Abstract
Gastrointestinal stromal tumors (GISTs) are the most common sarcoma of the gastrointestinal tract, with transformation typically driven by activating mutations of c-KIT and less commonly platelet-derived growth factor receptor alpha (PDGFRA). Successful targeting of c-KIT and PDGFRA with imatinib, a tyrosine kinase inhibitor (TKI), has had a major impact in advanced GIST and as an adjuvant and neoadjuvant treatment. If treatment with imatinib fails, further lines of TKI therapy have a role, but disease response is usually only measured in months, so strategies to maximize the benefit from imatinib are paramount. Here, we provide an overview of the structure and signaling of c-KIT coupled with a review of the clinical trials of imatinib in GIST. In doing so, we make recommendations about the duration of imatinib therapy and suggest how best to utilize imatinib in order to improve patient outcomes in the future.
Introduction
Gastrointestinal tumors (GIST) are mesenchymal tumors thought to be derived from interstitial cells of Cajal that coordinate peristalsis within the gastrointestinal tract, and occur most commonly in the stomach. The incidence of GIST is approximately 15 per million per year.Citation1 Immunohistochemically, most GISTs show positive staining for c-KIT (CD117 antigen), DOG1, CD34, and PKCθ, and on mutational analysis have characteristic activating mutations in c-KIT or PDGFRA. The mainstay of treatment for localized GIST is surgical resection. GIST has been shown to be chemotherapy-insensitive, so patients with inoperable or advanced GIST had a dismal prognosis before imatinib was developed. Imatinib, previously known as STI571, is an adenosine triphosphate (ATP)-competitive inhibitor of tyrosine protein kinases. It was the product of a rational drug development program for inhibitors against Bcr-Abl, the proto-oncogene identified to be the driver for chronic myeloid leukemia, and was an emphatic success.Citation2,Citation3 In addition, imatinib was shown to inhibit c-KIT in mast cell leukemia harboring an activating mutation of c-KIT,Citation4 and inhibition of growth in the first GIST cell line, GIST882, was subsequently demonstrated.Citation5 These combined findings led rapidly to a compassionate use program in patients with GIST. The first patient with GIST to be treated with imatinib was a 50-year-old lady who had rapidly progressing disease with liver and intra-abdominal metastasis, and was found to carry an exon 11 activating mutation of c-KIT. Within four weeks of starting imatinib, her liver lesions had significantly reduced and there was a complete metabolic response by fluorodeoxyglucose positron emission tomography criteria.Citation6 Subsequently, imatinib has become the first -line treatment for locally advanced/metastatic GIST and as adjuvant or neoadjuvant therapy. Most patients eventually cease to benefit from imatinib, and treatment with second-line and third-line tyrosine kinase inhibitors is used. A critical question remains as to what is the appropriate duration of imatinib therapy in these different contexts. The duration of successful treatment depends on efficacy and tolerability. In this review, we provide a structure-functional analysis of the most prevalent imatinib target, c-KIT, and combine this with clinical trial data to provide an overview of the activity, resistance, and tolerability of imatinib, and thus the optimal duration of imatinib treatment.
Structure and signaling of c-KIT
Structure of c-KIT
The viral oncogene v-c-KIT was first identified as the transforming gene of Hardy-Zuckerman 4 feline sarcoma virus in 1986.Citation7 Soon afterwards, the cellular homolog, c-KIT, was cloned, and found to be located on chromosome 4q11 comprising 21 exons.Citation8 c-KIT is a transmembrane receptor tyrosine kinase that is normally activated by engagement of stem cell factor with the extracellular domain (in part coded for by exon 9) of the receptor, resulting in homodimerization. The dimerization results in transphosphorylation of multiple tyrosine (Y) residues,Citation9 leading to allosteric activation of the kinase and formation of phosphotyrosine-containing protein binding sites.
Like many protein kinases, c-KIT has a bilobed kinase domain, with the pockets for substrate and ATP binding (catalytic site, coded for by exons 13 and 14) lying between the two lobes ().Citation10 The lobes are able to move relative to each other, opening or closing the catalytic site, and this movement can be defined by the relative positions of the α-C helix. The glycine-rich loop (G-loop), the catalytic lysine (K), and the activation loop (A-loop) are key for the positioning of ATP and subsequent transfer of a phosphate group to the substrate. The juxtamembrane region, which is coded by exon 11, is autoinhibitory and maintains the closed kinase position. Upon transphosphorylation of the juxtamembrane region, the kinase can adopt the open active state which is further stabilized by transphosphorylation of the A-loop, a sequence coded for by exons 17 and 18.
Figure 1 Structural features of the catalytic c-KIT domain. (A) Inactive conformation of the c-KIT kinase domain (PDB code 1T45) with important motifs highlighted. The catalytic site is bordered by a variety of motifs required for activity. The glycine-rich loop (lime), HRD-motif (red), and K623 (pink) coordinate adenosine triphosphate binding and phosphotransfer. There is a charged interaction between K623 and E640 in the α-C helix (teal) that helps stabilizes the conformation. Because the inhibitory juxtamembrane (JM) region is bound (orange), the A-loop (blue) is in an “out” position. (B) Imatinib binds the inactive conformation of c-KIT (PDB codes 1T45 and 1T46). Compared with the active state (green), the imatinib-bound structure of c-KIT (red) has a shift of the α-C helix and DFG-motif to form an inactive conformation of the kinase.
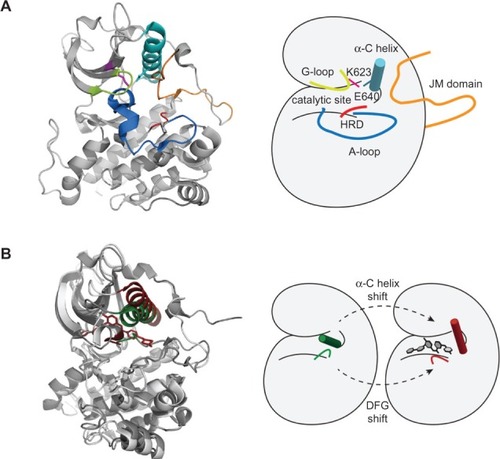
At the start of the A-loop is an aspartate (D), phenylalanine (F), and glycine (G) sequence (DFG motif) which, when the A-loop is in its phosphorylated activated state, has the F811 (F of the DFG) facing inwards (so-called “DFG-in”) to the nucleotide binding pocket and helping to coordinate ATP binding (). In the dephosphorylated inactive A-loop state, the F811 faces away from the nucleotide binding pocket, and it is this form that imatinib binds to and stabilizes, thus sustaining an inactive kinase.Citation11,Citation12
c-KIT-activating and imatinib-desensitizing mutations
Many c-KIT mutations have been linked to oncogenic transformation or imatinib resistance, and these mutations are localized on a number of hot spots within the c-KIT protein, namely exons 9, 11, 13, and 17 (). Up to 85% of GIST samples taken at the time of diagnosis carry mutations in c-KIT, most commonly in exons 9 and 11, that tend to confer constitutive activation and imatinib sensitivity. In contrast, mutations of exons 13 and 17 (nucleotide binding pocket and A-loop) tend to cause imatinib insensitivity, which can occur de novo but are usually identified in patients who have progressed on treatment after an initial response. Therefore, molecular characterization of c-KIT in GIST patients provides useful information on imatinib sensitivity, an explanation for resistance, and may help guide imatinib dosing and scheduling, although there is still much unknown ().
