Abstract
Biliary tract cancer (BTC) is a group of relatively rare tumors with a poor prognosis. The current standard of care consists of doublet chemotherapy (platinum plus gemcitabine); however, even with cytotoxic therapy, the median overall survival is less than 1 year. The genetic basis of BTC is now more clearly understood, allowing for the investigation of targeted therapy. Combinations of doublet chemotherapy with antiepidermal growth factor receptor agents have provided modest results in Phase II and Phase III setting, and responses with small molecule inhibitors are limited. Moving forward as we continue to characterize the genetic hallmarks of BTC, a stepwise, strategic, and cooperative approach will allow us to make progress when developing new treatments.
Introduction
Biliary tract cancers (BTC) encompass a group of tumors, generally of the adenocarcinoma histologic subtype, that include both intra- and extrahepatic cholangiocarcinoma and gallbladder carcinoma. The incidence of BTC varies greatly throughout the world, ranging from as low as 0.1–0.2 individuals per 100,000 in Australia to 96 individuals per 100,000 in Thailand,Citation1 and this rate is closely linked with the geographic distribution of risk factors. In the United States, it is estimated that more than 12,000 cases will be diagnosed in 2013,Citation2 and the incidence of intrahepatic cholangiocarcinoma specifically appears to be on the rise.Citation2,Citation3 The high mortality of this disease is due to its late presentation, as the majority of patients come to clinical attention with metastatic disease; thus, less than 15% of patients are candidates for potential curative surgery.Citation4
Risk factors can be stratified based on location within the biliary tree. Specific risks for gallbladder cancer include large symptomatic gallstones, obesity, and the combination of chronic infection with Salmonella typhi and cholelithiasis.Citation5 One of the strongest risk factors for cholangiocarcinoma is primary sclerosing cholangitis, with a lifetime risk in excess of 10%.Citation6 Additional risk factors include bile duct adenoma, Caroli’s disease, multiple biliary papillomatosis, and infection with parasites (ie, Opisthorchis viverrini in Southeast Asia, and Clonorchis sinensis in Japan).Citation7 Furthermore, diabetes and smoking are known independent risk factors for intrahepatic cholangiocarcinomaCitation8 in addition to viral hepatitis.Citation9 Gallbladder cancer is more common in females, whereas cholangiocarcinoma is seen more often in men and is likely secondary to the higher incidence of gallstones in females and primary sclerosing cholangitis in males. This review will highlight our current understanding of the genetic basis of BTC with an in-depth focus on emerging targeted therapies as defined by each genetic subtype.
Current standard of care
Historically, progress in the discovery of new chemotherapeutic regimens for advanced BTC has been slow. One of the first randomized trials showed an overall survival (OS) benefit with 5-fluorouracil/leucovorin and etoposide compared to best supportive care with a median OS of 6 months versus 2.5 months, respectively; P < 0.01.Citation10 As gemcitabine emerged as a treatment option for pancreatic cancer, providers begin to extrapolate its use to BTC, which was supported by Phase II trials of gemcitabine in advanced BTC, demonstrating response rates (RRs) greater than 20%.Citation11,Citation12 Combinations of cisplatin or oxaliplatin together with gemcitabine showed greater activity, as evidenced by comparatively improved RRs and progression-free survival (PFS) rates. This benefit of combination chemotherapy was firmly established by a randomized controlled trial of cisplatin and gemcitabine compared with gemcitabine alone.Citation13 In the largest randomized biliary tract trial to date,Citation13 402 patients were enrolled between 2002 and 2004. OS was significantly increased in the combination arm versus with the single agent, gemcitabine (11.7 months versus 8.1 months).Citation13 Based on these results, the combination of cisplatin and gemcitabine was established as the new standard of care in advanced, unresectable BTC. Additional clinical trials have evaluated oxaliplatin in combination with gemcitabine (GemOx) and have yielded similar PFS, OS, and RR to cisplatin/gemcitabine combinations.Citation14,Citation15 Given the favorable side effect profile, combined GemOx is a reasonable alternative.
Looking forward, ways to improve the outcome of patients with advanced disease may be include incorporating novel “targeted” agents with traditional chemotherapy in order to maximize treatment efficacy and minimize the potential for toxicity, resulting in improved quality of life.Citation16 This approach has been facilitated in other tumor types by understanding the genetic characteristics within each individual tumor that may predict responses to a defined molecular target. We will discuss the genetic features of BTC followed by the clinical trials attempting to capitalize on these mutations.
