Abstract
Cataplexy is defined as episodes of sudden loss of voluntary muscle tone triggered by emotions generally lasting <2 minutes. Cataplexy is most commonly associated with and considered pathognomonic for narcolepsy, a sleep disorder affecting ~0.05% of the general population. Knowledge of the pathophysiology of cataplexy has advanced through study of canine, murine, and human models. It is now generally considered that loss of signaling by hypothalamic hypocretin/orexin-producing neurons plays a key role in the development of cataplexy. Although the cause of hypocretin/orexin neuron loss in narcolepsy with cataplexy is unknown, an autoimmune etiology is widely hypothesized. Despite these advances, a literature review shows that treatment of cataplexy remains limited. Multiple classes of antidepressants have been commonly used off-label for cataplexy in narcolepsy and are suggested for this use in expert consensus guidelines based on traditional practice, case reports, and small trials. However, systematic research evidence supporting antidepressants for cataplexy is lacking. The single pharmacotherapy indicated for cataplexy and the guideline-recommended first-line treatment in Europe and the US is sodium oxybate, the sodium salt of gamma-hydroxybutyrate. Clinical trial evidence of its efficacy and safety in cataplexy is robust, and it is hypothesized that its therapeutic effects may occur through gamma-aminobutyric acid receptor type B-mediated effects at noradrenergic, dopaminergic, and thalamocortical neurons. Additional possible mechanisms for cataplexy therapy suggested by preliminary research include antagonism of the histamine H3 autoreceptor with pitolisant and intravenous immunoglobulin therapy for amelioration of the presumed autoimmune-mediated hypocretin/orexin cell loss. Further research and development of therapeutic approaches to cataplexy are needed.
Cataplexy: definition and characteristics
Cataplexy is defined as episodes of sudden, transient loss of voluntary muscle tone (usually bilateral, but case reports have identified unilateral casesCitation1) triggered by strong emotions. While laughter is the most typical trigger, other triggers include happiness, elation, fright, anger, startle, stress and, less frequently, pain and orgasm, although episodes may also occur spontaneously.Citation2–Citation4 Episodes are typically brief, generally lasting <2 minutes, followed by rapid return of normal muscle tone/function, and range from mild or barely noticeable to severe, with complete postural collapse. While any or all voluntary muscles can be affected (with the exception of the diaphragm and the extraocular muscles of the eye), patients remain conscious and continue to breathe and to move their eyes.Citation3 The most common manifestations are neck weakness, causing head drop; partial or complete ptosis; facial weakness with sagging of the jaw with or without dysarthria; and trembling or buckling of the knees.Citation2,Citation5,Citation6 Positive motor symptoms such as muscle twitching or small jerks of the face or limbs also occur, sometimes contributing to misdiagnosis.Citation2,Citation5 Patients typically sense the onset of an episode, allowing them to sit or brace themselves before its occurrence, thus reducing the risk of injury, although in one survey, up to half of patients reported some kind of injury from their cataplexy.Citation2 Duration of a cataplectic attack generally lasts from a few seconds to several minutes. However, more commonly after the abrupt discontinuation of antidepressant medication (tricyclic antidepressants [TCAs], serotonin reuptake inhibitors [SSRIs], or serotonin-norepinephrine reuptake inhibitor [SNRI]), attacks of cataplexy typically are more frequent and/or more intense (rebound phenomena) and can last up to several hours, at which time they are designated as status cataplecticus.Citation7,Citation8 Frequency of episodes is variable, ranging from <1 episode per year to several per day, but most patients have several episodes per week.Citation2,Citation5,Citation6,Citation9
with cataplexy is a sleep disorder that is traditionally characterized by a symptom pentad that includes, in addition to cataplexy, excessive daytime sleepiness (EDS), sleep paralysis, hypnagogic hallucinations, and disrupted nighttime sleep.Citation10 Narcolepsy with cataplexy is estimated to affect 0.03% to 0.05% of the general population,Citation11 and onset occurs typically in childhood or adolescence.Citation6,Citation12 Although EDS is present in all patients with narcolepsy and is often the initial presenting symptom, cataplexy occurs in ~70% of patients;Citation3,Citation13 cataplexy is considered pathognomonic for narcolepsy and after sleepiness is the primary behavioral marker.
The third edition of the International Classification of Sleep Disorders (ICSD-3) classifies narcolepsy as either type 1 or type 2, with the presence of cataplexy incorporated into the definition of type 1.Citation13 Type 1 narcolepsy is formally defined in the ICSD-3 as EDS that persists for ≥3 months with “positive” electrophysiological sleep studies that includes the finding of an average sleep-onset latency ≤8 minutes on the MSLT following a nocturnal polysomnogram that was negative for any comorbid sleep disorders, and two or more sleep-onset rapid eye movement (REM) periods on the MSLT (one of these sleep-onset REM periods may come from the preceding nocturnal polysomnogram) with clear historic evidence of cataplexy or low or absent levels of hypocretin/orexin in cerebrospinal fluid (CSF) along with the “positive” sleep studies.Citation13 Hypocretin-1 and hypocretin-2, also called orexin A and orexin B, respectively, are peptide neurotransmitters that facilitate wakefulness and enhance arousal mechanisms as well as stabilizing REM and non-REM sleep states.Citation14,Citation15 Type 2 narcolepsy, or narcolepsy without cataplexy, is defined as EDS persisting for ≥3 months, a positive result on the polysomnogram/Multiple Sleep Latency Test (MSLT), and normal or mildly decreased CSF hypocretin/orexin levels; hypocretin-1 is more stable than hypocretin-2 and thus is the peptide that is measured in CSF as a neurochemical marker of narcolepsy.Citation13
Although cataplexy is often absent as an early symptom of narcolepsy in children, retrospective data suggest that children with narcolepsy who initially present without cataplexy will likely develop cataplexy within 1 year of EDS onset.Citation16–Citation18 Additionally, the initial presenting cataplexy phenotype in children can differ from the presentation commonly seen in adults.Citation16,Citation19,Citation20 Relative to the exclusive loss of voluntary muscle tone in adults, early manifestations of cataplexy in children may variously include both hypotonia and abnormal movements, with prominent facial involvement (often referred to as “cataplectic facies”), including partial ptosis, masked-like facies with jaw slackening, facial grimaces, and perioral and tongue movements/protrusion all with or without dysarthria.Citation16,Citation19,Citation20 Furthermore, chorea-like or dystonic movements, gait disturbances, and complete falls can occur spontaneously without apparent emotional triggers.Citation16,Citation19,Citation20
Cataplexy or cataplexy-like symptoms have been observed in other conditions including Niemann–Pick type C disease, Prader–Willi syndrome, Coffin–Lowry syndrome, Moebius syndrome, Norrie disease, and Wilson’s disease.Citation21–Citation28 Although some of these conditions may also be accompanied by sleep disorders and hypocretin/orexin deficiency,Citation29–Citation31 they are characterized by distinct features including other specific neurological deficits or mental/cognitive changes, or both, such that there is little overlap in diagnosis with narcolepsy with cataplexy.