Figure 2 Relative frequency of c-KIT mutations in gastrointestinal stromal tumors. Top panel shows the relative frequency of mutations in treatment-naïve patients. Where the mutation results in more than one amino acid substitution/deletion/insertion, the most N-terminal residue is denoted. The total number of gastrointestinal stromal tumor samples in this data set is 7254 for which there were 3903 unique mutated samples. Mid-panel shows mRNA of c-KIT denoting the 21 exons. Lower panel shows the relative frequency of mutations in patients who had progressed on imatinib. Two exon hot spots are highlighted with a dotted orange line.
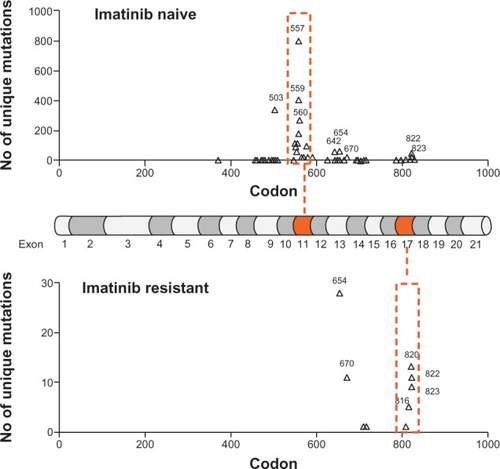
Table 1 Selected imatinib sensitizing and desensitizing mutations of c-KIT
c-KIT-binding domains and signaling
c-KIT is able to participate in multiple signaling pathways by recruiting adaptor proteins and phosphorylating substrates, some of which are themselves kinases. In this way, there is activation of signal transduction leading to biological responses, including cell survival, migration, and proliferation.Citation13 Important proximal c-KIT-interacting proteins include Src family kinases, the regulatory subunit of PI3K (p85), phospholipase Cγ, and several adaptor proteins, such as Grb2, Grb7 and APS.Citation14,Citation15 Common to each of these proteins is at least one of the following protein-binding domains that mediate the interaction with tyrosine phosphorylated c-KIT protein, ie, a pleckstrin homology, Src homology 2, or Src homology 3, as reviewed by Roskoski.Citation14 Immunohistochemistry and gene profiling of GIST primary tumors and cell lines combined with studies of chemical inhibition of c-KIT have helped to identify c-KIT-dependent signal transduction pathways (). Further, inhibition of these identified signaling targets has demonstrated anticancer phenotypes in cell lines and mouse cancer models (). Promising drugs for c-KIT signaling targets have been taken forward and tested in combination with imatinib in early clinical trials.
Table 2 c-KIT signaling molecules downstream
Table 3 Phenotypic effects of inhibition of signaling pathways associated with c-KIT
Imatinib in advanced GIST
Duration of imatinib therapy is determined by efficacy
The maximum tolerated dose of imatinib (400 mg twice daily) was demonstrated in a Phase I European Organisation for Research and Treatment of Cancer (EORTC) trial,Citation16 and activity was confirmed in two Phase II trials with overall response rates of 63%–73% (see for individual trial details).Citation17,Citation18 In fact, the results were so much better than for historical controls treated with chemotherapy that the subsequent Phase III trials were required to include imatinib in the control arms, so two different daily doses (400 mg and 800 mg) were compared. In both the European-Australasian trial and the North American Intergroup study, response rates were similar between the high-dose and low-dose imatinib arms, with a progression-free survival of approximately 18–24 months.Citation19,Citation20 In the European-Australasian trial, but not the North American Intergroup study, there was a statistically significant improvement in progression-free survival with the higher dose of imatinib,Citation19 and with extended follow-up, the progression-free survival was 22 months.Citation21 A pooled meta-analysis of these two trials demonstrated a small but significant improvement in progression-free survival in the high-dose arm (19 months versus 23 months, hazards ratio 0.89, P = 0.0195) but no difference in overall survival.Citation22 When the duration of response was analyzed for the common mutation subgroups, progression-free survival was longest for exon 11 mutations at 36 months and less favorable for exon 9 mutations or patients lacking both c-KIT and PDGF mutations, ie, the so-called wild-type variant.Citation22
Table 4 Landmark imatinib trials in patients with advanced gastrointestinal stromal tumors
Prolongation of imatinib therapy by dose escalation
Acquired resistance to imatinib can result as a consequence of amplification of c-KIT, new secondary mutations, or switching to distinct protumorigenic signaling pathways.Citation23 From a understanding of enzyme kinetics, amplification of the c-KIT gene and thus high expression of the c-KIT protein kinase would be predicted to require higher concentrations of inhibitor, although whether such tumors respond to higher-dose imatinib has not been confirmed by clinical data. Additionally, in silico modeling has predicted that the binding affinity of imatinib to the nucleotide binding pocket of exon 9-mutated c-KIT would be lower but might be subverted by dose escalation.Citation24 Clinical data from the EORTC and North American Intergroup studies, where patients were allowed to cross over to high-dose imatinib upon progression, revealed that approximately one third of these patients were able to regain disease control. The median progression-free survival following crossover to high-dose imatinib was three and five months in the Phase III EORTC and North American Intergroup trials, respectively.Citation25,Citation26 Subsequent analysis has demonstrated that the patients who responded to this dose escalation were indeed those that carried exon 9 mutations and often demonstrated primary resistance (progression in ≤3 months from initiation of imatinib).Citation27 Based on these data, a high dose of 800 mg/day should only be recommended routinely for patients who carry the exon 9 activating mutation.
