Abstract
Background
Midazolam (MDZ) has powerful hypnosis, amnesia, anti-anxiety and anticonvulsant effects. Studies have shown that prenatally developmental toxicity of diazepam can be observed in many organs/tissues. However, it remains elusive in male reproductive system.
Materials and methods
TM3 mouse Leydig progenitor cell line was used to determine whether MDZ has any unfavorable effects.
Results
Midazolam significantly decreased cell viability in dose- and time-dependent manners in TM3 cells. In flow cytometry analysis, midazolam significantly increased subG1 phase cell numbers, and annexin V/PI double staining assay further confirmed that MDZ induced apoptosis in TM3 cells. Moreover, MDZ significantly induced the expression of caspase-8 and -3 proteins and the phosphorylation of JNK, ERK1/2 and p38. Besides, MDZ didn’t activate Akt pathway in TM3 cells. Furthermore, the expressions of p-EIF2α, ATF4, ATF3 and CHOP were induced by midazolam, suggesting that midazolam could induce apoptosis through endoplasmic reticulum (ER) stress in TM3 cells. Additionally, the expressions of cyclin A, cyclin B and CDK1 were inhibited by midazolam through the regulation of p53 in TM3 cells, indicating that midazolam could regulate cell cycle to induce apoptosis.
Conclusion
Midazolam could activate caspase, MAPKs and ER stress pathways and impede Akt pathway and cell cycle to induce apoptosis in TM3 mouse Leydig progenitor cells.
Introduction
Midazolam (MDZ), a general benzodiazepine sedative and anesthetic agent, has powerful hypnosis, amnesia, anti-anxiety, anticonvulsant and skeletal muscle relaxant effects by improving Cl− channel gated through the γ-aminobutyric acid type A receptor in the central nervous system.Citation1 However, potential prenatal developmental toxicity of diazepam related to neurogenesis has been assessed in several studies.Citation2–Citation5 A previous study has also illustrated that MDZ could suppress cell viability and osteogenic differentiation in human mesenchymal stem cells.Citation6 It is highly possible that reproductive system will also be affected by anesthetic agent during fetal period, which should be profoundly investigated. In males, Leydig stem and progenitor cells are very important in testis development during the fetal period for normal reproductive functions.Citation7 Studies have demonstrated that ontogenesis of Leydig cell function involves at least two generations of cells. The first generation develops during fetal life, and these fetal Leydig cells are responsible for the masculinization of the male urogenital system.Citation7–Citation9 These cells regress thereafter, although fetal Leydig cells persevere in adult life and their number remains constant at least until 90 days postpartum in the rat.Citation8,Citation9 The second Leydig cell population appears during puberty and produces the hormone testosterone, which is required for the onset of spermatogenesis and maintenance of male reproductive functions.Citation8,Citation9 It is well known that various factors could affect Leydig stem/progenitor cell development during embryo and fetal periods, which would result in male infertility.Citation9 In fact, studies have shown that MDZ could regulate Leydig cell steroidogenesis and apoptosis.Citation10–Citation14
Apoptosis is characterized by some features, such as nuclear chromatin condensation, cytoplasmic shrinkage, dilated endoplasmic reticulum and membrane blebbing.Citation15 The biochemical symbol of apoptosis is the activation of caspases (cysteine aspartate-specific proteases), which are the members of the family of cysteine proteases related to ced-3, the “death gene” of the nematode Caenorhabditis elegans.Citation16,Citation17 There are at least two regulatory pathways for the caspase activation: extra-mitochondrial pathway (extrinsic pathway), which is mediated by death receptors,Citation18 and intra-mitochondrial pathway (intrinsic pathway), which is mediated by cytochrome c.Citation19 Studies have shown that receptor-associated adaptor proteins, such as FADD (Fas-associated death domain), facilitate close association of certain caspases to promote caspase auto-processing.Citation20–Citation22 Similar adaptor molecules, such as CED-4 homologue and Apaf-1 (Apoptotic protease-activating factor), may play key roles in promoting apoptosis by clustering caspases at intracellular sites.Citation23 On the other hand, mitochondria can sense apoptotic signals and convey them to activate adaptor Apaf-1 via the release of cytochrome c. Cytochrome c, Apaf-1 and procaspase-9 form a complex called apoptosome. The apoptosome formation can cause the autolytical cleavage of procaspase-9 to form active caspase-9, which can further cleave caspase-3, -6 and -7.Citation24
A defect in cell division can lead to developmental abnormalities as well as cancerous growth, and the eukaryotic cell cycle is controlled by the activities of cyclin-dependent kinases (CDKs) and cyclins.Citation25 A previous study has demonstrated that a correlation between the G2/M arrest and the induction of mitogen-activated protein kinases (MAPKs) is related to apoptosis.Citation26 MAPK pathway plays a key role in the progression of cancer, and can regulate cell growth, proliferation, differentiation, migration, apoptosis and so on.Citation27,Citation28 MAPKs are protein-serine/threonine kinases, including ERK1/2, JNK and p38. Each subgroup of MAPKs is activated through a cascade of phosphorylation events, beginning with the activation of MAPK kinase kinases (MAP3Ks) to regulate cell fate.Citation29 Study has demonstrated that MDZ triggers apoptosis by activating JNK and p38 in MA-10 mouse Leydig tumor cells.Citation12–Citation14
Another cell-fate-regulating pathway, the Akt signaling pathway, is a pro-survival pathway and acts by inhibiting apoptotic signal cascades and activating pro-survival signal.Citation30 The activation of Akt inhibits a number of pro-apoptotic Bcl-2 family members, including Bad, Bax and Bim.Citation31 Akt also positively regulates anti-apoptotic pathways, inducing the activation of NF-κB transcription factor, which promotes the transcription of a wide range of anti-apoptotic genes, in particular Bcl-2 and Bcl-x-L.Citation31 Scientific data have indicated that the attenuation of Akt has induced apoptosis in the human malignant testicular germ cells.Citation32
In protein homeostasis, unfolded protein response (UPR) will be stimulated in response to ER stress in cells.Citation33 Three signaling pathways, protein kinase RNA (PKR)-like ER kinase (PERK), activating transcription factor 6 (ATF6) and inositol-requiring protein-1α (IRE1α), will then be activated by UPR.Citation34 Study has shown that PERK activation could phosphorylate eukaryotic translation initiation factor 2α (eIF2α) to selectively induce the transcription factor ATF4, which could further increase the expression of pro-apoptotic CCAAT/enhancer-binding protein-homologous protein (CHOP) to induce cell apoptosis.Citation35 In addition, ATF6 transited to the Golgi will be cleaved by site-1 protease (S1P) and site-2 protease (S2P) to become an activated transcription factor ATF6.Citation35 Moreover, activated-IRE1 could remove a small intron from the mRNA encoding X-box binding protein 1 (XBP1), which will become an active transcription factor. Combination of ATF6 and IRE1 pathways can then induce CHOP expression to trigger apoptosis.Citation36 In addition, caspase-12 pathway could be stimulated by ER stress through an interaction with IRE1 to stimulate apoptosis.Citation37
The p53 protein is activated in response to numerous factors, including DNA damage, oxidative stress and osmotic shock, and deregulated expression of oncogenes.Citation38 p53 induces expression of genes involved in both death receptor-dependent (CD95 and DR5) and mitochondrion-dependent (PUMA, Bax, Bid) apoptosis pathways. The p53 protein can also inhibit the Bcl-2 family of proteins, which are involved in promoting cell survival.Citation39 Cyclin-dependent kinase inhibitor 1A (CDKN1A, also known as p21) is an important part of the p53 response. In the nucleus, p21 inhibits CDK1 and 2 and thereby blocks mitosis in response to DNA damage. In the cytoplasm, p21 blocks apoptosis by inhibiting CASP3.Citation40 It has been shown that TGFβ signals are transmitted through type I and II transmembrane serine/threonine receptors and inhibit cell proliferation by causing a G1 cell-cycle arrest through the activities of SMAD family member 2, 3, and 4 (SMAD2−4). This signaling pathway also regulates the expression of c-Myc, p15 and p21.Citation41
TM3 cells are developed from neonatal BALB/c mouse testicular interstitial cells with LH receptor and can respond to trophic hormones to produce testosterone.Citation42–Citation44 Also, TM3 cells have similar progenitor cell characteristics.Citation9,Citation44 Thus, TM3 cell line is suitable to study the apoptotic effect of MDZ and investigate the associated mechanisms. In the present study, we have observed that MDZ induced cell rounding up and detachment phenomena; decreased cell number; stimulated cell death; regulated cell cycle; and activated caspase MAPK, Akt, ER stress and p53 pathways in TM3 mouse Leydig progenitor cells. This study revealed how MDZ could regulate mouse Leydig progenitor cell development, thus enriching the knowledge on how exposure to anesthetic agents during the fetal period could possibly affect testicular normal development resulting in male abnormal reproductive functions.