Pathophysiology of narcolepsy with cataplexy
Role of hypocretin/orexin in narcolepsy
Located in the posterolateral hypothalamus, hypocretin/orexin neurons project to almost all brain areas and play critical roles in regulation of the sleep/wake cycle, as well as metabolism, feeding, reward, mobility, and autonomic tone ().Citation32–Citation36 Hypocretin/orexin neurons innervate noradrenergic, dopaminergic, serotonergic, histaminergic, and cholinergic brain regions and directly excite these neurons, as well as regulate release of glutamate and other neurotransmitters ().Citation34,Citation37–Citation39 Studies of neuronal sleep regulation in murine and other animal models have shown that hypocretin/orexin neurons discharge during wakening, promoting wakefulness and the return of postural muscle tone, and virtually cease discharge during sleep; their activity appears to suppress cortical deactivation and muscle atonia.Citation40,Citation41
Figure 1 Hypocretin/orexin neuron innervation.
Abbreviations: 5-HT, 5-hydroxytryptamine (serotonin); Ach, acetylcholine; DA, dopamine; GABA, gamma-aminobutyric acid; HA, histamine; NE, norepinephrine.
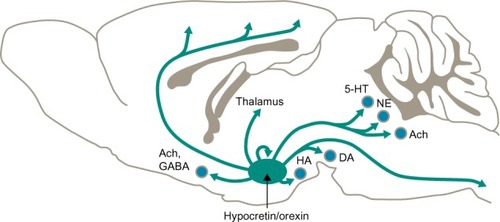
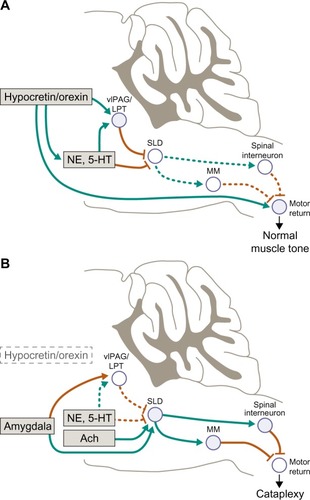
Figure 2 Atonia pathways triggering cataplexy.
Abbreviations: 5-HT, 5-hydroxytryptamine (serotonin); Ach, acetylcholine; LPT, lateral pontine tegmentum; MM, medial medulla; NE, norepinephrine; SLD, sublaterodorsal nucleus; vlPAG, ventrolateral periaqueductal gray.
The discovery that loss of signaling by these hypothalamic neuropeptides is the presumptive cause of narcolepsy in humans, dogs, and mice has been a major advance in our understanding of the regulation of sleep and wakefulness along with the control of REM-on and REM-off states.Citation37,Citation42–Citation44 While the underlying pathologic changes resulting in this loss of signaling are different among the models, genetic mutation of the gene encoding the hypocretin-2 protein in dogs,Citation42 knockout of genes encoding hypocretin receptors in mouse models,Citation43 and selective loss of hypocretin neurons likely due to an autoimmune response in humans,Citation44 the manifestations resulting from the loss of hypocretin signaling support this loss as the pathophysiologic basis of narcolepsy.
Narcolepsy with cataplexy is typically associated with the loss of 90% or more of hypocretin/orexin-producing neurons with low or undetectable CSF hypocretin-1/orexin A levels.Citation34 A CSF hypocretin/orexin level below 110 pg/mL is considered diagnostic for type 1 narcolepsy,Citation13 but a standard test for hypocretin/orexin is not available for general use in the clinical setting. Most patients with narcolepsy without cataplexy have CSF hypocretin/orexin levels in the normal range, although a reduced hypocretin/orexin concentration in this subgroup of patients is associated with worse narcolepsy symptoms than in patients with higher levels.Citation17 Mouse models suggest that cataplexy occurs when loss of hypocretin/orexin-producing neurons is ~95%, whereas early narcolepsy traits such as sleep fragmentation/disturbance and reduced wakefulness were observed even at low levels of neuronal destruction.Citation36,Citation45 Such a relationship was further supported by a case study suggesting that cataplexy may be on a narcolepsy spectrum that results from a decremental loss of hypocretin/orexin.Citation46
Although the etiopathogenesis of the destruction of these hypocretin/orexin-producing neurons has not been fully elucidated, it appears to result from a process selective for these neurons rather than generalized neuronal degeneration.Citation37,Citation47 A widely held hypothesis is that autoimmune processes contribute to the pathogenic mechanisms.Citation47,Citation48 Support for autoimmune mechanisms also comes from the observed association of narcolepsy with specific genotypes, including the human leukocyte antigen HLA-DQB1*06:02, which is found in ~95% of patients with what is currently classified as type 1 narcolepsy, 41% of patients with type 2, and only 18%–35% of the general population.Citation49 An observed association between T-cell receptor variants and loss of hypocretin/orexin-producing neurons further supports the autoimmune hypothesis,Citation50,Citation51 as do the reports of narcolepsy onset associated with seasonal Streptococcus infections, H1N1 influenza, and H1N1 vaccination in individuals with genetic predisposition to induction of autoimmune events. While researchers have yet to definitively determine the specific autoimmune mechanisms involved, a nucleoprotein that is present in both the H1N1 virus and the Pandemrix H1N1 vaccine has been identified that cross-reacts with the hypocretin receptor 2.Citation52 The additional finding of a possible immune response to the hypocretin-2 receptor after the Pandemrix H1N1vaccine in DQB1*0602-positive narcoleptic patients supports the autoimmune hypothesis.Citation52
Neurological pathways of cataplexy
Investigations of the neurophysiology of cataplexy have included studies in humans and animal models (ie, dogs and mice; ). The “REM sleep disassociation hypothesis” suggests that cataplexy and sleep paralysis are dysregulated manifestations, or intrusions into the waking state, of the skeletal muscle motor inhibition that normally occurs during REM sleep to prevent the acting out of dreams, while diaphragmatic breathing and extraocular muscles remain functional.Citation9 Indeed, studies in dogs and humans have suggested that brainstem circuitry is similar in both REM sleep and cataplexy episodes.Citation53,Citation54 However, this does not fully address the triggering of cataplexy by emotional stimulation, and this mechanism has also been an area of investigation and source of alternative hypotheses.Citation55–Citation58
Table 1 Cataplexy across species