Genetic basis of BTC
The standard for genetic profiling of tumors is evolving. Previously, approaches for genotyping tumors were limited to single gene mutations or a select group of predefined mutations (ie, polymerase chain reaction, Sanger sequencing, and mass spectrometry-based assays). These techniques, however, come with limitations; among these is the fact that these techniques are insensitive for inactivating tumor suppressor mutations.Citation17 As global, unbiased approaches such as whole genome sequencing have been utilized across many tumor types, previously unrecognized mutations have been uncovered.Citation18,Citation19 Thus, such comprehensive methods have not been applied to BTC; however, many genetic mutations in this disease have been uncovered and will be summarized ().
Table 1 Common mutations in biliary tract cancer
The complete genome of a number of cancer subtypes has been sequenced including pancreatic, esophageal, and lung cancer.Citation18,Citation20,Citation21 With respect to cholangiocarcinoma, the complete spectrum of genetic mutations has yet to be defined, as the entire genome has not been sequenced. Efforts thus far have been limited to exome sequencing of the entire genome of eight well-characterized, liver fluke-associated tumors.Citation22 Within this panel of tumors, 206 somatic mutations were identified in 187 genes using Sanger sequencing. The frequency of the most commonly mutated genes were TP53 (44%), KRAS (17%) SMAD4 (17%), and MLL (15%), which is similar to the results found in pancreatic cancer sequencing.Citation22 Certainly, the limitation here is the inability to extrapolate results to other geographic regions where the incidence of liver fluke-associated cholangiocarcinoma is minimal.
Previous efforts analyzing the frequency of mutations in cholangiocarcinoma have established a wide range of alterations contributing to the heterogeneity of tumor pathogenesis.Citation4 Mutations of the important mitogen-activated protein kinase intracellular signaling cascade with key effectors, RAS and RAF, are altered in BTC. Reported rates of KRAS mutations range from 9%–54% in intrahepatic tumors and 10%–22% in extrahepatic samples.Citation23–Citation27 Mutations in gallbladder carcinoma tend to be less frequent at 3%–38%.Citation23,Citation25,Citation28
Directly downstream from RAS, B-RAF mutations have changed the scope of metastatic melanoma.Citation29,Citation30 The range of mutations in BTC varies from 0%–20%.Citation31,Citation32 One particular series found BRAF to be mutated in approximately 20% of patients in both gallbladder and intrahepatic carcinomas.Citation26 It should be noted that these particular mutations were found to be mutually exclusive of KRAS mutations.
The epidermal growth factor receptor (EGFR) and HER-2 NEU (v-erb-b2 erythroblastic leukemia viral oncogene homolog 2, neuro/glioblastoma derived oncogene homolog [avian], also known as HER-2/NEU) are both involved in the pathogenesis of BTC.Citation4 Overexpression of EGFR is found in 38%–100% of BTC.Citation33 EGFR mutations of the tyrosine kinase domain have been found in intrahepatic and extrahepatic tumors (5%–15%)Citation34–Citation36 and in gallbladder carcinoma (9%–38%).Citation33,Citation35,Citation36 The majority of these mutations were found in the gene sequence coding for the tyrosine kinase domain found in exon 21. The most common mutation in nonsmall cell lung carcinoma is found in codon 858; this study found only a silent nucleotide substitution in that codon.Citation35
Mutations of the PIK3CA/mammalian target of rapamycin signaling pathway have also been found in varying percentages of BTC samples, with frequencies ranging from 0%–33%.Citation37–Citation39
In addition to constitutively activated molecular pathways, dysregulated metabolic enzymes are a potential driver of oncogenesis. Somatic mutations in the isocitrate dehydrogenase 1 (IDH1) and isocitrate dehydrogenase 2 (IDH2) genes commonly found among human leukemia, glioblastoma, and sarcomaCitation40 have recently been identified in subsets of biliary tract tumors.Citation37,Citation40,Citation41IDH1 and IDH2 are nicotinamide adenine dinucleotide phosphate-dependent enzymes that catalyze the oxidative decarboxylation of isocitrate to alpha-ketoglutarate. These somatic mutations lead to disruptive enzyme activity, allowing alpha-ketoglutarate to be more effectively converted to 2-hydroxyglutarate.Citation42 Elevated levels of 2-hydroxyglutarate are hypothesized to promote carcinogenesis by competitively inhibiting enzymes that use alpha-ketoglutarate as a cofactor.Citation42 Emerging data have identified that these mutations also occur in BTC; in one series of 87 patients with BTC, IDH mutations were found in 23% of intrahepatic cholangiocarcinoma samples.Citation40 In another analysis of 94 tumors, mutations of IDH1 and IDH2 were found in 28% of intrahepatic samples, but only in 7% of extrahepatic tumors.Citation41 One could postulate that the anatomic location of the tumor correlates to the genetic subtype; intrahepatic tumors have higher rates of IDH1/IDH2 mutations.