Materials and methods
Chemicals
MDZ was purchased from Roche Products Ltd (Welwyn Garden City, United Kingdom). Waymouth MB 752/1 medium, propidium iodide (PI), penicillin-streptomycin, RNase A, Folin and Ciocalteu’s phenol regent, ethylene diamine tetraacetic acid (EDTA), 30% acrylamide/bis-acrylamide solution, MTT and monoclonal antibody against β-actin were purchased from Sigma-Aldrich Co. (St Louis, MO, USA). Fetal bovine serum (FBS), Dulbecco’s Modified Eagle’s Medium (DMEM)/F12 and trypsin-EDTA were purchased from Thermo Fisher Scientific (Waltham, MA, USA). Gentamycin sulfate was purchased from A.G. Scientific (San Diego, CA, USA). Sodium chloride, potassium chloride, 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid and Tris base were purchased from JT Bacer (Phillipsburg, NJ, USA). Disodium hydrogen phosphate, potassium dihydrogen phosphate, and tissue culture grade sodium bicarbonate were purchased from Riedel-deHaen (Seelze, Germany). Sucrose was purchased from Panreac (Barcelona, Spain). Hydrochloric acid, sodium dodecyl sulfate (SDS), Tween 20 and dimethyl sulfoxide (DMSO) were purchased from Merck (Darmstadt, Germany). Isoton II was purchased from Beckman Coulter (Brea, CA, USA). Micro BCA protein assay kit was purchased from Thermo Fisher Scientific. Donkey anti-rabbit IgG conjugated with horseradish peroxidase and donkey anti-mouse IgG conjugated with horseradish peroxidase were purchased from PerkinElmer Inc. (Waltham, MA, USA). Annexin V-FITC apoptosis detection kit was purchased from Strong Biotech (Taipei, Taiwan). Polyclonal antibodies against cleaved caspase-8, cleaved caspase-9, phosphor-ERK1/2, ERK1/2, phosphor-JNK, JNK, phosphor-p38 MAPK, p38 MAPK, phosphor-Akt, Akt, cleaved caspase-12, Bax, cytochrome C and COX IV were purchased from Cell Signaling (Beverly, MA, USA). Monoclonal antibodies against cleaved caspase-3, p27, p53, phosphor-p53 (Ser 15), IRE1α, phosphor-EIF2α, PERK and ATF4 were purchased from Cell Signaling. Polyclonal antibodies against ATF3, CHOP, CDK1, cyclin A and phosphor-p21 (Thr 145) were purchased from Santa Cruz Biotechnology Inc. (Dallas, TX, USA). Polyclonal antibody against phosphor-IRE1α was purchased from Abcam (Cambridge, UK). Polyclonal antibodies against ATF6β, EIF2α and XBP1 were purchased from Abgent (San Diego, CA, USA). Polyclonal antibodies against phosphor-p27 (Ser 10), cyclin B and p21 were purchased from Gene Tex (Irvine, CA, USA). Enhanced chemiluminescence (ECL) detection kit was purchased from EMD Millipore (Billerica, MA, USA).
Cell culture
TM3 mouse Leydig progenitor cells were purchased from ATCC (Manassas, VA, USA) and maintained in DMEM/F12 medium supplemented with 10% FBS at 37°C in a humidified environment containing 95% air and 5% CO2 for all the following experiments.
Morphology observation
TM3 cells were seeded at a concentration of 6×105/mL in 6 cm Petri dishes with 2 mL culture medium and treated without or with different concentrations of MDZ (150 and 300 μM) for 24 hours. Cell morphology was observed under Olympus CK40 light microscopy and images were recorded by Olympus DP20 digital camera (Olympus, Tokyo, Japan).
MTT viability test
MTT assay is a colorimetric assay for assessing cell viability.Citation45 TM3 cells were seeded at a concentration of 8×103 cell/well. After 70%–80% confluence was obtained, cells were treated without or with different concentrations of MDZ (6, 30, 150 or 300 μM) for 1, 3, 6, 12 and 24 hours. The selected concentrations of MDZ were in accordance with those used regularly in clinical applications. At the end of experiments, each well had MTT (0.5 mg/mL) added and plates were incubated at 37°C for 4 hours. Then, the medium was discarded and 50 μL of DMSO was added into each well to dissolve the crystals by keeping the plates on a shaker at 37°C for 20 minutes in the dark. The cell viability was detected at a wavelength of 570 nm by VersaMax ELISA reader (Molecular Devices, Sunnyvale, CA, USA).Citation45
Cell cycle analysis
To investigate whether MDZ could induce death in TM3 mouse Leydig progenitor cells through apoptosis, their DNA contents were examined by PI staining through flow cytometric analysis. TM3 cells were seeded at a concentration of 6×105 in 6 cm Petri dishes with 2 mL culture medium and treated without or with different concentrations of MDZ (150 and 300 μM) for 12 and 24 hours. Cells were harvested by trypsin digestion, centrifuged and then washed with Isoton II and fixed with 70% ethanol for at least 2 hours at −20°C. After fixation, cells were washed with cold Isoton II and then collected after centrifugation. Cell suspensions were mixed with 100 μg/mL RNase and stained with 40 μg/mL PI solution for 30 minutes. The stained cells were analyzed at a wavelength of 488 nm for PI detection by FACScan flow cytometer (Becton Dickinson, Franklin Lakes, NJ, USA). Cells in subG1 phase have less DNA contents on cell cycle distribution, which is considered to be a result of DNA fragmentation and an outcome of cell apoptosis.