Biomarker-driven prolongation of treatment
The EORTC investigated the pharmacokinetics of imatinib in patients with advanced GIST who were enrolled into their Phase I and Phase II studies analyzing the effect of covariates. From their modeling, low clearance was correlated with low body weight and high granulocyte count, and low hemoglobin was correlated with a low volume of distribution. Further, there was a trend towards a 33% increase in imatinib clearance after 12 months.Citation28 Similarly, a post hoc analysis of a subset of 73 patients with advanced GIST in the US-Finnish Phase II trial treated daily with 400 mg or 600 mg imatinib, for whom data were available on plasma imatinib levels on days 1 and 29, was done to see if there was a correlation between drug exposure and clinical outcomes. Patients with a trough level of imatinib in the lower quartile were less likely to obtain clinical benefit (a composite endpoint of complete response, partial response, and stable disease) and had a decreased time to progression (lowest quartile versus other quartiles, 11.3 months versus >30 months, respectively; P = 0.003).Citation29
Therefore, these retrospective studies raised the possibility that treatment failure on imatinib could be as a result of lowered plasma imatinib levels in a subset of patients. Eechoute et al designed a prospective study in 50 patients to identify causes of reduced imatinib pharmacokinetics and to test the exposure-outcome hypothesis. Full pharmacokinetic profiles were performed at day 1 and then at one, six, and 12 months, and trough imatinib levels were taken at day 14 and monthly thereafter. There was almost a 30% reduction in the plasma trough imatinib level in the first three months of treatment, after which clearance seemed to plateau. However, the explanation for this clearance remains elusive and it cannot be convincingly attributed to reduced absorption, increased liver metabolism, or an increase in drug efflux.Citation30
Imatinib has been shown in vitro to reduce the proliferation and colony-forming capacity of hematopoietic progenitor cells (CD34+/CD38+) in both a c-KIT-dependent and c-KIT-independent manner.Citation31 In keeping with this observation, patients with chronic phase myeloid leukemia treated with imatinib commonly exhibit a macroscopic anemia, ie, a high mean cell volume (MCV).Citation32 Several groups have suggested that this rise in MCV correlates with response; for example, patients who had an elevated MCV (>100 fL) for at least six months were more likely to achieve a complete hematological response after 12 months of treatment with imatinib (increased MCV versus non-increased MCV; complete hematological response, 92.1% versus 69.6%, respectively; P = 0.011).Citation33
To assess whether elevated MCV as a predictive surrogate marker of imatinib response in chronic myeloid leukemia could be relevant in imatinib-treated patients with GIST, a preliminary retrospective analysis of 130 patients with locally advanced/advanced GIST treated with imatinib was performed at our institution. We found that 33 patients had a ≥10% increase in MCV three months after initiation of imatinib, and in patients with a ≥10% increase in MCV, there was almost a 50% prolongation in progression-free survival compared with those who did not have such an increase (37.1 months versus 24.3 months, respectively; P = 0.032).Citation34 Based on the above evidence, it is plausible that MCV is also a biomarker for imatinib exposure. Therefore, using plasma imatinib and/or MCV measurements, it may be possible to identify patients in whom there is increased clearance and who may benefit from dose-escalation strategies, potentially prolonging time to treatment failure and thus the duration of imatinib therapy. This hypothesis needs testing in prospective randomized trials.
Prolongation of imatinib treatment by intermittent dosing
The most frequent cause of imatinib failure is acquired secondary resistance mutations or toxicity. To address whether acquired resistance to imatinib in patients with advanced GIST can be delayed, the French Sarcoma Group designed a Phase III trial (BFR-14) that randomized patients to continuous or interrupted imatinib treatment following one, three, or five years of benefit from imatinib. Patients who discontinued imatinib were considerably more likely to progress than patients who were on continuous dosing, and due to the large number of patients with progressive disease in the interruption group (12 of 25 patients versus one of 25 in the continuous group), the randomization was stopped. In the interruption arm, all patients who were subsequently rechallenged with imatinib had at least stable disease after three months and 12 of 21 had a partial response or better. However, the volume of disease remained greater in the interruption arm and there was no obvious difference between time to secondary resistance or overall survival, although this study was not powered to detect such a difference.Citation35 A subsequent exploratory analysis of the BFR-14 trial was recently performed to identify predictive factors for progression-free survival during imatinib interruption and progression-free survival upon rechallenge with imatinib. The length of continuous imatinib before randomization (one, three, or five years) and the previous best response (complete response, partial response, or stable disease) correlated directly with progression-free survival once treatment was interrupted.Citation36 This is not surprising given that patients who were in the five-year group and/or had a complete response are essentially preselected as a biologically favorable subset. However, what is important to note is that all patients did eventually progress, telling us that viable GIST cells remain despite five years of treatment or a complete radiological response. Patients who progressed rapidly during interruption of imatinib were also more likely to have a short progression-free survival upon imatinib rechallenge, which probably reflects the biological characteristics of GIST, particularly in terms of acquired resistance. In summary, it would seem sensible to recommend continuous dosing of imatinib in the advanced setting. If patients are intolerant of imatinib despite dose modification, an argument could be made to cautiously adopt an interrupted dosing strategy.
Prolongation of imatinib therapy by use of combination strategies
Despite the success of imatinib as a single agent in the management of GIST, resistance is almost inevitable and there is a clear need for new agents with a different mode of action. Although new agents might be tested initially as single agents in imatinib-resistant patients, there is a strong rationale to identify treatments that synergize with imatinib in an attempt to prevent resistance. The ultimate goal of combining other treatments with imatinib is to prolong the treatment duration and potentially cure patients. Combination strategies have focused on targeting upregulated signaling pathways in imatinib-resistant patients or on reducing the stability of activated signaling proteins (see section on c-KIT binding domains and signaling).
Based on preclinical data in GIST cell models, the PI3K/Akt/mTOR signaling axis has been studied in early clinical trials. An international dose-finding study for BKM120, a PI3K inhibitor, in combination with imatinib is currently in accrual. Perifosine, an inhibitor of AKT, in combination with imatinib had minimal activity in 40 patients with imatinib-resistant GIST, although there was a suggestion that there may be a benefit in wild-type GIST.Citation37 In a Phase I–II study of imatinib together with RAD001, an mTOR inhibitor, in patients resistant to imatinib, the combination was shown to be tolerable and to have activity. In the 47 pretreated patients taking part, there was one patient (2%) with a partial response and 20 patients (43%) with stable disease, and further studies are planned.Citation38
The other main focus of clinical trials involving novel agents has been heat shock protein 90 and histone deacetylase inhibitors in combination with imatinib. In a Phase I trial of IPI-504, a heat shock protein 90 inhibitor, a 22% partial response rate and 78% stable disease rate was seen in patients with imatinib-resistant GIST as assessed by positron emission and computed tomography.Citation39 This led to a placebo-controlled Phase III trial which had to be terminated early due to 3 out of 4 deaths on treatment in the IPI-504 arm.Citation40 Panobinostat (LBH589) is a histone deacetylase inhibitor that has been tested in combination with imatinib. At the maximum tolerated dose of 20 mg three times weekly with imatinib 400 mg/day, there were seven grade 3 adverse events, no objective responses by Response Evaluation Criteria In Solid Tumors, and one partial response based on metabolic criteria.Citation41 In summary, while there have been a number of interesting mechanism-based combination drug studies with imatinib, disappointing efficacy or toxicity results have precluded their translation to clinical practice. This remains an area of intensive investigation, and further advances that may help prolong imatinib treatment and afford patient benefit are anticipated in the near future.