Clinical trials with targeted agents
The field of oncology has witnessed tremendous growth with respect to molecular targeted therapy within the last 10–15 years. While there have been significant breakthroughs in leukemia, lung cancer, and most recently melanoma, progress in BTC has lagged. Here, we will review a few key randomized clinical trials followed by a number of single-arm Phase II studies evaluating targeted agents in the first and setting line; an overview is provided in .
Table 2 Phase II and Phase III clinical trials investigating targeted agents in BTC
EGFR
Both small molecule inhibitors of the kinase domain and antibodies targeting the extracellular components of EGFR have been evaluated in BTC. A randomized Phase III study conducted in South Korea investigated the combination of GemOx with or without erlotinib in advanced BTC.Citation43 A total of 268 patients were recruited from eleven tertiary hospitals from 2009–2010. Although not statistically significant, an increase in PFS was seen, from 4.2 months in the chemotherapy-alone arm to 5.8 months in the combination group. There was a statistically significant increase in objective RR (16% versus 30%; P = 0.005); however, OS was equal in both groups at 9.5 months. A predefined subset analysis of patients specifically with intrahepatic cholangiocarcinoma revealed a greater increase in PFS for patients in the GemOx + erlotinib arm, suggesting that these patients may benefit from the addition of erlotinib. Mutation analysis was performed on 60 patient samples with adequate deoxyribonucleic acid for analysis; twelve patients (43%) were found to have overexpression of EGFR and six (10%) were found to a have a KRAS mutation. Despite overexpression in limited samples, EGFR/KRAS is not an established predictive biomarker in BTC.
Cetuximab has long been approved for KRAS wild-type colon cancer, which raises interest in studying this antibody in BTC. A recently completed randomized Phase II trial (BINGO [A multicenter, randomized phase II trial of gemcitabine and oxaliplatin alone or in combination with biweekly Cetuximab in the first-line treatment of advanced biliary cancer]Citation44) was presented at American Society of Clinical Oncology 2012. From 2007–2009, 150 patients with advanced BTC were randomized to the combination of GemOx + cetuximab versus GemOx alone. The PFS and OS in the combination versus GemOx alone arm was 6.0 months versus 5.3 months, and 11.0 months versus 12.4 months, respectively. Furthermore, RRs in the GemOx + cetuximab arm were only 23% compared to 29% in the GemOx arm. Analysis of KRAS mutational status has just been completed; in the 91 patient samples adequate for deoxyribonucleic acid analysis, KRAS and BRAF mutations were found in 19% and 5% of patients, respectively.Citation44 There was no statistically significant prognostic or predictive impact with respect to the KRAS mutation status, potentially explaining, to at least some degree, the lack of OS benefit with the addition of cetuximab.
In a smaller single-arm Phase II trial,Citation45 30 patients with advanced BTC were treated with the combination of GemOx and cetuximab. Results were notable for an objective response in 19 patients (63%), including three of whom achieved a complete response. Notably, nine initially unresectable patients were able to undergo surgical resection after response to treatment. Analysis of the tumors sample revealed KRAS mutations in 3/30 (10%); two of these patients had a partial response and the third had stable disease.
A recent Phase II randomized trial investigated GemOx + cetuximab versus GemOx alone.Citation47 A total of 122 patients were enrolled; the PFS and OS in the GemOx + cetuximab arm versus the GemOx arm were 7.1 months versus 4.0 months, and 10.3 versus 8.8 months, respectively. Interestingly, subgroup analysis suggested that patients with KRAS mutated tumors derived benefit from cetuximab with increased PFS and OS of 7.0 months versus 1.9 months, and 10.3 months versus 6.6 months, respectively. This is surprising given the known and well established paradigms of KRAS mutation and its effect on EGFR therapy in colorectal cancer. It is possible that this result may be explained by the small sample size and potential variability in Phase II trials. An alternative explanation is that undiscovered features drive or contribute to response to anti-EGFR therapy in BTC as well.