Annexin V/PI double staining assay
After harvesting the cells with trypsin and washing with 2 mL of culture medium, cell suspensions were centrifuged at 300× g for 10 minutes at 4°C. The pellets were resuspended with cold Isoton II and centrifuged again. The pellets were mixed with 100 μL staining solution for 15 minutes according to the user’s manual of Annexin V-FITC apoptosis detection kit from Strong Biotech. The stained cells were analyzed at 488 nm excitation, using 515 nm band pass filter for FITC detection and >600 nm band pass filter for PI detection, by FACScan flow cytometer (Becton Dickinson). The double-negative cells (viable), annexin V single-positive cells (early apoptotic), PI single-positive cells (necrotic), and double-positive cells (late apoptotic) could be illustrated in four quadrants.Citation46
Protein extraction and Western blot
Cells were seeded in 6 cm Petri dishes. After treatments, medium was transferred to 15 mL tubes and cells were washed with cold PBS, and then suspensions were centrifuged at 600× g for 10 minutes at 4°C. Attached cells were lysed by using 20 μL of lysis buffer with proteinase inhibitor. The pellets were resuspended with 10 μL of lysis buffer and mixed with cell lysates, and then the suspension was centrifuged at 12,000× g for 12 minutes at 4°C. The supernatants were collected and stored at −80°C. Protein concentrations of cell lysates were determined by the Lowry assay.Citation47
For Western blot, cell lysates were dissolved in 12% SDS-PAGE gel with standard running buffer at room temperature and electrophoretically transferred to polyvinyldifluoride membrane at 4°C. After blocking the membrane and incubating it with primary antibodies overnight at 4°C, the membrane was washed and incubated with HRP-conjugated secondary antibodies, and then detected by ECL kit through UVP EC3 BioImaging Systems (UVP, Upland, CA, USA).
Statistical analysis
The data are expressed as mean ± standard error of the mean (SEM) of three separate experiments. Statistical significance of differences between control and treatment groups was determined by one-way analysis of variance (ANOVA) and then LSD comparison. Statistical significance was considered as p<0.05 in all experiments.
Results
MDZ induced cell death through apoptosis in TM3 cells
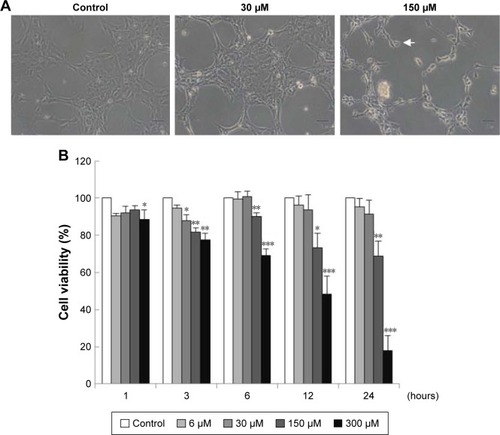
TM3 cells were treated without or with different concentrations of MDZ (30 and 150 μM) for 24 hours, and results showed that cell shrinkage with membrane blebbing could be observed by 150 μM MDZ treatment (), indicating that MDZ could induce TM3 cell death possibly through apoptosis. To confirm the cell death effect of MDZ on TM3 cells, MTT viability test was performed. TM3 cells were treated with 6, 30, 150 and 300 μM concentrations for 1, 3, 6, 12 and 24 hours, and results demonstrated that MDZ from 150 to 300 μM for 3 to 24 hours significantly decreased cell viability () (p<0.05). After treatment with 150 μM MDZ for 24 hours, cell viability of TM3 cells decreased to 74%±5.6% ().
Figure 1 Midazolam induced cell death through apoptosis in TM3 cells. (A) TM3 cells were treated without or with different concentrations of midazolam (30 and 150 μM) for 24 hours, and were observed under light microscopy (scale bar: 50 μm, arrow: membrane-blebbed cells). (B) TM3 cells were treated with 6, 30, 150 and 300 μM for 1, 3, 6, 12 and 24 hours. Cell viabilities were examined by MTT viability test. Results are presented as percentages of cell growth relative to control groups. Each data point represents the mean ± SEM of three separate experiments. *, ** and *** indicate statistical difference compared to control equivalent to p<0.05, p<0.01 and p<0.005, respectively.
MDZ regulated cell cycle to induce apoptosis in TM3 cells
To investigate whether MDZ could affect cell cycle to cause apoptosis, TM3 cells were treated with MDZ and the DNA contents were examined by flow cytometry. Results showed that treatment with 300 μM MDZ for 24 hours significantly increased cell percentage of subG1 phase, a sign of DNA fragmentation related to apoptosis, in TM3 cells () (p<0.05). In addition, treatments with 150 μM MDZ for 12 hours and 300 μM MDZ for 24 hours significantly increased the cell percentage of G2/M phase in TM3 cells () (p<0.05), implying a G2/M phase arrest. These data demonstrated that MDZ could regulate the distribution of cell cycle by increasing subG1 phase with G2/M phase arrest to induce apoptosis in TM3 cells.
Figure 2 Midazolam regulated cell cycle to induce apoptosis in TM3 cells. TM3 cells were treated with 150 and 300 μM midazolam for 12 and 24 hours and subG1 (A) and G2/M (B) phase cell numbers were detected. Cells were fixed and then stained with propidium iodide (PI) for cell cycle analysis. SubG1 phase means that cells contain less DNA content than normal cells, representing apoptosis. TM3 cells were treated with 150 and 300 μM midazolam for 24 hours. The apoptotic status of midazolam-treated cells was detected by Annexin V/PI double staining assay (C). The percentages of double-negative cells (viable cells), annexin V single-positive cells (early apoptotic cells), PI single-positive cells (necrotic cells) and annexin V and PI double-positive cells (late apoptotic cells) in each treatment are illustrated (D). The difference in the number of annexin V-positive cells (early apoptotic plus late apoptotic status) was then analyzed among treatments without or with midazolam (E). Each data point represents the mean ± SEM of three separate experiments. *, ** and *** indicate statistical difference compared to control equivalent to p<0.05, p<0.01 and p<0.005, respectively.