Cataplectic atonia is caused by inhibition of skeletal motor neuron activity and absence of deep tendon reflexes and the loss of the monosynaptic Hoffman reflex,Citation56 which results from increased inhibitory and reduced excitatory signaling of motor neurons in the brain and spinal cord.Citation34,Citation59,Citation60 Notably, loss of Hoffman reflex activity is common to cataplexy, laughter, and REM sleep.Citation56 Neurochemically, cataplexy is triggered by cholinergic activation and deactivation of monoaminergic systems primarily in the brainstem, especially those of adrenergic pathways, which may be caused by an imbalance of monoamines and acetylcholine.Citation55,Citation59 This inhibitory mechanism is characterized by intense activation of gamma-aminobutyric acid (GABA)–releasing neurons in the medial medulla and central nucleus of the amygdala, which, in turn, inhibits noradrenergic neurons that maintain waking muscle tone such as those in the ventrolateral periaqueductal gray, lateral pontine tegmentum, locus coeruleus, and dorsal raphe.Citation9,Citation58,Citation61 This action turns off release of noradrenaline to motor neurons (both alpha-motor neurons and spinal interneurons), leading to their reduced activity with emergence of cataplectic atonia.Citation9,Citation34 It has also been suggested that the emotionally-induced cataplectic atonia may result from glutamatergic excitatory activation of neurons in the sublaterodorsal tegmental nucleus, which generally regulate muscle atonia during REM sleep;Citation62 in normal individuals, that is, those with normal hypocretin levels, this excitation during waking would be blocked by a hypocretin-mediated GABAergic effect. Other brain regions involved in the neural circuitry of cataplexy may also include basal forebrain, hypothalamus, and limbic structures, although the precise roles of these regions are yet to be fully elucidated.Citation34,Citation57,Citation58,Citation63
Cataplexy treatment
Clinical management of cataplexy is limited by etiological uncertainty as well as by challenges in the clinical identification and diagnosis of the underlying narcolepsy; diagnosis is often delayed by many years after initial symptom onset.Citation64 In particular, there are no standard measures for identifying or assessing cataplexy, increasing the challenges not only of its identification but also of evaluation during treatment. Thus, the recognition of cataplexy has primarily been based on clinical interview and patient self-reports. With evaluation of cataplexy during clinical trials or in the clinical setting relying on patient recall and/or diaries, the manner of assessment often varies and may not always include a full description of frequency and severity. Although self-administered cataplexy questionnaires have been developed for eliciting information on occurrence,Citation65,Citation66 they may be limited by a high burden of administration (one of the validated questionnaires consists of 51 questionsCitation65) and/or use only as a screening tool since their sensitivity to treatment effects has not been demonstrated. However, despite these challenges, the pharmacological management of cataplexy has a long history and a potentially promising future.
Pharmacological management: historical overview
Multiple classes of pharmacological agents have been used for treatment of narcolepsy, including stimulants, antidepressants, and hypnosedatives. The majority of these agents have not been rigorously tested for either safety or efficacy in cataplexy. Use of amphetamines was among the earliest approaches to narcolepsy treatment; they were first used for this purpose in 1935 based on their strong wake-promoting effects.Citation67 Although these drugs have consistently been used and are generally considered effective for reducing EDS, their lack of efficacy for cataplexy was recognized early during their clinical use.Citation68 Methylphenidate, with a similar mechanism of action and somewhat better safety profile compared with the amphetamines, has been widely used for narcolepsy since the 1950s,Citation69 but also like the amphetamines, there have been no demonstrable effects vis-à-vis improvement of cataplexy.
TCAs such as clomipramine, imipramine, desipramine, and protriptyline have been used as a therapeutic approach for narcolepsy for many years, and their efficacy for cataplexy was reported as early as 1960.Citation70,Citation71 Since then, evidence from case reports and small open-label studies has further supported their beneficial effects for improving cataplexy.Citation72–Citation77 It is important to note that these drugs have never been evaluated in larger and more formal randomized, placebo-controlled clinical trials. Although the mechanism of action of these drugs for cataplexy remains unknown, it has been thought that it may be related to their augmentation of noradrenergic signaling and/or the relative suppression of REM sleep.Citation78
Current therapeutic approaches
Treatment recommendations for cataplexy are included as part of the overall guidelines for the treatment of narcolepsy issued by the American Academy of Sleep MedicineCitation79 and European Federation of Neurological Societies.Citation80 Both of these guidelines recommend several drugs as being of potential benefit for the treatment of cataplexy, but only sodium oxybate is recommended as a first-line treatment for cataplexy based on high levels of evidence obtained from randomized controlled clinical trials. Sodium oxybate is indicated for the treatment of cataplexy associated with narcolepsyCitation81 and is currently the only medication with such an indication. Additionally, both guidelines recommend sodium oxybate as a first-line therapy for treatment of EDS in narcolepsy,Citation79,Citation80 for which it is indicated,Citation81 and the American Academy of Sleep Medicine guidelines also recommend sodium oxybate as a standard treatment for disrupted sleep, although it is not approved by the US Food and Drug Administration or the European Medicines Agency for use for the treatment of this symptom. Both guidelines further suggest that sodium oxy-bate be considered for hypnagogic hallucinations and sleep paralysis;Citation79,Citation80 as is the case for disrupted nighttime sleep, it is not specifically indicated for these symptoms, and the level of evidence is lower than for cataplexy and EDS.
As mentioned in the guidelines, the evidentiary basis for therapeutic alternatives to sodium oxybate for the treatment of cataplexy is limited. Suggested alternatives include TCAs, particularly clomipramine, and newer antidepressants such as SSRIs, the SNRI venlafaxine, and norepinephrine reuptake inhibitor reboxetine (not available in the US).Citation79,Citation80 The monoamine oxidase type B inhibitor selegiline is also noted for its efficacy in cataplexy, although both guidelines warn of limitations due to its safety profile including the potential for drug and food interactions.Citation79,Citation80 The European guidelines identify TCAs as the most effective of the alternatives to sodium oxybate for cataplexy, whereas the American guidelines do not state any general preferences among the second-line drug classes.
