Abstract
We applied 13C magnetic resonance spectroscopy (MRS), a nonradioactive, noninvasive brain imaging technique, to quantify the oxidation of [1-13C] acetate in a conventional clinical magnetic resonance imaging (MRI) scanner in five consecutive elderly subjects at various clinical stages of Alzheimer’s disease (AD) progression. [1-13C] acetate entered the brain and was metabolized to [5-13C] glutamate and glutamine, as well as [1-13C] glutamate and glutamine, and the final glial oxidation product, 13C bicarbonate, at a linear rate. Calculation of the initial slope was similar in a single subject, examined twice, 1 month apart (test-re-test 8%). Mean rate of cerebral bicarbonate production in this elderly group was 0.040 ± 0.01 (n = 5). Assuming that the rate of conversion of acetate to bicarbonate is a reflection of glial metabolic rate and that glial metabolic rate is a surrogate marker for ‘neuroinflammation’, our preliminary results suggest that [1-13C] MRS may provide biomarkers for diseases, believed to involve microglia and other cells of the astrocyte series. Among these is AD, for which novel drugs which ameliorate the damaging effects of neuroinflammation before symptoms of dementia appear, are in advanced development. The value of 13C MRS as an early, noninvasive biomarker may lie in the conduct of cost-effective clinical trials.
Introduction
In Alzheimer’s disease (AD), incidence and prevalence increase with age. AD is the sixth leading cause of death in the US, and there are no effective therapies for the disease. Development of disease-modifying treatments for AD has been both expensive and unsuccessful to date. A shortcoming of current largely neuronal biomarkers, magnetic resonance imaging (MRI), amyloid imaging, and cerebrospinal fluid biomarkers, is the absence of an accepted biomarker for glial activation.Citation1,Citation2 Neuroinflammation, also termed glial activation, has long been implicated in the pathology of AD.Citation3 Recent research has shown that there are early pathological changes in the brains of persons with predementia memory decline, which can even be detected in asymptomatic individuals who may eventually develop AD.Citation4,Citation5 This phenomenon occurs early in the course of AD possibly before the more extensive neuron damage monitored by clinical tests. Most AD clinical trials depend on measures of rates of change in highly variable and insensitive clinical and cognitive measures. Therefore, there is an urgent need for appropriate biomarkers that can serve as outcome endpoints to detect progression of disease.
Magnetic resonance spectroscopy (MRS) is a powerful method for measuring brain metabolism. Noninvasive proton MRS has been used to measure brain chemical contents staticallyCitation6–Citation8 while minimally invasive carbon MRS has been used to measure brain metabolic processes in action, dynamically.Citation9 With appropriate infusion of brain exogenous labeled substrates, neuronal and glial metabolic rates have been reported in numerous neurodegenerative diseases.Citation10–Citation14 It is accepted that acetate, which enters the brain tricarboxylic acid cycle via an astrocyte-specific enzyme, can be used as a tracer for astrocyte or glial oxidative metabolism.Citation15–Citation17 Both C1 and C2 labeled acetate have been used successfully to study glial metabolism in intact human brains.Citation10,Citation13,Citation14 In aging, evidence of upregulation of astrocytes (glia) metabolism was demonstrated with an increase of 25% in older subjects compared with younger adults using labeled C2 acetate as exogenous substrate.Citation18
In this preliminary communication, we employed a short protocol developed previouslyCitation19 to directly measure glial dysfunction in Alzheimer’s patients using C1 acetate as an exogenous labeled substrate.
Methods
Patients
The studies were approved by the local internal review board (Huntington Memorial Hospital), and all patients gave written informed consent. A total of 24 MRI/MRS studies were performed on six subjects of whom two had AD, two had mild cognitive impairment (MCI) and two were healthy older adults with mean age of 78 ± 3 years, and two were females.
Neuropsychological assessments
All subjects underwent extensive neuropsychological evaluation using the standard test battery to evaluate AD.Citation20 Briefly: premorbid estimates of intellectual functioning were performed (Wechsler Test of Adult Reading). The core neuropsychological battery included the Wechsler Adult Intelligence Scales, third ed. (WAIS-III) and the Wechsler Memory Scales, third ed. (WMS-III) Domains of Attention/Concentration/Working Memory (WAIS-III Digit Span, Letter–Number Sequencing and Arithmetic), Psychomotor/Information processing (WAIS-III Symbol Search and Digit Symbol, Stroop word reading and color naming), Language functions (COWAT-FAS and Animals, WAIS-III Information), Verbal Memory (WMS-III Logical Memory I and II, California Verbal Learning Test-II), Nonverbal Memory (Brief Visual Memory Test-Revised, Rey Osterreich Complex Figure Test-Delay), Visuospatial/construction (WAIS-III Block Design, Picture Completion, Ruff Figural Fluency, Judgment of Line Orientation, Rey Osterreich Complex Figure Test-Copy) Executive Functions (Delis–Kaplan Executive Functions System – Tower Test), and Motor Functioning (Purdue Pegboard) were assessed.