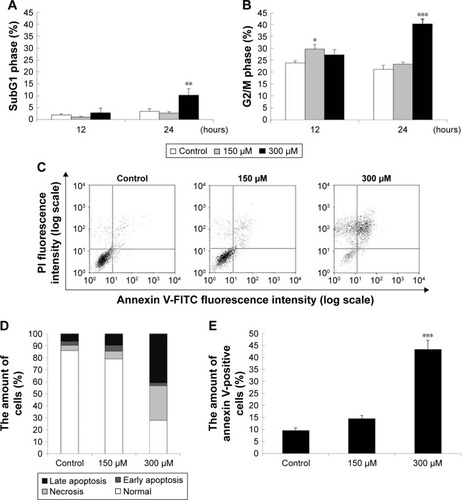
To further confirm that MDZ induced TM3 cell apoptosis, annexin V/PI double staining assay followed by flow cytometry were performed. Data showed that the number of annexin V-positive (early and late apoptosis) cells significantly increased with 300 μM MDZ treatment for 24 hours in TM3 cells () (p<0.05). These results confirm that MDZ induced TM3 cell death through apoptosis.
MDZ activated caspase cascade to induce apoptosis in TM3 cells
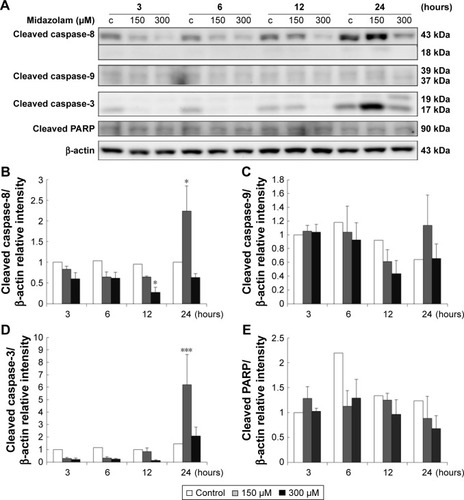
Caspase cascade, including extrinsic and intrinsic pathways, is an important inducer of the apoptotic pathway.Citation48 Our data have shown that MDZ could induce apoptosis in TM3 cells. Thus, Western blotting analysis was used to determine whether MDZ could induce apoptosis through caspase extrinsic and intrinsic pathways. Results showed that treatment with 150 μM MDZ for 24 hours significantly increased the expression of cleaved caspase-8 and -3 () (p<0.05), while the expressions of cleaved caspase-9 and PARP were not affected in TM3 cells () (p>0.05). These results showed that MDZ could activate caspase-8 and -3, but not caspase-9 and PARP, to induce apoptosis in TM3 cells.
Figure 3 The involvement of the caspase cascade in midazolam-induced apoptosis in TM3 cells. TM3 cells were treated without or with different concentrations of midazolam (150 and 300 μM) for 3, 6, 12 and 24 hours. Cleaved caspase-8 (43/18 kDa), -9 (39/37 kDa), -3 (17/19 kDa) and cleaved PARP (85–90 kDa) were detected by Western blot analysis (A). Immunoblot represents the observations from one single experiment repeated at least three times. The integrated optical densities (IOD) of cleaved caspase-8 (B), -9 (C), -3 (D) and cleaved PARP (E) proteins were normalized with β-actin (43 kDa) in each lane. Each data point represents the mean ± SEM of three separate experiments. * and *** indicate statistical difference compared to control equivalent to p<0.05 and p<0.005, respectively, (c = control).
MDZ stimulated MAPK pathways to induce apoptosis in TM3 cells
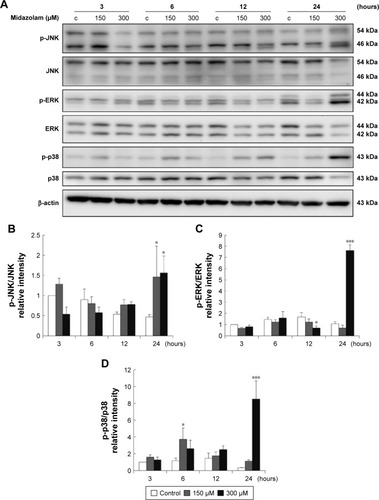
Studies have shown that the MAPK pathways could regulate cell growth, proliferation, apoptosis, and other biological processes.Citation27–Citation29 To determine whether MDZ-induced TM3 cell apoptosis would be regulated by MAPK pathways, the related proteins were analyzed by Western blot. Results showed that treatment with 300 μM MDZ for 24 hours significantly stimulated the expressions of phosphor-JNK (), phosphor-ERK () and phosphor-p38 () in TM3 cells (p<0.05). Moreover, treatment with 150 μM MDZ for 6 and 24 hours significantly induced the expression of phosphor-p38 is expressed at 6 hours and phosphor-JNK is expressed at 24 hours, respectively () (p<0.05). These results showed that MDZ could stimulate MAPK pathways to induce apoptosis in TM3 cells.
Figure 4 The involvement of MAPK pathways in midazolam-induced apoptosis in TM3 cells. TM3 cells were treated without or with different concentrations of midazolam (150 and 300 μM) for 3, 6, 12 and 24 hours. Phosphor-JNK (54/46 kDa), JNK, phosphor-ERK (44/42 kDa), ERK, phosphor-p38 (43 kDa) and p38 were detected by Western blot analysis (A). Immunoblot represents the observations from one single experiment repeated at least three times. The integrated optical densities (IOD) of phosphor-JNK (B), phosphor-ERK (C) and phosphor-p38 (D) proteins were normalized with β-actin (43 kDa) in each lane. Each data point represents the mean ± SEM of three separate experiments. * and *** indicate statistical difference compared to control equivalent to p<0.05 and p<0.005, respectively, (c = control).
Involvement of Akt pathways in MDZ-induced apoptosis in TM3 cells
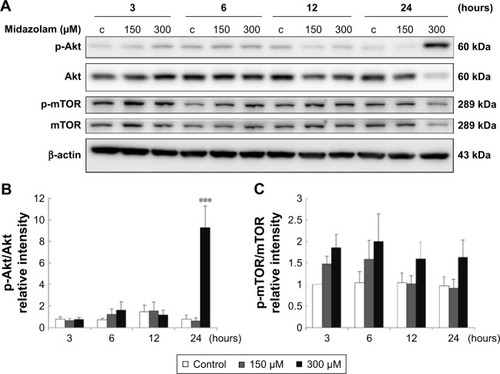
Previous studies have indicated that Akt pathway is a pro-survival pathway and acts by inhibiting apoptotic signal cascades and activating pro-survival signal cascades.Citation30–Citation32 To investigate whether MDZ could induce apoptosis in TM3 cells by inhibiting Akt pathway, the expressions of Akt, phosphor-Akt, mTOR and phosphor-mTOR (downstream target protein of Akt) were analyzed by Western blot. Results showed that 300 μM MDZ treatment for 24 hours significantly induced the expression of phosphor-Akt () (p<0.05). However, MDZ could not induce the expression of phosphor-mTOR () (p>0.05). These results illustrated the stimulation of Akt, but not mTOR, by MDZ, suggesting that the activation of Akt pathway could not be turned on to protect TM3 cells.