Overall, the currently available treatments for cataplexy act symptomatically, and there is no evidence suggesting that they target the hypocretin/orexin system ().Citation82 Sodium oxybate, which is the sodium salt of gamma-hydroxybutyrate, was observed to induce REM sleep followed by an increase in slow-wave non-REM sleep and was subsequently investigated for the treatment of narcolepsy on the hypothesis that improvement of nocturnal sleep would reduce EDS and possibly other narcolepsy symptoms, including cataplexy.Citation83,Citation84 Although the mechanism of action of sodium oxybate is unknown, it is hypothesized that its therapeutic effects on cataplexy and EDS are mediated through GABA type B (GABAB) activity, potentially impacting noradrenergic and dopaminergic neuronal function as well as that of thalamocortical neurons.Citation81,Citation85 However, a clinical comparison with the prototypical GABAB agonist baclofen (a racemic mixture of R- and S-isomers of baclofen) showed that while sodium oxybate reduced EDS and cataplexy, baclofen had no effect on these narcolepsy symptoms. These results suggest that the efficacy of sodium oxybate may derive from mechanisms more complex than direct GABAB agonism.Citation86,Citation87
Table 2 Commonly used anticataplectic medications and their pharmacological properties
The mechanism of action of the antidepressants in narcolepsy with cataplexy is generally related to their ability to inhibit reuptake of monoamines,Citation9,Citation87,Citation88 with a high correlation between receptor affinity and potency of antidepressants for their effects on cataplexy.Citation89–Citation91 The TCAs are nonspecific monoamine reuptake inhibitors with effects that promote the availability and activity of serotonin, noradrenaline and, for some agents, dopamine. The TCAs most commonly used in narcolepsy – clomipramine, imipramine, desipramine, and protriptyline – exert, in addition to monoamine reuptake inhibition, anticholinergic actions that may contribute to their effect on cataplexy but may also cause important side effects such as constipation, dry mouth, blurred vision, restless legs, and alterations in cardiac conduction.Citation9,Citation87 While inhibition of serotonin reuptake by SSRIs reduces cataplexy, very selective SSRIs such as escitalopram or fluoxetine are usually not as effective for cataplexy as venlafaxine, an SNRI that is used mainly because it has demonstrated a short onset of action.Citation87 However, abrupt withdrawal of venlafaxine may precipitate rebound cataplexy.Citation8 The monoamine oxidase type B inhibitor selegiline increases availability of the monoamine neurotransmitter dopamine, but its use is limited by potential side effects, as also noted in the narcolepsy treatment guidelines.Citation89,Citation90
As suggested by the high level of evidence used to support its recommendation as a first-line therapy, sodium oxybate has demonstrated efficacy for the treatment of cataplexy and other narcolepsy symptoms in multiple randomized controlled clinical trials.Citation92–Citation94 In particular, the median percent decrease in the number of weekly cataplexy attacks was ~70% after 4 weeks of treatment with 9 g per night, and no rebound cataplexy was observed in patients who had their sodium oxybate abruptly discontinued.Citation93,Citation94 Long-term studies showed that these effects on cataplexy were maintained for 12 months and up to 4 years.Citation95,Citation96 Two systematic reviews and meta-analyses of sodium oxybate provided further support for the robustness of its efficacy for reducing cataplexy episodes, with the greatest effects observed at the highest dose of 9 g per night.Citation97,Citation98 These meta-analyses also reported that sodium oxybate was generally well tolerated, with most adverse events of mild-to-moderate severity. Furthermore, a retrospective analysis of sodium oxybate in a pediatric population suggested that in children and adolescents aged 4 through 16 years, treatment significantly reduced the median frequency of cataplexy episodes from 38 per week to <1 per week (n=14; P<0.001) and reduced cataplexy severity relative to baseline (P<0.001; sodium oxybate is not indicated for use in patients <18 years old).Citation99 It should also be noted that sodium oxybate has the potential for serious adverse effects (eg, central nervous system depression, abuse/misuse, respiratory depression and sleep-disordered breathing, depression and suicidality, other behavioral/psychiatric adverse reactions, and parasomnias)Citation81 and when used at higher than therapeutic doses, whether accidentally or intentionally, it can lead to acute or fatal intoxication.Citation100
In contrast to sodium oxybate, randomized controlled trials evaluating antidepressants for narcolepsy are sparse. A 2008 Cochrane Database System Review of the effects of antidepressant drugs on narcolepsy symptoms, including cataplexy, found only three crossover and two parallel trials of “unclear” methodological quality, resulting in the conclusion that there is “scarce evidence” that antidepressants have a positive effect on cataplexy.Citation101 Although there have not been any additional, more formal evaluations of these drugs for cataplexy, or indeed for narcolepsy in general, TCAs continue to be used off-label for cataplexy on the basis of historical precedent, anecdotal evidence from case reports, and guideline suggestions of their potential efficacy. Off-label use of other antidepressants (SSRIs and SNRIs) for cataplexy is similarly based primarily on case reportsCitation102–Citation106 and their listing in guidelines.Citation79,Citation80 The well-recognized side effects associated with antidepressants must also be considered, including anticholinergic effects of TCAs, as noted above, sexual dysfunction, and exacerbation of other sleep disorders caused by most antidepressants of all classes.Citation106–Citation108 Although onset of action in cataplexy is rapid, rebound cataplexy, which can be dramatic and rapid, has been observed to occur with cessation of these treatments, occurring as early as the day following treatment interruption.Citation7,Citation8,Citation109,Citation110
Ongoing clinical cataplexy treatment research
Histamine H3-receptor antagonism using direct receptor antagonists or inverse agonists
In addition to the catecholamines, tuberomammillary histaminergic neurons play a crucial role in maintenance of wakefulness but remain active during cataplexy, helping to preserve consciousness.Citation34,Citation111 Indeed, these neurons appear to be increased in narcolepsy, perhaps as a compensatory response to hypocretin/orexin loss and the resulting deficit in excitatory adrenergic drive.Citation112 Pitolisant is an inverse agonist of the histamine H3 autoreceptor, which theoretically reduces H3 inhibitory activity below basal rates and thereby functions more effectively than H3 antagonists to activate histaminergic neuronal activity in the brain and promote wakefulness. While it reduced EDS in patients with narcolepsy in a small clinical trial,Citation113 a post hoc analysis of another clinical trial reported that pitolisant for 8 weeks resulted in statistically significant reductions in the rate of cataplexy from baseline compared with placebo (P<0.05) and was not “noninferior” relative to modafinil in terms of improvement in daytime sleepiness.Citation114 Pitolisant was also shown to slightly improve cataplexy severity and frequency in a case series of four teenagers with narcolepsy.Citation115 The mechanism by which pitolisant may have positive effects on cataplexy is not clear since histaminergic activity is maintained during cataplexy and likely contributes to maintenance of wakefulness during these events.Citation111 Nevertheless, based on the preliminary results, a Phase III, randomized, controlled trial was initiated to specifically evaluate the effects of pitolisant on cataplexy as a primary outcome compared with placebo (ClinicalTrials.gov identifier NCT01800045).