Prolongation of imatinib treatment duration by local therapy
Surgery has traditionally played a palliative role in the advanced disease setting. However, several single-institution studies have investigated the role of surgery as a part of multimodality management of advanced GIST. One of the rationales for resecting oligometastatic disease or single sites of progressive disease is to eliminate imatinib-resistant clones, leaving behind only sensitive disease and prolonging imatinib therapy.
In a retrospective single-institution analysis of 69 patients who underwent surgery for advanced GIST while being treated with tyrosine kinase inhibitors, it was demonstrated that there was an association between the presurgical treatment response status (stable disease, limited progression, or generalized progression) and 12-month progression-free survival (80%, 33%, and 0%, respectively).Citation42 In two further studies, progression-free survival on imatinib following surgery for focal disease progression was eight and 11 months, which compares favorably with the outcomes seen in patients who are switched to sunitinib upon disease progression.Citation43–Citation45 These data would suggest that there is no role for elective surgery in patients with generalized progression on imatinib, but that resection of focally progressive disease could be considered to prolong the duration of therapy. However, randomized controlled trials are required to answer this question.
Radiofrequency ablation can also be used to treat metastatic lesions. In 13 imatinib-treated GIST patients with metastasis mainly to the liver, radiofrequency ablation was successful in 92% of cases, with a median progression-free survival of 28 months and minimal morbidity.Citation46 More specifically, in a separate study of nine patients who had focal progression of metastatic disease in the liver or soft tissue (single or limited sites) on imatinib (or sunitinib) therapy and underwent percutaneous radiofrequency ablation, all patients had successful ablation of the targeted lesion, three patients remained stable post radiofrequency ablation after a median follow-up of 13.6 months on treatment, while six progressed systemically after a median of 4.7 months.Citation47 These preliminary results point to a possible benefit of radiofrequency ablation as an adjunct to imatinib in order to prolong the duration of treatment in patients with focal progression.
Imatinib in localized GIST
Adjuvant imatinib
Whilst surgery remains the key therapeutic maneuver for patients with primary GIST, the risk of recurrence following complete resection remains high.Citation48 This is likely due to the persistence of microscopic disease following surgery. In 2001, a National Institutes of Health consensus group proposed a scheme to estimate the risk of metastatic disease following resection of the primary GIST based on primary tumor site, size, and mitotic rate.Citation49 The Armed Forces Institute of Pathology in the US built upon this initial work, based on observation of 1784 patients, and categorized the risk of recurrence as low, intermediate, or high.Citation48,Citation50 Subsequently, a prospectively validated prognostic nomogramCitation51 was developed to predict the risk of recurrence, which was shown to have improved accuracy and furthermore allowed prediction in individual patients rather than limited discrete categories. Most recently, the Scandinavian group developed prognostic contour maps to estimate the risk of recurrence using pooled data from 2560 patients.Citation52 This work shows that size and mitotic rate are the two most important prognostic variables, but also highlights the importance of intraoperative tumor rupture as an adverse prognostic feature.
The high risk of recurrence following surgery and subsequent development of metastatic disease has led to exploration of the role of adjuvant imatinib in order to reduce the risk of recurrence, and initially, a number of single-arm and Phase II studies of adjuvant imatinib for a duration of 12–26 months suggested benefit. The American College of Surgeons Oncology Group did a large multicenter, randomized, double-blind, placebo-controlled Phase III trial (Z9001) that took five years to complete between 2002 and 2007.Citation53 Eligible patients were those who had complete gross resection of GIST and tumors ≥3 cm in size. There was central pathological review and patients started treatment within 84 days of surgery; 713 patients were randomized and assigned to imatinib 400 mg/day or placebo for one year. The initial primary endpoint was overall survival, which was subsequently amended to recurrence-free survival due to problems with the initial trial design. A statistically significant improvement in recurrence-free survival was seen during a median follow-up of 19.7 months, with an estimated one-year recurrence-free survival of 98% in the imatinib group versus 83% in the placebo group, with an overall hazard ratio of 0.35 (0.22–0.53 95% confidence interval; P < 0.0001). In an unplanned subset analysis, it was shown that tumor size, particularly over 10 cm, was an independent predictor of benefit from adjuvant imatinib. Patients were not stratified according to tumor site or mitotic rate, the latter because the technique for measuring mitotic rate was not standardized at that time. Of the patients who stopped treatment prematurely, those in the imatinib group were more likely to stop due to adverse events whereas those in the placebo group were more likely to stop due to tumor recurrence. Interestingly, the rate of recurrence in patients in the imatinib group was seen to increase six months after stopping adjuvant treatment, suggesting benefit from longer treatment with imatinib. Overall survival in both groups was similar, and likely to be due to the fact that crossover to the active drug was allowed upon disease recurrence. This pivotal study led to approval of use of imatinib for one year in high-risk patients.
At the same time as the US study are two further Phase III adjuvant imatinib studies, ie, the EORTC 62024 study, which randomized no treatment versus two years of adjuvant imatinib and is due to be reported later this year, and the Scandinavian Sarcoma Group’s XVIII/AIO trial. This latter study was a collaboration between Scandinavian and German investigators and explored the effects of adjuvant imatinib in patients with high-risk GIST (modified National Institute of Health consensus criteria) given for one year versus three years. The trial took four years to recruit 400 patients between 2004 and 2008, with 200 in each arm and a median follow-up of 54 months.Citation54 Patients in the three-year arm had both a significant improvement in relapse-free survival compared with the one-year arm (five-year survival 65.6% versus 47.9%, respectively; P < 0.001) and longer overall survival (92% versus 81.7% respectively; P < 0.02). Subgroup analysis demonstrated that GIST patients with exon 11 mutation of c-KIT derived benefit from three years of adjuvant imatinib but those with exon 9 or PDGFRA mutation did not; however, numbers in these two latter groups were small. This landmark study has led to the adoption of three years of adjuvant imatinib as the recommended duration of treatment.Citation55 However, there are a number of outstanding questions that require further investigation.