Figure 5 The involvement of Akt pathways in midazolam-induced apoptosis in TM3 cells. TM3 cells were treated without or with different concentrations of midazolam (150 and 300 μM) for 3, 6, 12 and 24 hours. Phosphor-Akt (60 kDa), Akt, phosphor-mTOR (289 kDa) and mTOR were detected by Western blot analysis (A). Immunoblot represents the observations from one single experiment repeated at least three times. The integrated optical densities (IOD) of phosphor-Akt (B) and phosphor-mTOR (C) proteins were normalized with β-actin (43 kDa) in each lane. Each data point represents the mean ± SEM of three separate experiments. *** indicates statistical difference compared to control equivalent to p<0.005, (c = control).
Involvement of ER stress pathways in MDZ-induced apoptosis in TM3 cells
Studies have shown that the misfolded proteins could induce ER stress to restore protein homeostasis, and if the stress is prolonged, apoptotic cell death ensues.Citation33–Citation37 To examine whether MDZ could also regulate ER stress pathways to induce apoptosis in TM3 cells, the ER stress-related proteins, including ATF6, IRE1α, cleaved caspase-12, PERK and other downstream proteins, were analyzed by Western blot analysis.
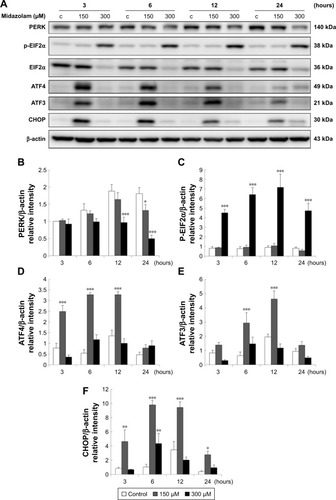
The expressions of ATF6, p-IRE1α and XBP1 were not affected by MDZ () (p>0.05), but the expression of cleaved caspase-12 was reduced by treatment with 300 μM MDZ for 3, 12 and 24 hours in TM3 cells () (p<0.05). We further observed the involvement of PERK-related pathway in TM3 cells. The expression of PERK was decreased by MDZ at 300 μM concentration for 12 and 24 hours, and 150 μM for 24 hours () (p<0.05). However, the expression of phosphor-EIF2α was significantly increased after treatment with 300 μM MDZ for 3, 6, 12 and 24 hours () (p<0.05). MDZ at 150 μM concentration significantly elevated the expression of ATF4 at 3, 6 and 12 hours () (p<0.05) and ATF3 at 6 and 12 hours () (p<0.05). MDZ at 150 μM from 3 to 24 hours significantly elevated the expression of CHOP () (p<0.05), and at 300 μM increased the expression of CHOP in 6 hours treatment () (p<0.05).
Figure 6 The involvement of ER stress pathways in midazolam-induced apoptosis in TM3 cells. TM3 cells were treated without or with different concentrations of midazolam (150 and 300 μM) for 3, 6, 12 and 24 hours. ATF6β (76 kDa), phosphor-IRE1α (110 kDa), IRE1α (130 kDa), XBP1 (28 kDa) and cleaved caspase-12 (42 kDa) were detected by Western blot analysis (A). Immunoblot represents the observations from one single experiment repeated at least three times. The integrated optical densities (IOD) of ATF6β (B), phosphor-IRE1α (C), XBP1 (D) and cleaved caspase-12 (E) proteins were normalized with β-actin (43 kDa) in each lane. Each data point represents the mean ± SEM of three separate experiments. * and ** indicate statistical difference compared to control equivalent to p<0.05 and p<0.01, respectively, (c = control).
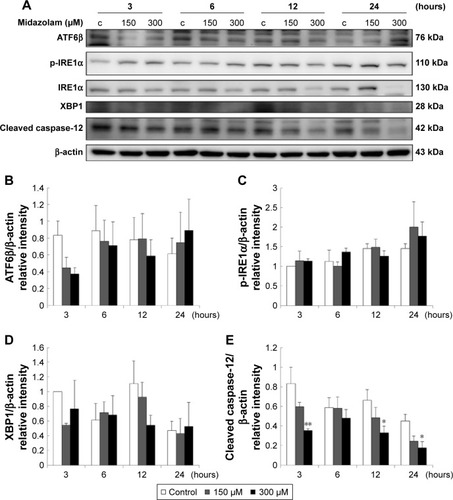
Figure 7 The involvement of ER stress pathways in midazolam-induced apoptosis in TM3 cells. TM3 cells were treated without or with different concentrations of midazolam (150 and 300 μM) for 3, 6, 12 and 24 hours. PERK (140 kDa), phosphor-EIF2α (38 kDa), EIF2α (36 kDa), ATF4 (49 kDa), ATF3 (21 kDa) and CHOP (30 kDa) were detected by Western blot analysis (A). Immunoblot represents the observations from one single experiment repeated at least three times. The integrated optical densities (IOD) of PERK (B), phosphor-EIF2α (C), ATF4 (D), ATF3 (E) and CHOP (F) proteins were normalized with β-actin (43 kDa) in each lane. Each data point represents the mean ± SEM of three separate experiments. *, ** and *** indicate statistical difference compared to control equivalent to p<0.05, p<0.01 and p<0.005, respectively, (c = control).
These results suggest that MDZ could induce apoptosis by activating ER stress-related PERK pathway in TM3 cells. Interestingly, these data showed that the activation of caspase cascade and MAPK pathways was observed at 24 hours treatment of MDZ, and the activation of ER stress pathways occurred at 3 hours treatment, which was earlier compared to caspase cascade and MAPK pathways, implying that MDZ could activate ER stress pathways prior to the caspase cascade and MAPK pathways to induce apoptosis in TM3 cells.
Involvement of cell cycle in MDZ-induced apoptosis in TM3 cells
Previous study has demonstrated a correlation between the G2/M arrest and the induction of apoptosis.Citation26 According to previous experiments, MDZ at 300 μM for 24 hours could regulate G2/M arrest in TM3 cells (). Thus, whether MDZ could induce apoptosis through cell cycle regulation or the expression of cyclin A, cyclin B and CDK1 proteins was examined by Western blot analysis.