Hypocretin/orexin replacement therapy
Compensating for hypocretin/orexin deficiency through the use of hypocretin/orexin peptide supplementation or cell replacement therapies may provide a rational approach to narcolepsy with cataplexy therapy.Citation82 Potential techniques include delivery of hypocretin/orexin peptides via intranasal, intravenous, intracisternal, or intracerebroventricular modes; use of prodrugs or agonists; or by genetic engineering or cell replacement techniques.Citation82 While these techniques are still in their early stages of development, the few available human studies have shown potential benefits for sleep and wakefulness, but the effects on cataplexy were not evaluated.Citation116,Citation117 However, in a canine model of narcolepsy, repeated systemic administration of hypocretin-1/orexin-A consolidated waking and sleeping periods and abolished cataplexy completely for periods of ≥3 days.Citation118 Further evaluation of these techniques may confirm the benefits of this therapeutic approach and may also provide insight into the underlying mechanisms of narcolepsy and cataplexy.
GABAB agonist
Baclofen, a GABAB agonist that has been suggested to improve nighttime sleep in patients with several neurological conditions including narcolepsy, has not demonstrated efficacy for cataplexy or EDS associated with narcolepsy.Citation86 However, a more recent study in a mouse model of narcolepsy showed that R-baclofen, an enantiomer with a three-fold higher affinity for GABAB receptors than the clinically available racemate, had greater efficacy than placebo in reducing cataplexy-like activity and non-REM sleep disturbances.Citation119 Further research is needed to determine if these observations translate into clinical benefits for the treatment of cataplexy.
Immunomodulation
The autoimmune hypothesis of narcolepsy with cataplexy provides a rationale for use of immunomodulation therapy,Citation82 which is exemplified by experimental use of intravenous immunoglobulin therapy, although it has only been evaluated in case studies in adults and children.Citation120–Citation128 While benefits were not consistently demonstrated in all studies, several studies did report that, if administered shortly after disease onset, intravenous immunoglobulin therapy may be effective in reducing narcolepsy symptoms including cataplexy and may have long-term benefits.Citation123–Citation127 These initial results suggest that further, more formal evaluation of intravenous immunoglobulin may be warranted.
In summary, despite advances in our knowledge of narcolepsy, treatment of cataplexy remains challenging. Only one medication, sodium oxybate, has been approved, has a high level of evidence of sustained efficacy for the treatment of cataplexy, and should be considered a first-line therapy. Other drugs, such as antidepressants, some of which have been traditionally used for narcolepsy with cataplexy for more than 50 years, still lack adequate evidence supporting their use, especially for chronic treatment of cataplexy. While formal studies can more clearly evaluate their efficacy and characterize those patients for whom they may be appropriate, they remain important components of treatment and should be considered as second-line therapy. The limited availability of treatment options for cataplexy suggests a need for development and evaluation of new approaches to the management of cataplexy. Ideally, drugs are needed that target cataplexy as well as other narcolepsy symptoms while maintaining an acceptable tolerability profile.
Acknowledgments
E Jay Bienen, PhD, and Larry Deblinger of the Curry Rock-efeller Group, LLC, provided medical writing assistance in developing this manuscript. This review was funded by Jazz Pharmaceuticals, Inc.
Disclosure
Dr Swick is an employee of Neurology and Sleep Medicine Consultants; has received consultancy fees and/or honoraria from Jazz Pharmaceuticals, Inc., Vanda Pharmaceuticals, XenoPort Pharmaceuticals, UCB Pharma, Merck, and Aerial BioPharma; has received grant/research funding from Jazz Pharmaceuticals, Inc., Aerial BioPharma, GSK Pharmaceuticals, Otsuka Pharmaceuticals, Teva Pharmaceuticals, Vanda Pharmaceuticals, UCB Pharma, XenoPort Pharmaceuticals, and Merck; and is on the speakers bureaus for Jazz Pharmaceuticals, Inc., XenoPort Pharmaceuticals, Teva Pharmaceuticals, Merck, and UCB Pharma.
References
- McCartyDEA case of narcolepsy with strictly unilateral cataplexyJ Clin Sleep Med201061757620191942
- OvereemSvan NuesSJvan der ZandeWLDonjacourCEvan MierloPLammersGJThe clinical features of cataplexy: a questionnaire study in narcolepsy patients with and without hypocretin-1 deficiencySleep Med2011121121821145280
- National Institute of Neurological Disorders and StrokeNarcolepsy Fact SheetNIH publication 13-1637 last updated January 5, 2015 Available from: http://www.ninds.nih.gov/disorders/narcolepsy/detail_narcolepsy.htm#241213201Accessed August 9, 2015
- PoryazovaRKhatamiRWerthEBassettiCLWeak with sex: sexual intercourse as a trigger for cataplexyJ Sex Med2009682271227719493288
- SturzeneggerCBassettiCLThe clinical spectrum of narcolepsy with cataplexy: a reappraisalJ Sleep Res200413439540615560774
- OkunMLLinLPelinZHongSMignotEClinical aspects of narcolepsy-cataplexy across ethnic groupsSleep2002251273511833858
- Martinez-RodriguezJIranzoASantamariaJStatus cataplecticus induced by abrupt withdrawal of clomipramineNeurologia2002172113116 Spanish11864561
- WangJGreenbergHStatus cataplecticus precipitated by abrupt withdrawal of venlafaxineJ Clin Sleep Med20139771571623853567
- DauvilliersYSiegelJMLopezRTorontaliZAPeeverJHCataplexy – clinical aspects, pathophysiology and management strategyNat Rev Neurol201410738639524890646