All subjects underwent mini-mental state examination (MMSE). Criteria for MCI cut-off from normal included absence of dementia (MMSE ≥ 23) essentially preserved activities of daily living, the presence of cognitive and/or memory complaints, and impairment >1 standard deviation below age norms on one or more immediate or delayed memory tests or ≥2 nonmemory tests.Citation21 Criteria for AD classification included MMSE score <23, impairment in activities of daily living, and ≥2 standard deviations below age norms on a majority of memory tests.
Neuroimaging and spectroscopy
Examinations were performed on a GE 1.5 T MRI scanner equipped with non-proton MRS capability as previously prescribed.Citation22 Localized MRI was acquired with a 30-slice T2-weighted fast spin echo imaging sequence with echo time (TE) = 96 milliseconds, repetition time (TR) = 4 seconds, slice thickness = 5 mm, 1 excitation, and 256 × 192 data acquisition matrix using a single channel quadrature head coil for both transmitting and receiving. These localizer images were used to prescribe two single voxel proton MRS acquisitions of posterior cingulate grey matter and white matter (PROBE-p TE = 35 milliseconds, TR = 1.5 seconds, 8 mL voxel, 128 averages). For proton decoupled 13C MRS, scanning protocol is similar to that previously described.Citation19,Citation23 Briefly, prior to the start of the infusion protocol, a natural abundance 13C MRS was acquired for 30 minutes of five blocks pulse, to acquire data averaging a Waltz-4 bi-level proton decoupling and nuclear OverHouser (NOE) scheme with a power level of 5 W during decoupling and 0.9 W during the NOE period. Subsequently, intravenous administration of stable isotope enriched 1–13C acetate (Cambridge Isotopes Laboratories, Andover, MA; FDA IND 59,950) was performed with the subjects lying comfortably outside the MRI scanner for 60 minutes. The patients were then swiftly positioned back in the MRI scanner and data acquisition was performed for another 60 minutes.
Data and statistical analysis
Automated spectral processing for single voxel proton MRS data was performed using the commercially available LCModel softwareCitation24 using a standard 1.5T reference basis set for semi-quantification. The results are reported as metabolic ratios.
For 13C MRS time series data were processed as previously described using an observer-independent automatic IDL-based software developed in house and SAGE (GE Healthcare, Milwaukee, WI).Citation14 The rate of bicarbonate production was determined using the difference between the amount of measured bicarbonate level pre- and post-infusion over the time from the start of infusion to the end of data acquisition.Citation19
Simple statistical analysis was performed using linear regression, and the goodness of linear fit is reported as R2.
Results
Subject characteristics, MRS, and neuropsychological evaluation results are shown in .
Table 1 Compilations of subjects’ characteristics, proton, carbon MRS, and neuropsychological assessment results
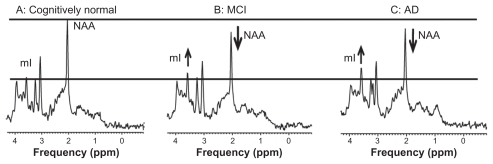
Proton MRS shows increased myoinositol to creatine ratio and decreased N-acetyl aspartate to myoinositol ratio, similar to results previously reported.Citation5 Typical proton MRS spectra in cognitively normal, MCI, and AD subjects are shown in .
Figure 1 Proton MRS spectra from healthy elderly (A), an elderly subject with MCI (B) and an AD patient (C). Spectra were scaled to creatine peak intensity.
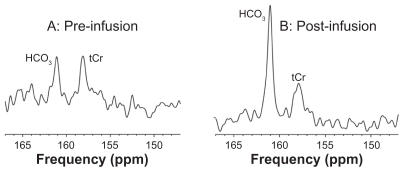
Glial metabolism of 13C acetate was demonstrated by increased 13C enrichment of cerebral bicarbonate (HCO3, 161 ppm) compared with pre-infusion (). The unenriched total creatine resonance (tCr, 158 ppm) was also observed.
Figure 2 Typical spectra (147–167 ppm region) of 13C MRS in an AD subject at baseline (A) and at 1 hour after start of infusion (B).