Similar to the mechanism of action of cetuximab, panitumumab differs from cetuximab only in the fact that it is a fully humanized antibody as opposed to a chimeric antibody. A recent trialCitation48 evaluated the combination of GemOx, capecitabine, and panitumumab. A total of 46 patients were enrolled at a single institution; importantly, this was the first “KRAS marker driven trial,” excluding patients who are KRAS mutant.Citation48 The RR was 33%, the PFS was 8.3 months, and the median OS was 10 months.
The chemotherapeutic backbone of gemcitabine and irinotecan is much less studied in BTC; nonetheless, the combination was evaluated with panitumumab in a Phase II study that remains open for accrual.Citation46 To date 26/42 patients have been enrolled with nine objective responses, including three complete responses in 21 evaluable patients. The median OS is 12.7 months; there have been no treatment-related deaths.
VEGF
The vascular endothelial growth factor (VEGF) is the most potent angiogenic factor currently identified.Citation50 The entire family consists of six members, of which VEGF-A is the most extensively studied. Binding of VEGF to its receptor leads to activation of key downstream cellular signaling molecules. Approved in 2004, bevacizumab is indicated for use in the treatment of metastatic colon cancer,Citation51 which is in addition to more recent approvals for use in nonsmall cell lung cancer, glioblastoma multiforme, and renal cell carcinoma. Its role in BTC was somewhat unknown until a small study found that VEGF immunoreactivity is an independent and negative predictor of extrahepatic BTC.Citation52 Based on this rationale, the combination of GemOx and bevacizumab was evaluated in a Phase II trial. A total of 35 patients were evaluated; the objective RR was 40%, the median PFS was 7.0 months, and the OS was 12.7 months.Citation53 Despite the addition of bevacizumab, toxicity was quite manageable with no grade 3 or grade 4 bleeding events documented.
Sorafenib has been investigated as a first-line and second-line therapy in BTC. In a Phase II multi-institutional trial among 31 evaluable patients,Citation54 there were no confirmed objective responses. Ten patients (32%) were documented as having stable disease; the median PFS was 3 months and the OS was 9 months.Citation54 There was a high rate of grade 3/4 toxicities reported in this trial, including thromboembolism, hand–foot syndrome, and hyperbilirubinemia.
The use of sorafenib as a single agent after disease progression on standard therapies demonstrated minimal activity with an objective RR of 2%, a PFS of 2.3 months, and an OS of 4.4 months.Citation55
Sunitinib is yet another orally administered inhibitor of multiple tyrosine kinases, including VEGF, which has shown activity in a number of cancers including renal cell carcinoma. In an open label Phase II, single-arm, multicenter trial, sunitinib was evaluated as a second-line treatment.Citation56 A total of 56 patients were evaluated; the objective RR was 8.9%. The median duration of disease control was 2.4 months and the median OS was 4.8 months. Toxicity was notable for greater than 46% of patients experiencing a grade 3 or grade 4 adverse event; thus, combined with marginal efficacy, the role of sunitinib in BTC is limited.
MEK
The RAS/RAF/MEK/ERK pathway has been demonstrated to be constitutively activated in a wide variety of tumors including BTC.Citation57 Activated RAS triggers phosphorylation and activation of RAF kinase, which subsequently phosphorylates MEK 1 and MEK 2, leads to the activation of ERK-1 and ERK-2. Phosphorylated ERK translocates into the nucleus where it activates key cellular functions. As ERK-1 and ERK-2 are the only known MEK substrates, MEK has been identified as a logical target of inhibition, and in a number of studies, both in vitro and in vivo systems have established the importance of MEK as a cancer target.Citation58 Based on this rationale, a multi-institutional Phase II study evaluated the MEK inhibitor, selumetinib, in 28 patients as a second-line therapy in patients with metastatic BTC.Citation59 The overall RR was 12%; additionally, 17 patients (68%) had stable disease leading to a disease control rate of 80%. Furthermore, the median PFS was 3.7 months and the OS was 9.8 months, which are greater than the rates observed in historical controls.
HER-2/NEU
Yet another key extracellular receptor with genetic relevance to BTC is HER-2/NEU,Citation60 which is most extensively studied and clinically applicable in breast cancer. Lapatinib is a dual small molecule inhibitor of both HER-2/NEU and EGFR. A Phase II trial was performed in hepatocellular carcinoma and BTC with lapatinib as a single agent in the second line.Citation61 In the 17 patients with BTC, there were no objective responses seen; the median PFS was 1.8 months and the OS was 5.2 months.