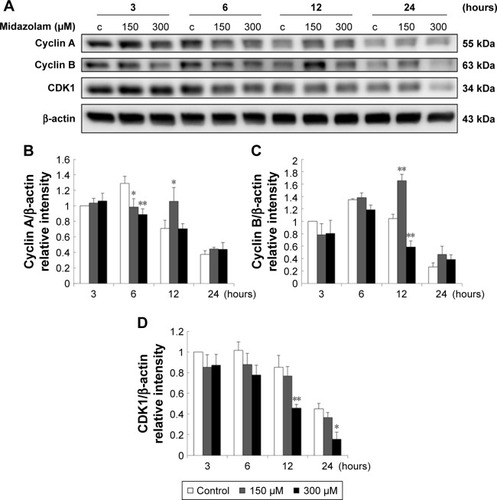
Results showed that MDZ at 150 and 300 μM concentrations for 6 hours decreased the expression of cyclin A () (p<0.05), but 150 μM for 12 hours increased the expression of cyclin A () (p<0.05). Interestingly, MDZ at 150 μM for 12 hours increased the expression of cyclin B () (p<0.05), but 300 μM for 12 hours decreased the expression of cyclin B () (p<0.05). Moreover, MDZ at 300 μM for 12 and 24 hours significantly decreased the expression of CDK1 () (p<0.05). These data showed that MDZ could regulate the expressions of cyclin A and cyclin B to reduce the expression of CDK1, suggesting that MDZ could actually induce G2/M arrest to induce apoptosis in TM3 cells.
Figure 8 The involvement of cell cycle related proteins in midazolam-induced apoptosis in TM3 cells. TM3 cells were treated without or with different concentrations of midazolam (150 and 300 μM) for 3, 6, 12 and 24 hours. Cyclin A (55 kDa), cyclin B (63 kDa) and CDK1 (34 kDa) were detected by Western blot analysis (A). Immunoblot represents the observations from one single experiment repeated at least three times. The integrated optical densities (IOD) of cyclin A (B), cyclin B (C) and CDK1 (D) proteins were normalized with β-actin (43 kDa) in each lane. Each data point represents the mean ± SEM of three separate experiments. * and ** indicate statistical difference compared to control equivalent to p<0.05 and p<0.01, respectively, (c = control).
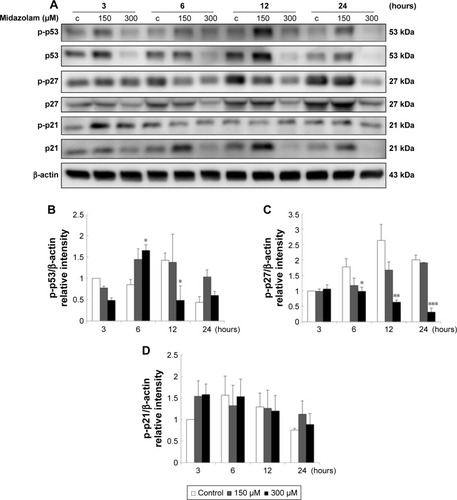
We further explored the possible role of p53 pathway upstream to the cell cycle regulation in MDZ-treated TM3 cells. MDZ at 300 μM for 6 hours significantly induced the expression of phosphor-p53 () (p<0.05), but 300 μM MDZ for 12 hours decreased its expression () (p<0.05). The expression of phosphor-p27 was reduced by 300 μM MDZ at 6, 12 and 24 hour treatments () (p<0.05). However, the expression of phosphor-p21 was not affected by MDZ () (p>0.05). These data suggested that MDZ could possibly regulate p53 pathway to cause G2/M arrest, and then induce apoptosis in TM3 cells.
Figure 9 The involvement of p53 pathway in midazolam-induced apoptosis in TM3 cells. TM3 cells were treated without or with different concentrations of midazolam (150 and 300 μM) for 3, 6, 12 and 24 hours. Phosphor-p53 (53 kDa), p53, phosphor-p27 (27 kDa), p27, phosphor-p21 (21 kDa) and p21 were detected by Western blot analysis (A). Immunoblot represents the observations from one single experiment repeated at least three times. The integrated optical densities (IOD) of phosphor-p53 (B), phosphor-p27 (C) and phosphor-p21 (D) proteins were normalized with β-actin (43 kDa) in each lane. Each data point represents the mean ± SEM of three separate experiments. *, ** and *** indicate statistical difference compared to control equivalent to p<0.05, p<0.01 and p<0.005, respectively, (c = control).
Discussion
Apoptosis is characterized by a series of dramatic transformations of cellular architecture, and the weakening of cell cytoskeleton is regulated by the activation of caspases, causing morphology changes such as cell shrinkage and membrane blebbing.Citation49 Our morphological results showed that MDZ could induce cell shrinkage and membrane blebbing, indicating MDZ could influence cytoskeleton and morphology changes to induce cell death related to apoptosis in TM3 Leydig progenitor cells.
The significant increase of subG1 phase after treatment with MDZ indicated that MDZ could cause DNA fragmentation to induce apoptosis in TM3 cells. Interestingly, MDZ could also induce G2/M phase arrest in TM3 cells. Studies have shown that the increase of subG1 phase and G2/M phase arrest could cause cell death through apoptosis.Citation6,Citation50 Hence, our data suggested that MDZ-induced apoptosis was associated with the regulation of cell cycle. The annexin V/PI double staining assay also indicated that the induction of apoptosis by MDZ occurs in a dose-dependent manner in TM3 cells, suggesting that MDZ could truly induce cell apoptosis. According to these observations, it is highly possible that MDZ would induce TM3 cell apoptosis through cell cycle arrest.
It is well known that apoptosis is mainly initiated by extrinsic and intrinsic signals, and then by the activation of the caspase cascade.Citation51 We have illustrated that MDZ could activate caspase pathways to induce MA-10 cell apoptosis.Citation13,Citation14 In the present study, we found that MDZ could also induce apoptosis in TM3 cells with the activation of caspase-8 and -3, but not the cleavages of caspase-9 and PARP. Interestingly, the higher dosage of MDZ (300 μM) did not affect the expressions of caspases and PARP. It has been shown that PARP cleavage can be used as a phenotype for cell apoptosis and an indirect evidence of the activation of caspase-3 to induce apoptosis through DNA fragmentation.Citation52 Therefore, these data suggested that TM3 cells might need a longer time with a higher dosage of MDZ to activate the caspase cascade, which should be investigated in detail to reveal an accurate mechanism.
Apoptotic pathways are regulated by many pathways, and one among them is MAPK pathway including JNK, ERK1/2 and p38 MAPK. MAPK pathways may respond to cellular stress to regulate cell survival or apoptosis.Citation27 Regulation of MAPK pathways by MDZ remains unclear in previous studies, although one of the studies has shown that MDZ diminishes phosphorylation of p38 MAPK in neutrophils.Citation53 We have illustrated that MDZ could activate JNK and p38 to induce MA-10 cell apoptosis.Citation13,Citation14 In the present study, activities of JNK, ERK and p38 were elevated by MDZ in TM3 cells. Our data suggested that MDZ could activate MAPK pathways to induce apoptosis in TM3 cells. Interestingly, MDZ could reduce the expression of total MAPKs, suggesting that MDZ could quickly induce the phosphorylation of MAPKs, lead to instability of MAPKs, and then result in degradation of MAPKs, or MDZ could affect the expression of MAPKs at translational level.
