 (95% CI 0.72–1.63, P=0.7) suggested that there was no significant difference between levetiracetam and placebo.
Table 2 Most common adverse events reported among included RCTs
QoL
Apart from efficacy and safety profile, four trials used the 31-item QoL in epilepsy questionnaireCitation34 to evaluate the improvement of the QoL.Citation20,Citation23,Citation29,Citation30 Cereghino et al found that for the overall health-related QoL, there was no significant improvement; however, concerning three of seven items of seizure worry, cognitive functioning, and overall QoL, the effect was obvious.Citation20 Berkovic et al reported in terms of total score; 38.3% of patients treated with levetiracetam had obvious improvement in overall QoL since the start of the study, by contrast, only 28.6% of patients with placebo showed improvement.Citation30 In the trial of Noachtar et al, except for the social functioning, all of the other subscale scores were higher in the levetiracetam group than in the placebo group.Citation29 Zhou et al also reported that patients benefited from levetiracetam with regard to QoL according to their study.Citation23 Levisohn et al explored the cognitive effect by using the Leiter International Performance Scale-revised attention and memory (Leiter-R AM),Citation35 Wide Range Assessment of Memory and Learning (second edition, WRAML-2),Citation36 and neither Leiter-R AM nor WRAML-2 showed statistically significant differences between the levetiracetam and placebo groups in changes from baseline to the end of the evaluation period in any of the index scores.Citation28
Publication bias
Publication bias evaluation revealed that there was no potential bias across included studies, with Egger’s test of P=0.81 and Begg’s test of P=0.6.
Discussion
In this meta-analysis, we explored the efficacy, tolerability, and safety profile of levetiracetam based on 17 RCTs. Pooled estimates suggested that levetiracetam had a favorable efficacy for 50%, 75%, and 100% seizure reduction from baseline. For 50% reduction from baseline, dosages of 60 mg/kg/day, 1,000, 2,000, and 3,000 mg/day performed substantially better than placebo; furthermore, the difference was statistically significant. Four trials reported responder rate among levetiracetam group to be substantially higher at 1,000 mg/day,Citation2,Citation18–Citation20 while only one trial reported no statistically significant difference when compared to placebo.Citation27 As for dosage of 3,000 mg/day, more than half RCTs reported significant improvement in patients treated with levetirace-tam,Citation20,Citation24,Citation25,Citation27 whereas only two trials observed no significant difference between levetiracetam and placebo.Citation22,Citation31 Regarding efficacy among children, two of three trials described favorable responder rate at a dose of 60 mg/kg/day, which was equivalent to 3,000 mg/day for adults.Citation10,Citation26 According to our analysis, it seemed 1,000 mg/day was the optimal dosage for responder rate in most RCTs. In trial conducted by Boon et al, however, patients treated with 2,000 mg/day had significantly greater responder rate than those treated with 1,000 mg/day (P=0.018).Citation19 For seizure freedom rate, patients treated with levetiracetam at 60 mg/kg/day, 1,000, 2,000, and 3,000 mg/day performed significantly better than with placebo, and there were three of six trials at 3,000 mg/dayCitation20,Citation24,Citation25 and one of three trialsCitation28 involving children observed that levetiracetam had significant greater seizure freedom rate.
As for the adverse profile, it seemed that somnolence, asthenia, dizziness, infection, nasopharyngitis, anxiety, and irritability were more common in patients treated with levetiracetam and significantly higher than patients with placebo. However, according to the description of studies, most of these AEs were mild or moderate and did not affect the treatment. Regarding the other six side effects reported by more than three trials of abdominal pain, accident injury, headache, flu syndrome, rash, and diarrhea, results from our analysis suggested that they were more common among patients with placebo than levetiracetam. In most studies, SAE was any AE that was fatal, life-threatening, or permanently or severely disabling or incapacitating, which resulted in prolonged hospitalization. SAEs were not substantially higher in patients treated with levetiracetam, in fact it was even lower (RR =0.87), and subgroup analysis suggested that the results were comparable through different dosages. Withdrawal induced by AEs was significantly higher in levetiracetam (RR =1.92, P<0.05), and subgroup analysis showed that except 2,000 mg/day, for all of the other dosages, there was no statistically significant difference between levetiracetam and placebo. Some studies reported that compared to adults, children are prone to suffer from behavioral side effects such as aggression hostility and nervousness;Citation11,Citation32,Citation33 however, because only two trials involved children (the other involved children <4 years, and lasted a period of 7 days; it was difficult to observe behavior-related side effects), we did not perform comparison between children and adults.
Regarding QoL, different measurements used across studies made it difficult to combine the data and to perform a meta-analysis. However, according to studies, it seemed that levetiracetam has some positive effects on QoL, but it is difficult to be sure of the real-life impact of these changes; thus, these conclusions remain to be validated in future.