Clinically defined normal, MCI, and AD patients (MMSE normal = 28–30, MCI = 23–27, AD < 23) was accompanied by reduction in NAA/mI (−36% ± 0.05; P = 0.01) as previously described.Citation5
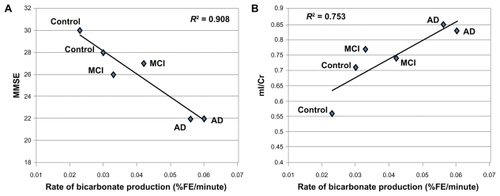
In contrast, 13C MRS showed a progressive increase in enrichment of the glial-oxidation product, 13C-bicarbonate (). The increase in glial metabolic activity showed that %fractional enrichment of bicarbonate was inversely correlated with clinical grade (MMSE) (R2 = 0.908) () and MRS-glial biomarker (mI/Cr) (R2 = 0.753) (). Weak or no correlation was observed for neuronal biomarker (NAA/Cr) and glial metabolic activity (R2 = 0.11) (not shown).
Figure 3 Significant correlation of the rate of bicarbonate productions in % fractional enrichment (FE) of bicarbonate per minute and the clinical measure, mini-mental state examination (MMSE) with R2 = 0.908 (A) and glial cell density from proton MRS (mI/Cr) with R2 = 0.753 (B).
Conclusions
Our preliminary results directly demonstrate progressively elevated glial metabolic rate in patients with MCI and AD in proportion to the elevation in glial marker myoinositol of conventional proton MRS, and inversely correlated with a clinical measure of cognitive decline, MMSE. Although the number of patients in this preliminary study is small, the data could contribute to the ongoing debate supporting the general belief that activation of glia observed in animal models, may occur in human MCI and AD.
Neuroscientists have made extensive use of the term ‘neuroinflammation’ to describe a complex process that occurs when glia cells undergo activation in response to stimuli such as viral infection,Citation25,Citation26 undesirable substances,Citation27,Citation28 injury,Citation29 and illnesses.Citation30 Activated microglia and glia cells have been reported in AD.Citation3,Citation31,Citation32 It is possible that our observation of an increase in glial activation is synonymous with neuroinflammation. Additional studies are required to confirm that relationship. If confirmed, 13C MRS after infusion of 1–13C acetate as employed here would provide a practical approach to defining neuroinflammation in human brain. Extending the present observation to larger numbers of well-defined patients and in longitudinal studies which document the onset of glial hyperactivity in comparison with the earliest evidence of neuronal loss (proton MRS or 13C MRS using 1–13C glucose as exogenous substrate are valid techniquesCitation33) could provide direct information on whether neuroinflammation is the immediate cause of MCI and AD or merely a secondary effect of neuronal injury. A conclusion in favor of the former mechanism would support the renewed impetus for preventative therapies, which target neuroinflammation in the increasing numbers of patients diagnosed with early AD. Human use of 13C MRS, as demonstrated here, is a safe, nonradioactive technique which can be performed on conventional clinical MRI scanners with only minor modification, and would be a valuable tool for such future studies.
Acknowledgment
The authors thank Norman Chien, MD for referral of AD subjects, Dr Michael Harrington, Ms Sherri Lee, and the Fuller Seminary students for their assistance in preliminary subject screening (N = 3) and Mr Thomas Warren for his assistance in the infusion protocol. The study is funded by NIDA (K25DA21112, NS) and L.K. Whittier Foundation (BDR, KH, and TT).
Disclosure
The authors declare no conflicts of interest in this work.