Combinations
Both EGFR overexpression and angiogenesis have been associated with poor outcomes in BTC; thus, a combination approach was trialed in the Phase II setting as first-line therapy.Citation62 A total of 53 patients were evaluated with the combination of erlotinib and bevacizumab between 2006 and 2008. In 49 evaluable patients, the objective RR was 12% and median OS was 9.9 months. Compared to the standard of care, these results are underwhelming; however, there may be a role for future combinations with cytotoxic chemotherapy.
A promising novel combination is that of gemcitabine plus S-1, an oral drug that combines three pharmacological agents: tegafur, a prodrug of 5-fluorouracil; 5-chloro-2,4-dihydroxypyridine, which inhibits dihydroxypyridine dehydrogenase activity; and potassium oxonate, which reduces gastrointestinal toxicity.Citation63 In a Phase II trial, gemcitabine + S-1 (GS) was compared to single agent S-1.Citation64 A total of 51 patients were randomized to the combination arm and 50 patients were randomized to the S-1 arm from 2009 to 2010. The median PFS was 7.1 months and 4.2 months, and the OS was 12.5 months versus 9.5 months in the combination versus single-agent groups, respectively. Of note, two treatment-related deaths occurred in the GS arm. The authors concluded that GS warrants head-to-head comparisons with gemcitabine/cisplatin in a randomized Phase III trial.
In summary, there have been two randomized controlled trials with targeted agents, namely GemOx + erlotinib and GemOx + cetuximab with RRs nearing 30%; however, neither of these combination treatments led to a significant increase in OS. Phase II studies in the first- and second-line have yielded modest results,Citation65 highlighting the drive for emerging targets.
Future direction
Given the favorable RR of selumetinib in the second-line setting, additional testing of MEK inhibition in BTC is worthwhile. Last year, the MEK inhibitor trametinib was shown to have a statistically significant increase in OS in metastatic melanoma when compared to standard chemotherapy;Citation66 thus, early phase testing of this molecule in BTC is feasible. There are no completed trials investigating PIK3CA inhibition in BTC; however, two trials are evaluating inhibitors, BYL719 and SF1126.
Yet another promising oncologic target is c-met, which is a high-affinity receptor for hepatocyte growth factor. In addition, c-met activates the EGFR pathway, which is known to be upregulated in BTC, as discussed previously.Citation67 In one series, c-met was found to be expressed in 35% of cases.Citation68 In another series, immunohistochemical analysis of c-met in patient BTC samples revealed that tumors with a high level of c-met expression tended to have a worse prognosis.Citation67 C-met inhibitors are in development and vandetanib has been approved for advanced medullary thyroid cancer; these agents are yet to be evaluated in vivo in BTC.Citation69
Preclinical data using IDH1/IDH2 inhibitors are emerging in leukemia and malignant glioma; given the frequency of mutations in cholangiocarcinoma, this is promising.Citation70,Citation71 Additional targets and enrolling trials are depicted in and .
Figure 1 Pathways and targeted therapy in BTC.
Abbreviations: BTC, biliary tract cancer; PDGFR, platelet-derived growth factor receptor; EGFR, epidermal growth factor receptor; HER-2, human epidermal growth factor receptor 2; VEGFR, vascular endothelial growth factor receptor; AKT, protein kinase; mTOR, mammalian target of rapamycin; MEK, mitogen-activated protein kinase.
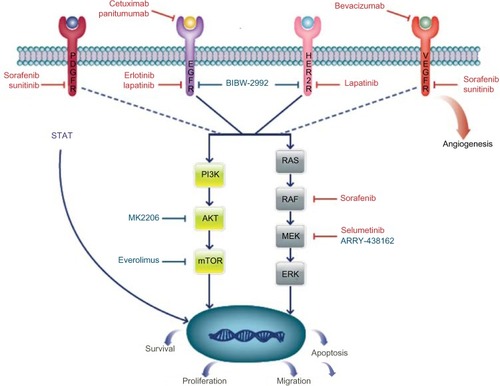
Table 3 Clinical trials currently enrolling in BTC
Conclusion
Treatment for advanced BTC is currently anchored by the backbone of platinum-based doublet chemotherapy, which has a proven survival benefit. The genetic heterogeneity of this disease combined with its lower incidence as compared with other more common genetically heterogeneous tumor types (lung, colon, and breast cancer) has hindered progress in the development of novel targeted therapy. Our efforts should be focused on identifying those patients who would best benefit from specific targeted agents in collaborative efforts. The first step is to more clearly define the complete mutational and genetic spectrum, which can be accomplished via whole genome efforts, as demonstrated in lung cancer.Citation72 From there, predictive biomarkers can be established in order to maximize clinical response for each individual patient, essentially defining the model of “personalized medicine.” A stepwise, strategic, and cooperative approach will allow us all to make progress when developing new treatments.