Akt signaling pathway is a pro-survival pathway and acts by inhibiting apoptotic signal cascades and activating pro-survival signal.Citation30 It has been reported that the activation of PI3K/Akt pathway could be observed in normal cells and the formation of some cancers.Citation54 In addition, activation of Akt not only inhibits pro-apoptotic ability of Bad, Bax and Bim, but also enhances anti-apoptotic ability of NF-κB transcription factor promoting the transcription of anti-apoptotic genes to inhibit apoptosis.Citation31 Our previous study has shown that MDZ could induce MA-10 cell apoptosis by inhibiting Akt pathway.Citation13,Citation14 However, present results showed that MDZ could elevate the phosphorylation of Akt in TM3 cells, and the G2/M arrest of cells was observed at the same time. Study has illustrated that the activation of Akt pathway occurs in response to DNA damage and aids in DNA repair and cell survival.Citation31 According to these observations, it is highly possible that MDZ would induce TM3 cell apoptosis through cell cycle arrest, and a portion of cells might survive by activating Akt pathway. It is reported that MDZ protects neuroblastoma cells through Akt-phosphorylation in reactive oxygen species derived cellular injury.Citation55 However, our data showed that the expression of phosphor-mTOR, the downstream target protein of Akt, was not changed by MDZ treatment, implying that the protective effect of Akt pathway on TM3 cells could not be activated and, hereafter, cell apoptosis occurred.
Different physiological and pathological perturbations lead to accumulation of unfolded or misfolded proteins in the ER lumen, which is termed ER stress.Citation33,Citation56 ER stress triggers UPR to increase protein folding capacity and decrease unfolded protein load.Citation33,Citation56 If ER stress is chronically prolonged, cell death often occurs through apoptosis which is induced by PERK, ATF6 and IRE1α.Citation33 In the present study, MDZ did induce the activation of PERK downstream proteins, including EIF2α, ATF4, ATF3 and CHOP, in TM3 cells. Thus, MDZ could stimulate MAPK pathways and ER stress pathway, but not Akt pathway, to induce TM3 cell apoptosis.
Eukaryotic cell cycle is tightly controlled by cyclins and CDKs, and imbalance in the regulation of cell cycle would cause developmental abnormalities and cancer growth.Citation25 It was reported that chemo-drugs caused DNA damage in cancer cells, leading to cell cycle arrest, such as G2/M arrest, and then inducing cell apoptosis.Citation57,Citation58 In our study, MDZ could induce G2/M phase arrest in TM3 cells. In addition, MDZ modulated cyclin A and cyclin B, and then caused the inactivation of CDK1, suggesting that MDZ could regulate cell cycle-related proteins to induce G2/M arrest in TM3 cells. Our data further illustrated that MDZ elevated the phosphorylation of p53 in TM3 cells. These data suggested that p53 activation could facilitate MDZ-induced TM3 cell apoptosis through G2/M arrest.
It has been shown that MDZ in different forms or in different culture media among various cell types would have different cytotoxicity effects.Citation59 Interestingly, we have illustrated that MDZ at 150 μM concentration could significantly reduce MA-10 cell viability to induce apoptosis,Citation13,Citation14 while higher dosage of MDZ (300 μM) in the present study was needed to induce the same effect in TM3 cells. Besides, cell viability of MA-10 cells decreased to 47% with 150 μM MDZ for 24-hour treatment, but cell viability of TM3 cells only decreased to 26%.Citation13,Citation14 Therefore, MA-10 mouse Leydig tumor cells were more sensitive to MDZ than TM3 mouse Leydig progenitor cells. The different phenomena as to how and why MDZ has different effects on MA-10 mouse Leydig tumor cells and TM3 mouse Leydig progenitor cells need further investigations.
Conclusion
MDZ could induce cell apoptosis by activating ER stress (at 3 hours), caspases (at 24 hours) and MAPK pathways (at 24 hours), and by impeding cell cycle (at 6 hours) and Akt pathway (at 24 hours) in TM3 mouse Leydig progenitor cells. These results imply the misuse of MDZ could cause Leydig progenitor cell death affecting male reproductive functions.
Acknowledgments
This work was supported by Chi Mei-NCKU Hospital grant (CMNCKU10621) (FCK and BMH) and Ministry of Science and Technology (MOST 105-2320-B-006-028) (BMH), Taiwan, Republic of China.
Disclosure
The authors report no conflicts of interest in this work.
References
- RevesJGFragenRJVinikHRGreenblattDJMidazolam: pharmacology and usesAnesthesiology19856233103243156545
- LaegreidLClinical observations in children after prenatal benzodiazepine exposureDev Pharmacol Ther1990153–41861881983095
- LaegreidLOlegårdRConradiNHagbergGWahlströmJAbrahamssonLCongenital malformations and maternal consumption of benzodiazepines: a case-control studyDev Med Child Neurol19903254324411972364
- DolovichLRAddisAVaillancourtJMPowerJDKorenGEinarsonTRBenzodiazepine use in pregnancy and major malformations or oral cleft: meta-analysis of cohort and case-control studiesBMJ199831771628398439748174
- SueharaTMorishitaJUekiMUenoMMaekawaNMizobuchiSEffects of sevoflurane exposure during late pregnancy on brain development of offspring micePaediatr Anaesth2016261525926645425
- ZhangTShaoHXuKQKuangLTChenRFXiuHHMidazolam suppresses osteogenic differentiation of human bone marrow-derived mesenchymal stem cellsEur Rev Med Pharmacol Sci20141891411141824867522
- ChenYCChenYHPanBSChangMMHuangBM2017. Functional study of Cordyceps sinensis and cordycepin in male reproduction – a reviewJ Food Drug Anal201725119720528911537
- HabertRLejeuneHSaezJMOrigin, differentiation and regulation of fetal and adult Leydig cellsMol Cell Endocrinol20011791–2477411420130
- ChenHGeRSZirkinBRLeydig cells: From stem cells to agingMol Cell Endocrinol20093061–291619481681
- BraestrupCSquiresRSpecific benzodiazepine receptors in rat brain characterized by high-affinity (3H) diazepam bindingProc Natl Acad Sci U S A19777493805380920632
- PapadopoulosVBrownASRole of the peripheral-type benzodiazepine receptor and the polypeptide diazepam binding inhibitor in steroidogenesisJ Steroid Biochem Mol Biol1995531–61031107626442
- SoECChangYTHsingCHPoonPWLeuSFHuangBMThe effect of midazolam on mouse Leydig cell steroidogenesis and apoptosisToxicol Lett2010192216917819857560
- SoECLinYXTsengCHMidazolam induces apoptosis in MA-10 mouse Leydig tumor cells through caspase activation and the involvement of MAPK signaling pathwayOncoTargets Ther20147211221