References
- KimSSwaminathanSShenLGenome-wide association study of CSF biomarkers Abeta 1–42 t-tau, and p-tau181p in the ADNI cohortNeurology2011761697921123754
- ShawLMVandersticheleHKnapik-CzajkaMQualification of the analytical and clinical performance of CSF biomarker analyses in ADNIActa Neuropathol2011121559760921311900
- KrauseDLMullerNNeuroinflammation, microglia and implications for anti-inflammatory treatment in Alzheimer’s diseaseInt J Alzheimers Dis2010201073280620798769
- JackCRJrKnopmanDSJagustWJHypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascadeLancet Neurol20109111912820083042
- GodboltAKWaldmanADMacManusDGMRS shows abnormalities before symptoms in familial Alzheimer diseaseNeurology200666571872216534109
- RossBDBlumlSCowanRDanielsenEFarrowNGruetterRIn vivo magnetic resonance spectroscopy of human brain: the biophysical basis of dementiaBiophys Chem1997681–31611729468618
- RossBDDanielsenERBlumlSProton magnetic resonance spectroscopy: the new gold standard for diagnosis of clinical and sub-clinical hepatic encephalopathy?Dig Dis.199614Suppl 130398872450
- RossBTranTBhattacharyaPWattersonDMSailasutaNApplication of NMR spectroscopy in medicinal chemistry and drug discoveryCurr Top Med Chem20111119311420809893
- RossBLinAHarrisKBhattacharyaPSchweinsburgBClinical experience with 13C MRS in vivoNMR Biomed2003166–735836914679500
- LebonVPetersenKFClineGWAstroglial contribution to brain energy metabolism in humans revealed by 13C nuclear magnetic resonance spectroscopy: elucidation of the dominant pathway for neurotransmitter glutamate repletion and measurement of astrocytic oxidative metabolismJ Neurosci20022251523153111880482
- RothmanDLBeharKLHyderFShulmanRGIn vivo NMR studies of the glutamate neurotransmitter flux and neuroenergetics: implications for brain functionAnnu Rev Physiol20036540142712524459
- BlumlSMorenoAHwangJHRossBD1-(13)C glucose magnetic resonance spectroscopy of pediatric and adult brain disordersNMR Biomed2001141193211252037
- BlumlSMoreno-TorresAShicFNguyCHRossBDTricarboxylic acid cycle of glia in the in vivo human brainNMR Biomed20021511511840547
- SailasutaNAbulseoudOHarrisKCRossBDGlial dysfunction in abstinent methamphetamine abusersJ Cereb Blood Flow Metab201030595096020040926
- Van den BergCJKrzalicLMelaPWaelschHCompartmentation of glutamate metabolism in brain. Evidence for the existence of two different tricarboxylic acid cycles in brainBiochem J196911322812905808317
- WyssMTMagistrettiPJBuckAWeberBLabeled acetate as a marker of astrocytic metabolismJ Cereb Blood Flow Metab2011June 8 [Epub ahead of print.]
- MuirDBerlSClarkeDDAcetate and fluoroacetate as possible markers for glial metabolism in vivoBrain Res198638023363403756485
- BoumezbeurFMasonGFde GraafRAAltered brain mitochondrial metabolism in healthy aging as assessed by in vivo magnetic resonance spectroscopyJ Cereb Blood Flow Metab201030121122119794401
- SailasutaNTranTTHarrisKCRossBDSwift Acetate Glial Assay (SAGA): an accelerated human (1)(3)C MRS brain exam for clinical diagnostic useJ Magn Reson2010207235235520934362
- StraussEHShermanEMSSpreenOA compendium of neuropsychological tests3rd edNew YorkOxford University Press2006
- PetersenRCMild cognitive impairment as a diagnostic entityJ Intern Med2004256318319415324362
- SailasutaNRobertsonLWHarrisKCGropmanALAllenPSRossBDClinical NOE 13C MRS for neuropsychiatric disorders of the frontal lobeJ Magn Reson2008195221922518829354
- SailasutaNAbulseoudOHernandezMHaghaniPRossBDMetabolic abnormalities in abstinent methamphetamine dependent subjectsSubst Abuse20102010492020485533
- ProvencherSWEstimation of metabolite concentrations from localized in vivo proton NMR spectraMagn Reson Med19933066726798139448
- EdenAPriceRWSpudichSFuchsDHagbergLGisslenMImmune activation of the central nervous system is still present after >4 years of effective highly active antiretroviral therapyJ Infect Dis2007196121779178318190258
- Von GiesenHJWittsackHJWenserskiFKollerHHefterHArendtGBasal ganglia metabolite abnormalities in minor motor disorders associated with human immunodeficiency virus type 1Arch Neurol20015881281128611493169
- SekineYOuchiYSugiharaGMethamphetamine causes microglial activation in the brains of human abusersJ Neurosci200828225756576118509037
- Miguel-HidalgoJJThe role of glial cells in drug abuseCurr Drug Abuse Rev200921728219630738
- HiltonGDStoicaBAByrnesKRFadenAIRoscovitine reduces neuronal loss, glial activation, and neurologic deficits after brain traumaJ Cereb Blood Flow Metab200828111845185918612315
- GraberDJHickeyWFHarrisBTProgressive changes in microglia and macrophages in spinal cord and peripheral nerve in the transgenic rat model of amyotrophic lateral sclerosisJ Neuroinflammation20107820109233
- EikelenboomPBateCVan GoolWANeuroinflammation in Alzheimer’s disease and prion diseaseGlia200240223223912379910
- McGeerEGMcGeerPLInflammatory processes in Alzheimer’s diseaseProg Neuropsychopharmacol Biol Psychiatry200327574174912921904
- LinAPShicFEnriquezCRossBDReduced glutamate neurotransmission in patients with Alzheimer’s disease – an in vivo (13)C magnetic resonance spectroscopy studyMAGMA2003161294212695884