- NishinoSClinical and neurobiological aspects of narcolepsySleep Med20078437339917470414
- LongstrethWTJrKoepsellTDTonTGHendricksonAFvan BelleGThe epidemiology of narcolepsySleep2007301132617310860
- DauvilliersYMontplaisirJMolinariNAge at onset of narcolepsy in two large populations of patients in France and QuebecNeurology200157112029203311739821
- American Academy of Sleep MedicineThe International Classification of Sleep Disorders – Third Edition (ICSD-3)Darien, ILAmerican Academy of Sleep Medicine2014
- AdamantidisARZhangFAravanisAMDeisserothKde LeceaLNeural substrates of awakening probed with optogenetic control of hypocretin neuronsNature2007450716842042417943086
- EspanaRAScammellTESleep neurobiology from a clinical perspectiveSleep201134784585821731134
- SerraLMontagnaPMignotELugaresiEPlazziGCataplexy features in childhood narcolepsyMov Disord200823685886518307264
- AndlauerOMooreHtHongSCPredictors of hypocretin (orexin) deficiency in narcolepsy without cataplexySleep20123591247125522942503
- NevsimalovaSPiskoJBuskovaJNarcolepsy: clinical differences and association with other sleep disorders in different age groupsJ Neurol2013260376777523070467
- PlazziGPizzaFPalaiaVComplex movement disorders at disease onset in childhood narcolepsy with cataplexyBrain2011134Pt 1234803492
- PizzaFFranceschiniCPeltolaHClinical and polysomnographic course of childhood narcolepsy with cataplexyBrain2013136Pt 123787379524142146
- VanierMTNiemann-Pick diseasesHandb Clin Neurol20131131717172123622394
- TobiasESTolmieJLStephensonJBCataplexy in the Prader-Willi syndromeArch Dis Child200287217012138077
- NelsonGBHahnJSStimulus-induced drop episodes in Coffin-Lowry syndromePediatrics20031113e197e20212612271
- FryssiraHKountoupiSDelaunoyJPThomaidisLA female with Coffin-Lowry syndrome and “cataplexy”Genet Couns200213440540912558110
- TyagiAHarringtonHCataplexy in association with Moebius syndromeJ Neurol2003250111011112528006
- VosslerDGWylerARWilkusRJGardner-WalkerGVlcekBWCataplexy and monoamine oxidase deficiency in Norrie diseaseNeurology1996465125812618628463
- PortalaKWestermarkKEkseliusLBromanJESleep in patients with treated Wilson’s disease. A questionnaire studyNord J Psychiatry200256429129712470321
- NevsimalovaSBuskovaJBruhaRSleep disorders in Wilson’s diseaseEur J Neurol201118118419020550561
- KanbayashiTAbeMFujimotoSHypocretin deficiency in Niemann-Pick type C with cataplexyNeuropediatrics2003341525312690569
- OyamaKTakahashiTShojiYNiemann-Pick disease type C: cataplexy and hypocretin in cerebrospinal fluidTohoku J Exp Med2006209326326716778374
- VankovaJStepanovaIJechRSleep disturbances and hypocretin deficiency in Niemann-Pick disease type CSleep200326442743012841368
- PeyronCTigheDKvan den PolANNeurons containing hypocretin (orexin) project to multiple neuronal systemsJ Neurosci199818239996100159822755
- CasonAMSmithRJTahsili-FahadanPMoormanDESartorGCAston-JonesGRole of orexin/hypocretin in reward-seeking and addiction: implications for obesityPhysiol Behav2010100541942820338186
- BurgessCRScammellTENarcolepsy: neural mechanisms of sleepiness and cataplexyJ Neurosci20123236123051231122956821
- MiedaMTsujinoNSakuraiTDifferential roles of orexin receptors in the regulation of sleep/wakefulnessFront Endocrinol (Lausanne)201345723730297
- InutsukaAInuiATabuchiSTsunematsuTLazarusMYamanakaAConcurrent and robust regulation of feeding behaviors and metabolism by orexin neuronsNeuropharmacology20148545146024951857
- PeyronCFaracoJRogersWA mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brainsNat Med20006999199710973318
- PeeverJHLaiYYSiegelJMExcitatory effects of hypocretin-1 (orexin-A) in the trigeminal motor nucleus are reversed by NMDA antagonismJ Neurophysiol20038952591260012611960
- DauvilliersYJaussentILecendreuxMCerebrospinal fluid and serum cytokine profiles in narcolepsy with cataplexy: a case-control studyBrain Behav Immun201426026624394344
- LeeMGHassaniOKJonesBEDischarge of identified orexin/hypocretin neurons across the sleep-waking cycleJ Neurosci200525286716672016014733
- TakahashiKLinJSSakaiKNeuronal activity of orexin and non-orexin waking-active neurons during wake-sleep states in the mouseNeuroscience2008153386087018424001
- LinLFaracoJLiRThe sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 geneCell199998336537610458611
- ChemelliRMWillieJTSintonCMNarcolepsy in orexin knockout mice: molecular genetics of sleep regulationCell199998443745110481909
- NishinoSRipleyBOvereemSLammersGJMignotEHypocretin (orexin) deficiency in human narcolepsyLancet200035591973940 Letter10615891
- TabuchiSTsunematsuTBlackSWConditional ablation of orexin/hypocretin neurons: a new mouse model for the study of narcolepsy and orexin system functionJ Neurosci201434196495650924806676
- PizzaFVandiSLiguoriRPrimary progressive narcolepsy type 1: the other side of the coinNeurology201483232189219025355832
- NishinoSOkuroMKotoriiNHypocretin/orexin and narcolepsy: new basic and clinical insightsActa Physiol (Oxf)2010198320922219555382
- PartinenMKornumBRPlazziGJennumPJulkunenIVaaralaODoes autoreactivity have a role in narcolepsy?Lancet Neurol2014131110721073 Letter25316012
- MignotEHaydukRBlackJGrumetFCGuilleminaultCHLA DQB1*0602 is associated with cataplexy in 509 narcoleptic patientsSleep19972011101210209456467
- HallmayerJFaracoJLinLNarcolepsy is strongly associated with the T-cell receptor alpha locusNat Genet200941670871119412176
- FaracoJLinLKornumBRImmunoChip study implicates antigen presentation to T cells in narcolepsyPLoS Genet201392e100327023459209
- AhmedSSVolkmuthWDucaJAntibodies to influenza nucleoprotein cross-react with human hypocretin receptor 2Sci Transl Med20157294294ra105
- GulyaniSWuMFNienhuisRJohnJSiegelJMCataplexy-related neurons in the amygdala of the narcoleptic dogNeuroscience2002112235536512044453