First, GIST mutational status appears to be important, and there is a consensus that patients with the imatinib-insensitive D842V mutation of PDGFRA should not receive adjuvant imatinib.Citation56 What is less clear is whether patients with the exon 9 mutation should be treated with high-dose (800 mg/day) imatinib analogous to that recommended in the advanced setting. Second, opinions are split with regard to the rare wild-type GIST subgroup. Third, there is the issue of whether patients who fall into the intermediate-risk group should be offered adjuvant therapy and what improvements can be made to risk stratification. Finally, the trial data clearly support three years of adjuvant imatinib over one year but should the treatment duration be longer? We know from the BFR-14 trial in patients with advanced GIST that some patients who had a complete response to imatinib but then had interrupted therapy relapsed even after five years of treatment (see section on Prolongation of imatinib treatment by intermittent dosing). This may suggest the persistence of an as yet unidentified imatinib-resistant GIST cancer stem cell population.Citation57 The theoretical rationale for extending adjuvant imatinib beyond three years is counterbalanced by the possible development of imatinib-resistant mutations that would limit the efficacy of future therapies. There is a non-randomized Phase II trial of five years of adjuvant imatinib therapy currently in accrual (NCT00867113), which may help to define the optimal treatment duration for adjuvant imatinib.
Neoadjuvant/preoperative imatinib
The goal of neoadjuvant imatinib is to reduce the size of locally advanced GIST. This in turn may improve the likelihood of complete resection, reduce the risk of intraoperative complications including tumor rupture, reduce operative morbidity, improve the chance of function-sparing surgery, and hopefully improve long-term outcomes. Candidates for such treatment may include those with challenging anatomical locations, eg, rectal, duodenal, and esophageal GIST, and those with larger tumor sizes. Decisions surrounding preoperative imatinib should be made on a case-by-case basis and be taken in the multidisciplinary setting. Preoperative biopsy should be utilized not only to confirm the diagnosis of GIST but also to ensure that the imatinib-resistant D842V mutation is not present.
There are few prospective data on the use of imatinib in the preoperative setting. The use of neoadjuvant imatinib was retrospectively analyzed as part of the larger aforementioned prospective BFR-14 study,Citation58 where 25 of 434 patients taking part in the study were identified to have a median tumor size of 15 cm with no metastatic disease and no prior surgery. Of these patients, 15 had a partial response (60%) following treatment with imatinib for a median of four (range 1.4–12.8) months and nine patients went on to surgery after a median of seven (range 3.4–12) months. Outcomes in patients who had surgery following preoperative imatinib were comparable with those with localized intermediate and high-risk GIST in the subgroup of operated patients; whereas those who did not undergo surgery behaved similarly to those with metastatic GIST. Caveats do apply, given that this is a retrospective analysis and selection bias is a concern. Further, patients who responded well to imatinib were more likely to be able to proceed to surgery than those who did not.
In 2009, a Phase II trial from the US evaluating the role of neoadjuvant imatinib was reported.Citation59 There were two groups of patients; those with primary GIST (n = 30) with a median tumor size of 9 cm and those with metastatic GIST. Patients were treated with neoadjuvant imatinib at a dose of 600 mg/day for 8–12 weeks prior to surgery and then continued adjuvant imatinib 600 mg/day for two years. Of these patients, 7% had a partial radiological response and 83% had stable disease. Complete resection was performed in the localized GIST group in 77% of patients, and most had single organ resection. Two-year progression-free survival was 83% which compares well with data from the Phase III adjuvant imatinib study reported by the American College of Surgeons Oncology Group.Citation53 However, recently reported extended follow-up of this trial demonstrated that a high proportion of patients relapsed after two years of adjuvant imatinib.Citation60 Following this, data from Canada were published on an open-label single-arm prospective Phase II study of imatinib 400 mg/day in patients with locally advanced or metastatic GIST which was potentially resectable.Citation61 Patients received imatinib for a maximum of 12 months to maximal tumor response, during which time there was the option of escalating the dose to 600 mg/day at week 7 if there was no evidence of radiological response. Of the 14 patients who took part, six had a partial response and eight had stable disease with no evidence of tumor progression. Eleven of these patients had complete resection at surgery. Seven patients had their imatinib dose increased to 600 mg/day and the median duration of imatinib treatment was nine months. The authors concluded that at least 6–12 months of preoperative imatinib should be given. Further data on imatinib is eagerly awaited from the German APOLLON study which is investigating preoperative imatinib for six months, the preliminary results of which were reported at the annual meeting of the American Society of Clinical Oncology in 2012.Citation62
It is our view that neoadjuvant imatinib should be continued until maximum tumor response as demonstrated by cessation of tumor shrinkage on radiological assessment. In order to identify the time point of maximal response, frequent radiological assessments should be made, initially after one month to demonstrate imatinib sensitivity and three-monthly thereafter. It would seem from the available data that the optimum duration of treatment lies between six and 12 months. Unanswered questions include whether patients with exon 9 mutation should be given imatinib 800 mg/day, and the duration for which adjuvant treatment should be given when neoadjuvant has already been used is uncertain, but three years in total seems rational.Citation63
Patient compliance and imatinib toxicity
An important patient factor when considering the optimal duration of imatinib therapy is compliance with treatment and issues affecting adherence. Whatever the indication, imatinib is usually used for prolonged periods of time, and may be regarded as a treatment for chronic disease. In order to optimize patient outcomes and allow long-term treatment with imatinib, the associated toxicities of treatment should be managed proactively. It is notable that a considerable number of patients taking part in clinical trials of imatinib in both the adjuvant and metastatic setting have discontinued treatment due to toxicity. In the SSGXVIII study, just over 25% of patients randomized to the three-year arm discontinued imatinib compared with 12.6% of patients in the one-year arm. Grade 3 or 4 events occurred in a third of patients and 13.6% of patients in the three-year arm discontinued imatinib due to adverse events.Citation54 This clearly demonstrates that prompt management of side effects, prudent dose modifications, and continued patient support whilst on imatinib are of vital importance in order to optimize long-term outcomes. Further, it is noteworthy that patients who take part in clinical trials are in general a well motivated group and may not be representative of the general population. Treatment of an unselected group of patients with chronic myeloid leukemia using imatinib showed adherence rates <90% in 26% of patients; moreover, 14% had adherence rates lower than 80%.Citation64
Common toxicities associated with imatinib include diarrhea, fatigue, skin rash, and edema, and there is evidence that the worst toxicities arise in the first 3–6 months of treatment, in part due to alterations in pharmacokinetics over time.Citation18,Citation28 Changes in pharmacokinetics may also account for the differences in toxicity seen between men and women and also for the increased toxicity observed in older patients. Certainly if toxicity is unexpectedly severe in an individual patient, there may be a role for therapeutic drug monitoring. There is also a dose relationship, with side effects being more severe and frequent in patients treated with high-dose imatinib, ie, >400 mg/day. Optimizing the management of side effects includes effective patient information and engagement, including user-friendly advice on medicines that may alleviate the most common side effects. Input from specialist nurses who are readily available to patients is critical. GIST support groups and the Internet are also valuable resources.