Two meta-analyses on levetiracetam for refractory partial-onset seizures were published earlier. One by Mbizvo et al included 11 RCTs,Citation32 in which comprised nine for adults and two for children and subgroup analyses were performed based on dosage. The difference between the present meta-analysis and theirs was that we analyzed the 75% and 100% reduction from baseline, after all, the goal of treatment for epilepsy is to achieve seizure freedom. Another difference was that other than the most common side effects and premature discontinuations that were reported, we analyzed SAEs. Besides, our meta-analysis included more trials than previous studies. In summary, our analyses revealed that levetiracetam was an effective anti-epileptic drug, and significantly superior to placebo regarding responder rate at 1000, 2000, and 3000 mg/day for adults and 60 mg/day for children, this was consistent with two earlier meta-analyses. Mbizvo et al found that doses of 2,000 and 4,000 mg/day levetiracetam had higher withdrawal rates,Citation32 and our analysis suggested that at 2,000 mg/day, levetiracetam had statistically significant higher dropout rate than placebo. Another meta-analysis by Costa et al involved levetiracetam for the treatment of refractory partial-onset seizures,Citation37 which primarily concentrated on comparison among several AEDs, and not only involved levetiracetam. Moreover, they did not provide subgroup analyses as well as detailed description regarding adverse events.
Limitations
Several limitations existed in the current meta-analysis. First, there were only three trials that involved children and one of them had a study period of only 7 days; hence, the results for children should be regarded with caution. Second, for the treatment of refractory generalized epilepsy, only two trials were included in this meta-analysis, and the results of analyses and comparison were susceptible to one of them. Third, several trials reported detailed efficacy for subtypes, but owing to insufficient data, it was difficult to perform analysis or comparison. Finally, all the included trials were placebo-controlled, thus our meta-analysis lacked comparison with other AEDs.
Conclusion
In summary, findings from the current meta-analysis suggested that levetiracetam at 1,000–3,000 mg/day (for children 60 mg/kg/day) is an effective AED for patients with refractory partial or generalized epilepsy, even in very young children. Moreover, levetiracetam has a favorable safety profile, and most of the AEs are mild or moderate. However, it seems that levetiracetam has a limited improvement in patients’ QoL.
Disclosure
The authors report no conflicts of interest in this work.
References
- ForsgrenLBeghiEOunASillanpääMThe epidemiology of epilepsy in Europe – a systematic reviewEur J Neurol200512424525310.1111/j.1468-1331.2004.00992.x15804240
- ShorvonSDThe epidemiology and treatment of chronic and refractory epilepsyEpilepsia199637Suppl 2S1S310.1111/j.1528-1157.1996.tb06027.x
- MacTLTranD-SQuetFOdermattPPreuxP-MTanCTEpidemiology, aetiology, and clinical management of epilepsy in Asia: a systematic reviewLancet Neurol20076653354310.1016/S1474-4422(07)70127-817509488
- KwanPBrodieMJEarly identification of refractory epilepsyN Engl J Med2000342531431910.1056/NEJM20000203342050310660394
- BettsTWaegemansTCrawfordPA multicentre, double-blind, randomized, parallel group study to evaluate the tolerability and efficacy of two oral doses of levetiracetam, 2,000 mg daily and 4,000 mg daily, without titration in patients with refractory epilepsySeizure200092808710.1053/seiz.2000.038010845730
- LynchBALambengNNockaKThe synaptic vesicle protein SV2A is the binding site for the antiepileptic drug levetiracetamProc Natl Acad Sci U S A2004101269861986610.1073/pnas.030820810115210974
- De SmedtTRaedtRVonckKBoonPLevetiracetam: part II, the clinical profile of a novel anticonvulsant drugCNS Drug Rev2007131577810.1111/j.1527-3458.2007.00005.x17461890
- OtoulCSmedtHDStockisALack of pharmacokinetic interaction of levetiracetam on carbamazepine, valproic acid, topiramate, and lamotrigine in children with epilepsyEpilepsia200748112111211510.1111/j.1528-1167.2007.01201.x17651416
- PellockJMGlauserTABebinEMPharmacokinetic study of levetiracetam in childrenEpilepsia200142121574157910.1046/j.1528-1157.2001.41300.x11879369
- Piña-GarzaJENordliDRJrRatingDYangHSchiemann-DelgadoJDuncanBAdjunctive levetiracetam in infants and young children with refractory partial-onset seizuresEpilepsia20095051141114910.1111/j.1528-1167.2008.01981.x19243423
- EgunsolaOChoonaraISammonsHMThippeswamyTSafety of levetiracetam in paediatrics: a systematic reviewPLoS One2016113e014968610.1371/journal.pone.014968626930201