Disclosure
Aram F Hezel serves as a consultant to Amgen and Bayer, and receives research funding from Amgen. The other author reports no conflicts of interest in this work.
References
- ShaibYEl-SeragHBThe epidemiology of cholangiocarcinomaSemin Liver Dis200424211512515192785
- SiegelRNaishadhamDJemalACancer statistics, 2013CA Cancer J Clin2013631113023335087
- PatelTIncreasing incidence and mortality of primary intrahepatic cholangiocarcinoma in the United StatesHepatology20013361353135711391522
- HezelAFDeshpandeVZhuAXGenetics of biliary tract cancers and emerging targeted therapiesJ Clin Oncol201028213531354020547994
- De GroenPCGoresGJLaRussoNFGundersonLLNagorneyDMBiliary tract cancersN Engl J Med1999341181368137810536130
- KornfeldDEkbomAIhreTSurvival and risk of cholangiocarcinoma in patients with primary sclerosing cholangitis. A population-based studyScand J Gastroenterol19973210104210459361178
- FavaGLorenziniIMolecular pathogenesis of cholangiocarcinomaInt J Hepatol2012201263054321994887
- ChaiteerakijRYangJDHarmsenWSRisk factors for intrahepatic cholangiocarcinoma: association between metformin use and reduced cancer riskHepatology201357264865523055147
- YuTHYuanRHChenYLYangWCHsuHCJengYMViral hepatitis is associated with intrahepatic cholangiocarcinoma with cholangiolar differentiation and N-cadherin expressionMod Pathol201124681081921423153
- GlimeliusBHoffmanKSjödénPOChemotherapy improves survival and quality of life in advanced pancreatic and biliary cancerAnn Oncol1996765936008879373
- GallardoJORubioBFodorMA phase II study of gemcitabine in gallbladder carcinomaAnn Oncol200112101403140611762811
- PenzMKornekGVRadererMPhase II trial of two-weekly gemcitabine in patients with advanced biliary tract cancerAnn Oncol200112218318611300321
- ValleJWasanHPalmerDHABC-02 Trial InvestigatorsCisplatin plus gemcitabine versus gemcitabine for biliary tract cancerN Engl J Med2010362141273128120375404
- AndréTTournigandCRosmorducOGERCOR GroupGemcitabine combined with oxaliplatin (GEMOX) in advanced biliary tract adenocarcinoma: a GERCOR studyAnn Oncol20041591339134315319238
- PrachtMLe RouxGSulpiceLChemotherapy for inoperable advanced or metastatic cholangiocarcinoma: retrospective analysis of 78 cases in a single center over four yearsChemotherapy201258213414122572213
- ZaberniggAGiesingerJMPallGQuality of life across chemotherapy lines in patients with cancers of the pancreas and biliary tractBMC Cancer20121239022950826
- GarrawayLABaselgaJWhole-genome sequencing and cancer therapy: is too much ever enough?Cancer Discov20122976676822969114
- BiankinAVWaddellNKassahnKSAustralian Pancreatic Cancer Genome InitiativePancreatic cancer genomes reveal aberrations in axon guidance pathway genesNature2012491742439940523103869
- Cancer Genome Atlas NetworkComprehensive molecular characterization of human colon and rectal cancerNature2012487740733033722810696
- DulakAMStojanovPPengSExome and whole-genome sequencing of esophageal adenocarcinoma identifies recurrent driver events and mutational complexityNat Genet201345547848623525077
- VogelsteinBPapadopoulosNVelculescuVEZhouSDiazLAKinzlerKWCancer genome landscapesScience201333961271546155823539594
- OngCKSubimerbCPairojkulCExome sequencing of liver fluke-associated cholangiocarcinomaNat Genet201244669069322561520
- RashidAUekiTGaoYTK-ras mutation, p53 overexpression, and microsatellite instability in biliary tract cancers: a population-based study in ChinaClin Cancer Res20028103156316312374683
- SutoTHabanoWSugaiTAberrations of the K-ras, p53, and APC genes in extrahepatic bile duct cancerJ Surg Oncol200073315816310738270
- TannapfelABenickeMKatalinicAFrequency of p16(INK4A) alterations and K-ras mutations in intrahepatic cholangiocarcinoma of the liverGut200047572172711034592
- TannapfelASommererFBenickeMMutations of the BRAF gene in cholangiocarcinoma but not in hepatocellular carcinomaGut200352570671212692057