In terms of long-term safety information, imatinib has been in use in patients with GIST or chronic myeloid leukemia for over 10 years and been investigated in numerous rigorous clinical trials. In general, imatinib is a safe drug to use in the long term as confirmed in the study by Blanke et al in which follow-up was for a median 63 months.Citation26 Concerns have been raised about the cardiotoxicity profile of imatinib.Citation65 However, these have not been borne out in the clinical trial setting, where an excess of cardiac events compared with the general population has not been detected. Imatinib-related edema has also been raised as a possible factor contributing to cardiotoxicity, but this has not been explored in a prospective fashion. Clearly, further prospective studies with cardiology input are needed to answer this question. Pre-existing cardiac disease should not be seen as a contraindication to treatment, although careful monitoring is advised. Changes in bone metabolism associated with hypocalcaemia, hypophosphatemia and low bone mineral density have been documented, so monitoring of bone health should be considered.Citation66,Citation67 Finally, although the mechanism is not well described, imatinib may also impact on glucose metabolism, with reports of symptomatic hypoglycemia.Citation68 In summary, imatinib has been shown to be safe and effective even when used over a prolonged period of time.
Summary
Through a thorough understanding of the structure and function of c-KIT and its downstream signaling pathways combined with knowledge of the efficacy and tolerability of imatinib from clinical trial data, we will hopefully be able to maximize the benefit of imatinib in patients with GIST. The evidence thus far demonstrates that the duration of imatinib therapy should be as long as possible in the metastatic setting, providing that the patient is still responding to treatment. Data in the early disease setting is less mature, but points to at least three years of adjuvant imatinib in order to optimize patient outcomes. Further research is required to enhance our understanding of this rare disease.
Acknowledgments
We would like to thank the Royal Marsden National Institute for Health Research Biomedical Research Centre for supporting this research.
Disclosure
The authors report no conflicts of interest in this work.
References
- GattaGvan der ZwanJMCasaliPGRare cancers are not so rare: the rare cancer burden in EuropeEur J Cancer201147172493251122033323
- DrukerBJTalpazMRestaDJEfficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemiaN Engl J Med2001344141031103711287972
- DrukerBJTamuraSBuchdungerEEffects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr-Abl positive cellsNat Med1996255615668616716
- HeinrichMCGriffithDJDrukerBJWaitCLOttKAZiglerAJInhibition of c-kit receptor tyrosine kinase activity by STI 571, a selective tyrosine kinase inhibitorBlood200096392593210910906
- TuvesonDAWillisNAJacksTSTI571 inactivation of the gastrointestinal stromal tumor c-KIT oncoprotein: biological and clinical implicationsOncogene200120365054505811526490
- JoensuuHRobertsPJSarlomo-RikalaMEffect of the tyrosine kinase inhibitor STI571 in a patient with a metastatic gastrointestinal stromal tumorN Engl J Med2001344141052105611287975
- BesmerPMurphyJEGeorgePCA new acute transforming feline retrovirus and relationship of its oncogene v-kit with the protein kinase gene familyNature198632060614154213007997
- YardenYKuangWJYang-FengTHuman proto-oncogene c-kit: a new cell surface receptor tyrosine kinase for an unidentified ligandEMBO J1987611334133512448137
- DiNittoJPDeshmukhGDZhangYFunction of activation loop tyrosine phosphorylation in the mechanism of c-Kit auto-activation and its implication in sunitinib resistanceJ Biochem2010147460160920147452
- MolCDLimKBSridharVStructure of a c-kit product complex reveals the basis for kinase transactivationJ Biol Chem200327834314613146412824176
- MolCDFabbroDHosfieldDJStructural insights into the conformational selectivity of STI-571 and related kinase inhibitorsCurr Opin Drug Discov Devel200475639648
- MolCDDouganDRSchneiderTRStructural basis for the autoinhibition and STI-571 inhibition of c-Kit tyrosine kinaseJ Biol Chem200427930316553166315123710
- LennartssonJRonnstrandLStem cell factor receptor/c-Kit: from basic science to clinical implicationsPhysiol Rev20129241619164923073628
- RoskoskiRJrSignaling by Kit protein-tyrosine kinase – the stem cell factor receptorBiochem Biophys Res Commun2005337111316129412
- JahnTSeipelPUrschelSPeschelCDuysterJRole for the adaptor protein Grb10 in the activation of AktMol Cell Biol200222497999111809791
- van OosteromATJudsonIVerweijJSafety and efficacy of imatinib (STI571) in metastatic gastrointestinal stromal tumours: a Phase I studyLancet200135892911421142311705489
- DemetriGDvon MehrenMBlankeCDEfficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumorsN Engl J Med2002347747248012181401
- VerweijJvan OosteromABlayJYImatinib mesylate (STI-571 Glivec, Gleevec) is an active agent for gastrointestinal stromal tumours, but does not yield responses in other soft-tissue sarcomas that are unselected for a molecular target. Results from an EORTC Soft Tissue and Bone Sarcoma Group Phase II studyEur J Cancer200339142006201112957454
- VerweijJCasaliPGZalcbergJProgression-free survival in gastrointestinal stromal tumours with high-dose imatinib: randomised trialLancet200436494401127113415451219
- BlankeCDRankinCDemetriGDPhase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase: S0033J Clin Oncol200826462663218235122
- CasaliPGVerweijJKotasekDImatinib mesylate in advanced gastrointestinal stromal tumors (GIST): survival analysis of the intergroup EORTC/ISG/AGITG randomized trial in 946 patientsEur J Cancer Suppl200532201202
- Gastrointestinal Stromal Tumor Meta-Analysis Group (MetaGIST)Comparison of two doses of imatinib for the treatment of unresectable or metastatic gastrointestinal stromal tumors: a meta-analysis of 1,640 patientsJ Clin Oncol20102871247125320124181
- PierottiMATamboriniENegriTPriclSPilottiSTargeted therapy in GIST: in silico modeling for prediction of resistanceNat Rev Clin Oncol20118316117021364689
- TamboriniEPriclSNegriTFunctional analyses and molecular modeling of two c-Kit mutations responsible for imatinib secondary resistance in GIST patientsOncogene200625456140614616751810