- HongSBTaeWSJooEYCerebral perfusion changes during cataplexy in narcolepsy patientsNeurology200666111747174916769955
- OvereemSLammersGJvan DijkJGCataplexy: ‘tonic immobility’ rather than ‘REM-sleep atonia’?Sleep Med20023647147714592141
- OvereemSReijntjesRHuyserWLammersGJvan DijkJGCorticospinal excitability during laughter: implications for cataplexy and the comparison with REM sleep atoniaJ Sleep Res200413325726415339261
- SchwartzSPonzAPoryazovaRAbnormal activity in hypothalamus and amygdala during humour processing in human narcolepsy with cataplexyBrain2008131Pt 251452218094020
- BurgessCROishiYMochizukiTPeeverJHScammellTEAmygdala lesions reduce cataplexy in orexin knock-out miceJ Neurosci201333239734974223739970
- DauvillersYBilliardMMontplaisirJClinical aspects and pathophysiology of narcolepsyClin Neurophysiol20031142000201714580598
- PeeverJControl of motoneuron function and muscle tone during REM sleep, REM sleep behavior disorder and cataplexy/narcolepsyArch Ital Biol2011149445446622205591
- SiegelJMNienhuisRFahringerHMNeuronal activity in narcolepsy: identification of cataplexy-related cells in the medial medullaScience19912525010131513181925546
- LuppiPHClementOSapinEThe neuronal network responsible for paradoxical sleep and its dysfunctions causing narcolepsy and rapid eye movement (REM) behavior disorderSleep Med Rev201115315316321115377
- NishinoSTaftiMReidMSMuscle atonia is triggered by cholinergic stimulation of the basal forebrain: implication for the pathophysiology of canine narcolepsyJ Neurosci1995157 Pt 1480648147623112
- ThorpyMJKriegerACDelayed diagnosis of narcolepsy: characterization and impactSleep Med20141550250724780133
- Anic-LabatSGuilleminaultCKraemerHCMeehanJArrigoniJMignotEValidation of a cataplexy questionnaire in 983 sleep-disorders patientsSleep199922177879989368
- MooreWRSilberMHDeckerPACataplexy Emotional Trigger Questionnaire (CETQ) – a brief patient screen to identify cataplexy in patients with narcolepsyJ Clin Sleep Med200731374017557451
- PrinzmetalMBloombergWThe use of benzedrine for the treatment of narcolepsyJAMA19351052020512054
- ParkesJDFentonGWLevo(–) amphetamine and dextro(+) amphetamine in the treatment of narcolepsyJ Neurol Neurosurg Psychiatry1973366107610814359162
- DalyDDYossREThe treatment of narcolepsy with methyl phenylpiperidylacetate: a preliminary reportProc Staff Meet Mayo Clin1956312362062513379528
- AkimotoHHondaYTakahashiYPharmacotherapy in narcolepsyDis Nerv Syst19602170470613681922
- HishikawaYIdaHNakaiKKanekoZTreatment of narcolepsy with imipramine (tofranil) and desmethylimipramine (pertofran)J Neurol Sci1966354534614380880
- ShapiroWRTreatment of cataplexy with clomipramineTrans Am Neurol Assoc197499991024463568
- GuilleminaultCRaynalDTakahashiSCarskadonMDementWEvaluation of short-term and long-term treatment of the narcolepsy syndrome with clomipramine hydrochlorideActa Neurol Scand19765417187936975
- ParkesJDSchachterMClomipramine and clonazepam in cataplexyLancet1979281511085108691833
- SchachterMParkesJDFluvoxamine and clomipramine in the treatment of cataplexyJ Neurol Neurosurg Psychiatry19804321711746766990
- LangdonNShindlerJParkesJDBandakSFluoxetine in the treatment of cataplexySleep1986923713733509809
- ChenSYCliftSJDahlitzMJDunnGParkesJDTreatment in the narcoleptic syndrome: self assessment of the action of dexamphetamine and clomipramineJ Sleep Res19954211311810607149
- WinokurAGaryKARodnerSRae-RedCFernandoATSzubaMPDepression, sleep physiology, and antidepressant drugsDepress Anxiety2001141192811568979
- MorgenthalerTIKapurVKBrownTPractice parameters for the treatment of narcolepsy and other hypersomnias of central originSleep200730121705171118246980
- BilliardMBassettiCDauvilliersYEFNS guidelines on management of narcolepsyEur J Neurol 2006/10200613101035104816987156
- Xyrem® [sodium oxybate] oral solution US prescribing informationPalo Alto, CAJazz Pharmaceuticals, Inc42014
- MignotENishinoSEmerging therapies in narcolepsy-cataplexySleep200528675476316477963
- BroughtonRMamelakMEffects of nocturnal gamma-hydroxybutyrate on sleep/waking patterns in narcolepsy-cataplexyCan J Neurol Sci19807123317388696
- MamelakMScharfMBWoodsMTreatment of narcolepsy with gamma-hydroxybutyrate. A review of clinical and sleep laboratory findingsSleep198691 Pt 22852893704454
- PardiDBlackJγ-Hydroxybutyrate/sodium oxybate. Neurobiology, and impact on sleep and wakefulnessCNS Drugs20062012993101817140279
- HuangYSGuilleminaultCNarcolepsy: action of two gamma-aminobutyric acid type B agonists, baclofen and sodium oxybatePediatr Neurol200941191619520267
- MignotEJA practical guide to the therapy of narcolepsy and hypersomnia syndromesNeurotherapeutics20129473975223065655
- GowdaCRLundtLPMechanism of action of narcolepsy medicationsCNS Spectr201419Suppl 1253425403789
- MignotERenaudANishinoSArrigoniJGuilleminaultCDementWCCanine cataplexy is preferentially controlled by adrenergic mechanisms: evidence using monoamine selective uptake inhibitors and release enhancersPsychopharmacology (Berl)1993113176827862832
- NishinoSArrigoniJSheltonJDementWCMignotEDesmethyl metabolites of serotonergic uptake inhibitors are more potent for suppressing canine cataplexy than their parent compoundsSleep19931687067128165384
- NishinoSFruhstorferBArrigoniJGuilleminaultCDementWCMignotEFurther characterization of the alpha-1 receptor subtype involved in the control of cataplexy in canine narcolepsyJ Pharmacol Exp Ther19932643107910848095546
- LammersGJArendsJDeclerckACFerrariMDSchouwinkGTroostJGammahydroxybutyrate and narcolepsy: a double-blind placebo-controlled studySleep19931632162208506453
- The US Xyrem Multi-Center Study GroupA randomized, double blind, placebo-controlled multicenter trial comparing the effects of three doses of orally administered sodium oxybate with placebo for the treatment of narcolepsySleep200225424911833860