- WatanabeHDateKItoiTHistological and genetic changes in malignant transformation of gallbladder adenomaAnn Oncol199910Suppl 413613910436806
- HanadaKTsuchidaAIwaoTGene mutations of K-ras in gallbladder mucosae and gallbladder carcinoma with an anomalous junction of the pancreaticobiliary ductAm J Gastroenterol19999461638164210364037
- ChapmanPBHauschildARobertCBRIM-3 Study GroupImproved survival with vemurafenib in melanoma with BRAF V600E mutationN Engl J Med2011364262507251621639808
- CurtinJAFridlyandJKageshitaTDistinct sets of genetic alterations in melanomaN Engl J Med2005353202135214716291983
- PaiRKMojtahedKPaiRKMutations in the RAS/RAF/MAP kinase pathway commonly occur in gallbladder adenomas but are uncommon in gallbladder adenocarcinomasAppl Immunohistochem Mol Morphol201119213314021307665
- GoldenbergDRosenbaumEArganiPThe V599E BRAF mutation is uncommon in biliary tract cancersMod Pathol200417111386139115181454
- PignochinoYSarottoIPeraldo-NeiaCTargeting EGFR/HER2 pathways enhances the antiproliferative effect of gemcitabine in biliary tract and gallbladder carcinomasBMC Cancer20101063121087480
- WhelerJJFalchookGSTsimberidouAMAberrations in the epidermal growth factor receptor gene in 958 patients with diverse advanced tumors: implications for therapyAnn Oncol201324383884223139256
- LeoneFCavalloniGPignochinoYSomatic mutations of epidermal growth factor receptor in bile duct and gallbladder carcinomaClin Cancer Res20061261680168516551849
- GwakGYYoonJHShinCMDetection of response-predicting mutations in the kinase domain of the epidermal growth factor receptor gene in cholangiocarcinomasJ Cancer Res Clin Oncol20051311064965216032426
- VossJSHoltegaardLMKerrSEMolecular profiling of cholangiocarcinoma shows potential for targeted therapy treatment decisionsHum Pathol20134471216122223391413
- DeshpandeVNduagubaAZimmermanSMMutational profiling reveals PIK3CA mutations in gallbladder carcinomaBMC Cancer2011116021303542
- XuRFSunJPZhangSRKRAS and PIK3CA but not BRAF genes are frequently mutated in Chinese cholangiocarcinoma patientsBiomed Pharmacother2011651222621051183
- BorgerDRTanabeKKFanKCFrequent mutation of isocitrate dehydrogenase (IDH)1 and IDH2 in cholangiocarcinoma identified through broad-based tumor genotypingOncologist2012171727922180306
- KippBRVossJSKerrSEIsocitrate dehydrogenase 1 and 2 mutations in cholangiocarcinomaHum Pathol201243101552155822503487
- PrensnerJRChinnaiyanAMMetabolism unhinged: IDH mutations in cancerNat Med201117329129321383741
- LeeJParkSHChangHMGemcitabine and oxaliplatin with or without erlotinib in advanced biliary-tract cancer: a multicentre, open-label, randomised, phase 3 studyLancet Oncol201213218118822192731
- MalkaDCerveraPHeuteau-FoulonSGemcitabine and oxalipla-tin (GEMOX) alone or with cetuximab in first-line treatment of advanced biliary cancers (ABC): exploratory analyses according to tumor KRAS/BRAF mutations and EGFR expression in a randomized phase II trial (BINGO) [abstract]J Clin Oncol201331Suppl4127
- GruenbergerBSchuellerJHeubrandtnerUCetuximab, gemcitabine, and oxaliplatin in patients with unresectable advanced or metastatic biliary tract cancer: a phase 2 studyLancet Oncol201011121142114821071270
- SohalDavendraA phase II trial of gemcitabine, irinotecan, and panitumumab in advanced cholangiocarcinoma, with correlative analysis of EGFR, KRAS, and BRAF: An interim report2012 ASCO abstract
- ChenLTChenJSChaoYKRAS mutation status-stratified randomized phase II trial of GEMOX with and without cetuximab in advanced biliary tract cancer (ABTC): the TCOG T1210 trial [abstract]J Clin Oncol201331Suppl4018
- JensenLHLindebjergJPloenJHansenTFJakobsenAPhase II marker-driven trial of panitumumab and chemotherapy in KRAS wild-type biliary tract cancerAnn Oncol20122392341234622367707