- ZalcbergJRVerweijJCasaliPGOutcome of patients with advanced gastro-intestinal stromal tumours crossing over to a daily imatinib dose of 800 mg after progression on 400 mgEur J Cancer200541121751175716098458
- BlankeCDDemetriGDvon MehrenMLong-term results from a randomized Phase II trial of standard- versus higher-dose imatinib mesylate for patients with unresectable or metastatic gastrointestinal stromal tumors expressing KITJ Clin Oncol200826462062518235121
- Debiec-RychterMSciotRLe CesneAKIT mutations and dose selection for imatinib in patients with advanced gastrointestinal stromal tumoursEur J Cancer20064281093110316624552
- JudsonIMaPPengBImatinib pharmacokinetics in patients with gastrointestinal stromal tumour: a retrospective population pharmacokinetic study over time. EORTC Soft Tissue and Bone Sarcoma GroupCancer Chemother Pharmacol200555437938615592836
- DemetriGDWangYWehrleEImatinib plasma levels are correlated with clinical benefit in patients with unresectable/metastatic gastrointestinal stromal tumorsJ Clin Oncol200927193141314719451435
- EechouteKFranssonMNReynersAKA long-term prospective population pharmacokinetic study on imatinib plasma concentrations in GIST patientsClin Cancer Res201218205780578722850565
- BartolovicKBalabanovSHartmannUInhibitory effect of imatinib on normal progenitor cells in vitroBlood2004103252352912969987
- DrukerBJGuilhotFO’BrienSGFive-year follow-up of patients receiving imatinib for chronic myeloid leukemiaN Engl J Med2006355232408241717151364
- SongMKChungJSSeolYMMean cell volume can be an early predictor for the cytogenetic response of chronic myeloid leukemia patients treated with imatinib?Leuk Res200933111459146219446878
- ConstantinidouAKrikelisDOlmosDEarly assessment of MCV to predict clinical outcome in patients with advanced gastrointestinal stromal tumors (GIST) receiving imatinibJ Clin Oncol201230 SupplS10086
- Le CesneARay-CoquardIBuiBNDiscontinuation of imatinib in patients with advanced gastrointestinal stromal tumours after 3 years of treatment: an open-label multicentre randomised Phase 3 trialLancet Oncol2010111094294920864406
- PatrikidouAChabaudSRay-CoquardIInfluence of imatinib interruption and rechallenge on the residual disease in patients with advanced GIST: results of the BFR14 prospective French Sarcoma Group randomised, Phase III trialAnn Oncol20132441087109323175622
- ConleyAPAraujoDLudwigJA randomized Phase II study of perifosine (P) plus imatinib for patients with imatinib-resistant gastrointestinal stromal tumor (GIST)J Clin Oncol200927Suppl 1510563
- SchoffskiPReichardtPBlayJYA Phase I-II study of everolimus (RAD001) in combination with imatinib in patients with imatinib-resistant gastrointestinal stromal tumorsAnn Oncol201021101990199820507881
- DemetriGDLe-CesneAvon MehrenMFinal results form a Phase III study of IPI-504 (retaspimycin hydrochloride) versus placebo in patients (pts) with gastrointestinal stromal tumors (GIST) following failure of kinase inhibitor therapiesAbstract presented at the Gastrointestinal Cancers SymposiumOrlando, FLJanuary 22–24, 2010
- WagnerAJMorganJAChughRInhibition of heat shock protein 90 (Hsp90) with the novel agent IPI-504 in metastatic GIST following failure of tyrosine kinase inhibitors (TKIs) or other sarcomas: clinical results from Phase I trialJ Clin Oncol200826Suppl 1510503
- BauerSHilgerRGrabellusFPhase I trial of panobinostat (P) and imatinib (IM) in patients with treatment-refractory gastrointestinal stromal tumors (GIST)J Clin Oncol201230 Suppl10032
- RautCPPosnerMDesaiJSurgical management of advanced gastrointestinal stromal tumors after treatment with targeted systemic therapy using kinase inhibitorsJ Clin Oncol200624152325233116710031
- MussiCRonellenfitschUJakobJPost-imatinib surgery in advanced/metastatic GIST: is it worthwhile in all patients?Ann Oncol201021240340819628568
- Al-BatranSEHartmannJTHeidelFFocal progression in patients with gastrointestinal stromal tumors after initial response to imatinib mesylate: a three-center-based study of 38 patientsGastric Cancer200710314515217922091
- DemetriGDvan OosteromATGarrettCREfficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trialLancet200636895441329133817046465
- JonesRLMcCallJAdamARadiofrequency ablation is a feasible therapeutic option in the multi modality management of sarcomaEur J Surg Oncol201036547748220060679
- DileoPRandhawaRVansonnenbergESafety and efficacy of percutaneous radio-frequency ablation (RFA) in patients (pts) with metastatic gastrointestinal stromal tumor (GIST) with clonal evolution of lesions refractory to imatinib mesylate (IM)J Clin Oncol200422Suppl 149024
- MiettinenMLasotaJGastrointestinal stromal tumors: review on morphology, molecular pathology, prognosis, and differential diagnosisArch Pathol Lab Med2006130101466147817090188
- FletcherCDBermanJJCorlessCDiagnosis of gastrointestinal stromal tumors: a consensus approachHum Pathol200233545946512094370
- MiettinenMLasotaJGastrointestinal stromal tumors: pathology and prognosis at different sitesSemin Diagn Pathol2006232708317193820
- GoldJSGonenMGutierrezADevelopment and validation of a prognostic nomogram for recurrence-free survival after complete surgical resection of localised primary gastrointestinal stromal tumour: a retrospective analysisLancet Oncol200910111045105219793678
- JoensuuHVehtariARiihimakiJRisk of recurrence of gastrointestinal stromal tumour after surgery: an analysis of pooled population-based cohortsLancet Oncol201213326527422153892
- DematteoRPBallmanKVAntonescuCRAdjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: a randomised, double-blind, placebo-controlled trialLancet200937396691097110419303137
- JoensuuHErikssonMSundby HallKOne vs three years of adjuvant imatinib for operable gastrointestinal stromal tumor: a randomized trialJAMA2012307121265127222453568
- [No authors listed]Gastrointestinal stromal tumors: ESMO clinical practice guidelines for diagnosis, treatment and follow-upAnn Oncol201223Suppl 7vii49vii5522997454
- CasaliPGFumagalliEGronchiAAdjuvant therapy of gastrointestinal stromal tumors (GIST)Curr Treat Options Oncol201213327728422743760
- ChenJGuoTZhangLCD133 and CD44 are universally overexpressed in GIST and do not represent cancer stem cell markersGenes Chromosomes Cancer201251218619522076958