- SoECChenYCWangSCMidazolam regulated caspase pathway, endoplasmic reticulum stress, autophagy, and cell cycle to induce apoptosis in MA-10 mouse Leydig tumor cellsOncoTargets Ther2016925192533
- WyllieAHGlucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activationNature198028457565555566245367
- CerrettiDPKozloskyCJMosleyBMolecular cloning of the interleukin-1 beta converting enzymeScience19922565053971001373520
- YuanJShahamSLedouxSEllisHMHorvitzHRThe C. elegans cell death gene ced-3 encodes a protein similar to mammalian interleukin-1 beta-converting enzymeCell19937546416528242740
- AshkenaziADixitVMDeath receptors: signaling and modulationScience19982815381130513089721089
- LiPNijhawanDBudihardjoICytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascadeCell19979144794899390557
- AhmadMSrinivasulaSMWangLCRADD, a novel human apoptotic adaptor molecule for caspase-2, and FasL/tumor necrosis factor receptor-interacting protein RIPCancer Res19975746156199044836
- YangXChangHYBaltimoreDAutoproteolytic activation of pro-caspases by oligomerizationMol Cell1998123193259659928
- StennickeHRRyanCASalvesenGSReprieval from execution: the molecular basis of caspase inhibitionTrends Biochem Sci20022729410111852247
- SrinivasulaSMAhmadMFernandes-AlnemriTAlnemriESAuto-activation of procaspase-9 by Apaf-1-mediated oligomerizationMol Cell1998179499579651578
- GuptaSMolecular steps of tumor necrosis factor receptor-mediated apoptosisCurr Mol Med20011331732411899080
- BoxemMCyclin-dependent kinases in C. elegansCell Div20061616759361
- WangYJiPLiuJBroaddusRRXueFZhangWCentrosome-associated regulators of the G(2)/M checkpoint as targets for cancer therapyMol Cancer20098819216791
- JohnsonGLLapadatRMitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinasesScience200229856001911191212471242
- LeeSHJaganathIBAtiyaNManikamRSekaranSDSuppression of ERK1/2 and hypoxia pathways by four Phyllanthus species inhibits metastasis of human breast cancer cellsJ Food Drug Anal201624485586528911625
- SonYCheongYKKimNHChungHTKangDGPaeHOMitogen-activated protein kinases and reactive oxygen species: How can ROS activate MAPK pathways?J Signal Transduct2011201179263921637379
- MarkmanBDienstmannRTaberneroJTargeting the PI3K/Akt/mTOR pathway – beyond rapalogsOncotarget20101753054321317449
- HeinALOuelletteMMYanYRadiation-induced signaling pathways that promote cancer cell survival (review)Int J Oncol20144551813181925174607
- ZhouCZhaoXMLiXFCurcumin inhibits AP-2gamma-induced apoptosis in the human malignant testicular germ cells in vitroActa Pharmacol Sin20133491192120023685957
- LuoKCaoSSEndoplasmic reticulum stress in intestinal epithelial cell function and inflammatory bowel diseaseGastroenterol Res Pract2015201532879125755668
- SandersonTHGallawayMKumarRUnfolding the unfolded protein response: unique insights into brain ischemiaInt J Mol Sci20151647133714225830481
- LogueSEClearyPSaveljevaSSamaliANew directions in ER stress-induced cell deathApoptosis201318553754623430059
- KaniaEPająkBOrzechowskiACalcium homeostasis and ER stress in control of autophagy in cancer cellsBiomed Res Int2015201535279425821797
- KitamuraMEndoplasmic reticulum stress and unfolded protein response in renal pathophysiology: Janus facesAm J Physiol Renal Physiol20082952F323F33418367660
- HanESMullerFLPérezVIThe in vivo gene expression signature of oxidative stressPhysiol Genomics200834111212618445702
- ReinhardtHCSchumacherBThe p53 network: cellular and systemic DNA damage responses in aging and cancerTrends Genet201228312813622265392
- CmielováJRezáčováMp21Cip1/Waf1 protein and its function based on a subcellular localization [corrected]J Cell Biochem2011112123502350621815189
- KamatoDBurchMLPivaTJTransforming growth factor-β signalling: role and consequences of Smad linker region phosphorylationCell Signal201325102017202423770288
- MatherJPEstablishment and characterization of two distinct mouse testicular epithelial cell linesBiol Reprod19802312432526774781
- GoldenbergRCFortesFSCristanchoJMModulation of gap junction mediated intercellular communication in TM3 Leydig cellsJ Endocrinol2003177232733512740021
- GeigersederCDoepnerRFThalhammerAKriegerAMayerhoferAStimulation of TM3 Leydig cell proliferation via GABA(A) receptors: a new role for testicular GABAReprod Biol Endocrinol200421315040802
- GreenDRReedJCMitochondria and apoptosisScience19982815381130913129721092
- van EngelandMRamaekersFCSchutteBReutelingspergerCPA novel assay to measure loss of plasma membrane asymmetry during apoptosis of adherent cells in cultureCytometry19962421311398725662
- LowryOHRosebroughNJFarrALRandallRJProtein measurement with the Folin phenol reagentJ Biol Chem1951193126527514907713
- CreaghEMMartinSJCaspases: cellular demolition expertsBiochem Soc Trans200129Pt 669670211709057
- TaylorRCCullenSPMartinSJApoptosis: controlled demolition at the cellular levelNat Rev Mol Cell Biol20089323124118073771
- Paul-SamojednyMSuchanekRBorkowskaPKnockdown of AKT3 (PKBgamma) and PI3KCA suppresses cell viability and proliferation and induces the apoptosis of glioblastoma multiforme T98G cellsBiomed Res Int2014201476818124967401
- OliveiraJBGuptaSDisorders of apoptosis: mechanisms for autoimmunity in primary immunodeficiency diseasesJ Clin Immunol200828S20S2818193340
- LazebnikYAKaufmannSHDesnoyersSPoirierGGEarnshawWCCleavage of poly(ADP-ribose) polymerase by a proteinase with properties like ICENature199437164953463478090205
- GhoriKO’DriscollJShortenGThe effect of midazolam on neutrophil mitogen-activated protein kinaseEur J Anaesthesiol201027656256520421794
- VivancoISawyersCLThe phosphatidylinositol 3-Kinase AKT pathway in human cancerNat Rev Cancer20022748950112094235
- ChongWSHyunCLParkMKMidazolam protects B35 neuroblastoma cells through Akt-phosphorylation in reactive oxygen species derived cellular injuryKorean J Anesthesiol201262216617122379573
- HetzCMartinonFRodriguezDGlimcherLHThe unfolded protein response: integrating stress signals through the stress sensor IRE1alphaPhysiol Rev20119141219124322013210
- KoromilasAERoles of the translation initiation factor eIF2alpha serine 51 phosphorylation in cancer formation and treatmentBiochim Biophys Acta20151849787188025497381
- WangDLippardSJCellular processing of platinum anticancer drugsNat Rev Drug Discov20054430732015789122
- ShityakovSSohajdaTPuskasIRoewerNForsterCBroscheitJAIonization states, cellular toxicity and molecular modeling studies of midazolam complexed with trimethyl-beta-cyclodextrinMolecules20141910168611687625338177