- Xyrem International Study GroupFurther evidence supporting the use of sodium oxybate for the treatment of cataplexy: a double-blind, placebo-controlled study in 228 patientsSleep Med20056541542116099718
- US Xyrem Multicenter Study GroupA 12-month, open-label, multicenter extension trial of orally administered sodium oxybate for the treatment of narcolepsySleep2003261313512627729
- US Xyrem Multicenter Study GroupSodium oxybate demonstrates long-term efficacy for the treatment of cataplexy in patients with narcolepsySleep Med20045211912315033130
- AlshaikhMKTriccoACTashkandiMMamdaniMStrausSEBahammamASSodium oxybate for narcolepsy with cataplexy: systematic review and meta-analysisJ Clin Sleep Med20128445145822893778
- Boscolo-BertoRVielGMontagneseSRaduazzoDIFerraraSDDauvilliersYNarcolepsy and effectiveness of gamma-hydroxybutyrate (GHB): a systematic review and meta-analysis of randomized controlled trialsSleep Med Rev201216543144322055895
- MansukhaniMPKotagalSSodium oxybate in the treatment of childhood narcolepsy-cataplexy: a retrospective studySleep Med20121360661022445591
- CorkeryJMLoiBClaridgeHGamma hydroxybutyrate (GHB), gamma butyrolactone (GBL) and 1,4-butanediol (1,4-BD; BDO): a literature review with a focus on UK fatalities related to non-medical useNeurosci Biobehav Rev201553527825843781
- VignatelliLD’AlessandroRCandeliseLAntidepressant drugs for narcolepsyCochrane Database Syst Rev20081CD00372418254030
- FreyJDarbonneCFluoxetine suppresses human cataplexy: a pilot studyNeurology19944447077098164831
- IzziFPlacidiFMarcianiMGEffective treatment of narcolepsy-cataplexy with duloxetine: a report of three casesSleep Med2009101153154 Letter18226953
- MollerLROstergaardJRTreatment with venlafaxine in six cases of children with narcolepsy and with cataplexy and hypnagogic hallucinationsJ Child Adolesc Psychopharmacol200919219720119364297
- RatkiewiczMSplaingardMTreatment of cataplexy in a three-year-old using venlafaxineJ Clin Sleep Med201391213411342 Case report24340297
- SonkaKKemlinkDPretlMCataplexy treated with escitalopram – clinical experienceNeuro Endocrinol Lett2006271–217417616648807
- KaracanIErectile dysfunction in narcoleptic patientsSleep198691 Pt 22272313704447
- JuYELarson-PriorLDuntleySChanging demographics in REM sleep behavior disorder: possible effect of autoimmunity and antidepressantsSleep Med201112327828321317035
- PoryazovaRSiccoliMWerthEBassettiCLUnusually prolonged rebound cataplexy after withdrawal of fluoxetineNeurology200565696796816186554
- RistanovicRKLiangHHornfeldtCSLaiCExacerbation of cataplexy following gradual withdrawal of antidepressants: manifestation of probable protracted rebound cataplexySleep Med200910441642118753005
- JohnJWuMFBoehmerLNSiegelJMCataplexy-active neurons in the hypothalamus: implications for the role of histamine in sleep and waking behaviorNeuron200442461963415157423
- ValkoPOGavrilovYVYamamotoMIncrease of histaminergic tuberomammillary neurons in narcolepsyAnn Neurol201374679480424006291
- LinJSDauvilliersYArnulfIAn inverse agonist of the histamine H(3) receptor improves wakefulness in narcolepsy: studies in orexin−/− mice and patientsNeurobiol Dis2008301748318295497
- DauvilliersYBassettiCLammersGJPitolisant versus placebo or modafinil in patients with narcolepsy: a double-blind, randomised trialLancet Neurol201312111068107524107292
- InocenteCArnulfIBastujiHPitolisant, an inverse agonist of the histamine H3 receptor: an alternative stimulant for narcolepsy-cataplexy in teenagers with refractory sleepinessClin Neuropharmacol2012352556022356925
- BaierPCHallschmidMSeeck-HirschnerMEffects of intra-nasal hypocretin-1 (orexin A) on sleep in narcolepsy with cataplexySleep Med2011121094194622036605
- WeinholdSLSeeck-HirschnerMNowakAHallschmidMGoderRBaierPCThe effect of intranasal orexin-A (hypocretin-1) on sleep, wakefulness and attention in narcolepsy with cataplexyBehav Brain Res201426281324406723
- JohnJWuMFSiegelJMSystemic administration of hypocretin-1 reduces cataplexy and normalizes sleep and waking durations in narcoleptic dogsSleep Res Online200031232811382896
- BlackSWMorairtySRChenTMGABAB agonism promotes sleep and reduces cataplexy in murine narcolepsyJ Neurosci201434196485649424806675
- FronczekRVerschuurenJLammersGJResponse to intravenous immunoglobulins and placebo in a patient with narcolepsy with cataplexyJ Neurol2007254111607160817762946
- PlazziGPoliFFranceschiniCIntravenous high-dose immunoglobulin treatment in recent onset childhood narcolepsy with cataplexyJ Neurol2008255101549155418769859
- ValkoPOKhatamiRBaumannCRBassettiCLNo persistent effect of intravenous immunoglobulins in patients with narcolepsy with cataplexyJ Neurol2008255121900190318825431
- LecendreuxMMaretSBassettiCMourenMCTaftiMClinical efficacy of high-dose intravenous immunoglobulins near the onset of narcolepsy in a 10-year-old boyJ Sleep Res2003124347348 Letter14633248
- DauvilliersYCarlanderBRivierFTouchonJTaftiMSuccessful management of cataplexy with intravenous immunoglobulins at narcolepsy onsetAnn Neurol200456690590815562415
- DauvilliersYFollow-up of four narcolepsy patients treated with intravenous immunoglobulinsAnn Neurol200660115316802296
- DauvilliersYAbrilBMasEMichelFTaftiMNormalization of hypocretin-1 in narcolepsy after intravenous immunoglobulin treatmentNeurology200973161333133419841387
- KnudsenSMikkelsenJDBangBGammeltoftSJennumPJIntravenous immunoglobulin treatment and screening for hypocretin neuron-specific autoantibodies in recent onset childhood narcolepsy with cataplexyNeuropediatrics201041521722221210337
- KnudsenSBiering-SorensenBKornumBREarly IVIg treatment has no effect on post-H1N1 narcolepsy phenotype or hypocretin deficiencyNeurology201279110210322722630