- BlayJYLe CesneARay-CoquardIProspective multicentric randomized Phase III study of imatinib in patients with advanced gastrointestinal stromal tumors comparing interruption versus continuation of treatment beyond 1 year: the French Sarcoma GroupJ Clin Oncol20072591107111317369574
- EisenbergBLHarrisJBlankeCDPhase II trial of neoadjuvant/adjuvant imatinib mesylate (IM) for advanced primary and metastatic/recurrent operable gastrointestinal stromal tumor (GIST): early results of RTOG 0132/ACRIN 6665J Surg Oncol2009991424718942073
- WangDZhangQBlankeCDPhase II trial of neoadjuvant/adjuvant imatinib mesylate for advanced primary and metastatic/recurrent operable gastrointestinal stromal tumors: long-term follow-up results of Radiation Therapy Oncology Group 0132Ann Surg Oncol20121941074108022203182
- DoyonCSiderisLLeblancGLeclercYEBoudreauDDubePProlonged therapy with imatinib mesylate before surgery for advanced gastrointestinal stromal tumor results of a Phase II trialInt J Surg Oncol2012201276157623316352
- HohenbergerPLangerCWendtnerCMNeoadjuvant treatment of locally advanced GIST: results of APOLLON, a prospective, open label Phase II study in KIT- or PDGFRA-positive tumorsJ Clin Oncol201230 Suppl10031
- ReichardtPBlayJYBoukovinasIAdjuvant therapy in primary GIST: state-of-the-artAnn Oncol201223112776278122831984
- MarinDBazeosAMahonFXAdherence is the critical factor for achieving molecular responses in patients with chronic myeloid leukemia who achieve complete cytogenetic responses on imatinibJ Clin Oncol201028142381238820385986
- KerkelaRGrazetteLYacobiRCardiotoxicity of the cancer therapeutic agent imatinib mesylateNat Med200612890891616862153
- BermanENicolaidesMMakiRGAltered bone and mineral metabolism in patients receiving imatinib mesylateN Engl J Med2006354192006201316687713
- BermanEGirotraMChengCEffect of long term imatinib on bone in adults with chronic myelogenous leukemia and gastrointestinal stromal tumorsLeuk Res201337779079423473999
- HambergPde JongFABoonstraJGvan DoornJVerweijJSleijferSNon-islet-cell tumor induced hypoglycemia in patients with advanced gastrointestinal stromal tumor possibly worsened by imatinibJ Clin Oncol20062418e30e3116782905
- LuxMLRubinBPBiaseTLKIT extracellular and kinase domain mutations in gastrointestinal stromal tumorsAm J Pathol2000156379179510702394
- MiettinenMLasotaJGastrointestinal stromal tumors – definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosisVirchows Arch2001438111211213830
- ConcaEMirandaCColVDAre two better than one? A novel double-mutant KIT in GIST that responds to imatinibMol Oncol3212013 [Epub ahead of print.]
- RoskoskiRJrStructure and regulation of Kit protein-tyrosine kinase – the stem cell factor receptorBiochem Biophys Res Commun200533831307131516226710
- ConcaENegriTGronchiAActivate and resist: L576P-KIT in GISTMol Cancer Ther2009892491249519723893
- TamboriniEBonadimanLGrecoAA new mutation in the KIT ATP pocket causes acquired resistance to imatinib in a gastrointestinal stromal tumor patientGastroenterology2004127129429915236194
- DuensingAMedeirosFMcConartyBMechanisms of oncogenic KIT signal transduction in primary gastrointestinal stromal tumors (GISTs)Oncogene200423223999400615007386
- RossiFEhlersIAgostiVOncogenic Kit signaling and therapeutic intervention in a mouse model of gastrointestinal stromal tumorProc Natl Acad Sci USA200610334128431284816908864
- BairdKDavisSAntonescuCRGene expression profiling of human sarcomas: insights into sarcoma biologyCancer Res200565209226923516230383
- FrancisPNamlosHMMullerCDiagnostic and prognostic gene expression signatures in 177 soft tissue sarcomas: hypoxia-induced transcription profile signifies metastatic potentialBMC Genomics200787317359542
- AstolfiANanniniMPantaleoMAA molecular portrait of gastrointestinal stromal tumors: an integrative analysis of gene expression profiling and high-resolution genomic copy numberLab Invest20109091285129420548289
- LiFGrowneyJBattalagineLQiuSManleyPMonahanJThe effect combining the KIT inhibitor imatinib with the PI3K inhibitor BKM120 or the dual PI3K/mTOR inhibitor BEZ235 on the proliferation of gastrointestinal stromal tumor cell linesAbstract 2239 presented at the 103rd Annual Meeting of the American Association for Cancer ResearchChicago, ILMarch 31–April 4, 2012
- FlorisGWozniakASciotRA potent combination of the novel PI3K inhibitor, GDC-0941, with imatinib in gastrointestinal stromal tumor xenografts: long-lasting responses after treatment withdrawalClin Cancer Res201319362063023231951
- ChangBSYangTCibasESFletcherJAAn in vitro cytologic assay for evaluation of the KIT signaling pathway in gastrointestinal stromal tumorsMod Pathol200720557958317396139
- PantaleoMANicolettiGNanniCPreclinical evaluation of KIT/PDGFRA and mTOR inhibitors in gastrointestinal stromal tumors using small animal FDG PETJ Exp Clin Cancer Res20102917321192792
- DuensingAJosephNEMedeirosFProtein kinase C theta (PKCtheta) expression and constitutive activation in gastrointestinal stromal tumors (GISTs)Cancer Res200464155127513115289315
- OuWBZhuMJDemetriGDFletcherCDFletcherJAProtein kinase C-theta regulates KIT expression and proliferation in gastrointestinal stromal tumorsOncogene200827425624563418521081
- BauerSYuLKDemetriGDFletcherJAHeat shock protein 90 inhibition in imatinib-resistant gastrointestinal stromal tumorCancer Res200666189153916116982758
- SmythTVan LooyTCurryJEThe HSP90 inhibitor, AT13387, is effective against imatinib-sensitive and -resistant gastrointestinal stromal tumor modelsMol Cancer Ther20121181799180822714264
- FlorisGDebiec-RychterMWozniakAThe heat shock protein 90 inhibitor IPI-504 induces KIT degradation, tumor shrinkage, and cell proliferation arrest in xenograft models of gastrointestinal stromal tumorsMol Cancer Ther201110101897190821825009
- MuhlenbergTZhangYWagnerAJInhibitors of deacetylases suppress oncogenic KIT signaling, acetylate HSP90, and induce apoptosis in gastrointestinal stromal tumorsCancer Res200969176941695019706776
- EdrisBWillinghamSBWeiskopfKAnti-KIT monoclonal antibody inhibits imatinib-resistant gastrointestinal stromal tumor growthProc Natl Acad Sci USA201311093501350623382202
- HeinrichMCCorlessCLBlankeCDMolecular correlates of imatinib resistance in gastrointestinal stromal tumorsJ Clin Ocol: Official Journal of the American Society of Clinical OncologyOct 102006242947644774