- LiberatiAAltmanDGTetzlaffJThe PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaborationEpidemiol Biostat Public Health200964e1e34
- DerSimonianRLairdNMeta-analysis in clinical trialsControl Clin Trials1986731771883802833
- MantelNHaenszelWStatistical aspects of the analysis of data from retrospective studies of diseaseJ Natl Cancer Inst195922471974813655060
- HigginsJPGreenSCochrane Handbook for Systematic Reviews of InterventionsChichester, UKJohn Wiley & Sons, Ltd2008
- BeggCBMazumdarMOperating characteristics of a rank correlation test for publication biasBiometrics1994504108810.2307/25334467786990
- EggerMSmithGDSchneiderMMinderCBias in meta-analysis detected by a simple, graphical testBMJ1997315710962963410.1136/bmj.315.7109.6299310563
- PeltolaJCoetzeeCJiménezFOnce-daily extended-release levetiracetam as adjunctive treatment of partial-onset seizures in patients with epilepsy: a double-blind, randomized, placebo-controlled trialEpilepsia200950340641410.1111/j.1528-1167.2008.01817.x19317886
- BoonPChauvelPPohlmann-EdenBOtoulCWroeSDose-response effect of levetiracetam 1,000 and 2,000 mg/day in partial epilepsyEpilepsy Res2002481–2778910.1016/S0920-1211(01)00323-011823112
- CereghinoJJBitonVAboukhalilBDreifussFGauerLJLeppikILevetiracetam for partial seizures: results of a double-blind, randomized clinical trialNeurology200055223624210908898
- TsaiJJYenDJHsihMSEfficacy and safety of levetiracetam (up to 2,000 mg/day) in Taiwanese patients with refractory partial seizures: a multicenter, randomized, double-blind, placebo-controlled studyEpilepsia2006471728116417534
- XiaoZLiJ-MWangX-FEfficacy and safety of levetiracetam (3,000 mg/Day) as an adjunctive therapy in Chinese patients with refractory partial seizuresEur Neurol200961423323910.1159/00019710919176965
- ZhouBZhangQTianLXiaoJStefanHZhouDEffects of levetiracetam as an add-on therapy on cognitive function and quality of life in patients with refractory partial seizuresEpilepsy Behav200812230531010.1016/j.yebeh.2007.10.00318024209
- WuXHongZWuXMulticenter double-blind, randomized, placebo-controlled trial of levetiracetam as add-on therapy in Chinese patients with refractory partial-onset seizuresEpilepsia200950339810.1111/j.1528-1167.2008.01729.x18657175
- Ben-MenachemEFalterUEfficacy and tolerability of levetiracetam 3,000 mg/d in patients with refractory partial seizures: a multicenter, double-blind, responder-selected study evaluating monotherapyEpilepsia200041101276128311051122
- GlauserTAAyalaREltermanRDDouble-blind placebo-controlled trial of adjunctive levetiracetam in pediatric partial seizuresNeurology200666111654166016641323
- InoueYYagiKIkedaAEfficacy and tolerability of levetiracetam as adjunctive therapy in Japanese patients with uncontrolled partial-onset seizuresPsychiatry Clin Neurosci2015691064064810.1111/pcn.1230025854635
- LevisohnPMMintzMHunterSJYangHJonesJN01103 Levetiracetam Study GroupNeurocognitive effects of adjunctive levetiracetam in children with partial-onset seizures: a randomized, double-blind, placebo-controlled, noninferiority trialEpilepsia200950112377238910.1111/j.1528-1167.2009.02197.x19702752
- NoachtarSAndermannEMeyvischPAndermannFGoughWBSchiemann-DelgadoJLevetiracetam for the treatment of idiopathic generalized epilepsy with myoclonic seizuresNeurology200870860710.1212/01.wnl.0000297512.18364.4018285535
- BerkovicSFKnowltonRCLeroyRFSchiemannJFalterUPlacebo-controlled study of levetiracetam in idiopathic generalized epilepsyNeurology200769181751176017625106
- ZhengXWuSXiaMLiQStudy on the therapeutic effect of levetiracetam as an additive therapy for refractory partial epilepsy and the relativity between levetiracetam and multidrug resistance gene: a randomised double-blind placebo-controlled trialChin J Contemp Neurol Neurosurg2009902173177
- MbizvoGKDixonPHuttonJLMarsonAGLevetiracetam add-on for drug-resistant focal epilepsy: an updated Cochrane ReviewCochrane Database Syst Rev20129CD00190110.1002/14651858.CD001901.pub2
- HalmaEde LouwAJAKlinkenbergSAldenkampAPIJffDMMajoieMBehavioral side-effects of levetiracetam in children with epilepsy: a systematic reviewSeizure201423968569110.1016/j.seizure.2014.06.00424981629
- CramerJAPerrineKDevinskyOBryant-ComstockLMeadorKHermannBDevelopment and cross-cultural translations of a 31-item quality of life in epilepsy inventoryEpilepsia199839181889578017
- FarmerCLeiter International Performance Scale-Revised (Leiter-R)New YorkSpringer New York2013
- DumontRWillisJOVeizelKZibulskyJWide Range Assessment of Memory and Learning2nd edHobokenJohn Wiley & Sons, Inc2014
- CostaJFareleiraFAscençãoRBorgesMSampaioCVaz-CarneiroAClinical comparability of the new antiepileptic drugs in refractory partial epilepsy: a systematic review and meta-analysisEpilepsia20115271280129110.1111/j.1528-1167.2011.03047.x21729036