- SohalDPMetzJMSunWToxicity study of gemcitabine, oxaliplatin, and bevacizumab, followed by 5-fluorouracil, oxaliplatin, bevacizumab, and radiotherapy, in patients with locally advanced pancreatic cancerCancer Chemother Pharmacol20137161485149123532207
- TolJPuntCJMonoclonal antibodies in the treatment of metastatic colorectal cancer: a reviewClin Ther201032343745320399983
- HurwitzHFehrenbacherLNovotnyWBevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancerN Engl J Med2004350232335234215175435
- HidaYMoritaTFujitaMVascular endothelial growth factor expression is an independent negative predictor in extrahepatic biliary tract carcinomasAnticancer Res1999193B2257226010472340
- ZhuAXMeyerhardtJABlaszkowskyLSEfficacy and safety of gemcitabine, oxaliplatin, and bevacizumab in advanced biliary-tract cancers and correlation of changes in 18-fluorodeoxyglucose PET with clinical outcome: a phase 2 studyLancet Oncol2010111485419932054
- El-KhoueiryABRankinCJBen-JosefESWOG 0514: a phase II study of sorafenib in patients with unresectable or metastatic gallbladder carcinoma and cholangiocarcinomaInvest New Drugs20123041646165121748296
- BengalaCBertoliniFMalavasiNSorafenib in patients with advanced biliary tract carcinoma: a phase II trialBr J Cancer20101021687219935794
- YiJHThongprasertSLeeJA phase II study of sunitinib as a second-line treatment in advanced biliary tract carcinoma: a multicentre, multinational studyEur J Cancer201248219620122176869
- LorussoPMAdjeiAAVarterasianMPhase I and pharmacodynamic study of the oral MEK inhibitor CI-1040 in patients with advanced malignanciesJ Clin Oncol200523235281529316009947
- YehTCMarshVBernatBABiological characterization of ARRY-142886 (AZD6244), a potent, highly selective mitogen-activated protein kinase kinase 1/2 inhibitorClin Cancer Res20071351576158317332304
- Bekaii-SaabTPhelpsMALiXMulti-institutional phase II study of selumetinib in patients with metastatic biliary cancersJ Clin Oncol201129172357236321519026
- NakazawaKDobashiYSuzukiSFujiiHTakedaYOoiAAmplification and overexpression of c-erbB-2, epidermal growth factor receptor, and c-met in biliary tract cancersJ Pathol2005206335636515892172
- RamanathanRKBelaniCPSinghDAA phase II study of lapatinib in patients with advanced biliary tree and hepatocellular cancerCancer Chemother Pharmacol200964477778319169683
- LubnerSJMahoneyMRKolesarJLReport of a multicenter phase II trial testing a combination of biweekly bevacizumab and daily erlotinib in patients with unresectable biliary cancer: a phase II Consortium studyJ Clin Oncol201028213491349720530271
- MiuraKShirasakaTYamaueHSasakiIS-1 as a core anticancer fluoropyrimidine agentExpert Opin Drug Deliv20129327328622235991
- MorizaneCOkusakaTMizusawaJRandomized phase II trial of gemcitabine plus S-1 combination therapy versus S-1 in advanced biliary tract cancer: results of the Japan Clinical Oncology Group study (JCOG0805) [abstract]J Clin Oncol2012Suppl 4255
- PhilipPAMahoneyMRAllmerCPhase II study of erlotinib in patients with advanced biliary cancerJ Clin Oncol200624193069307416809731
- FlahertyKTRobertCHerseyPMETRIC Study GroupImproved survival with MEK inhibition in BRAF-mutated melanomaN Engl J Med2012367210711422663011
- MiyamotoMOjimaHIwasakiMPrognostic significance of overexpression of c-Met oncoprotein in cholangiocarcinomaBr J Cancer2011105113113821673683
- AishimaSITaguchiKISugimachiKShimadaMSugimachiKTsuneyoshiMc-erbB-2 and c-Met expression relates to cholangiocarcinogenesis and progression of intrahepatic cholangiocarcinomaHistopathology200240326927811895493
- YoshikawaDOjimaHKokubuAVandetanib (ZD6474), an inhibitor of VEGFR and EGFR signalling, as a novel molecular-targeted therapy against cholangiocarcinomaBr J Cancer200910081257126619319137
- RohleDPopovici-MullerJPalaskasNAn inhibitor of mutant IDH1 delays growth and promotes differentiation of glioma cellsScience2013340613262663023558169
- WangFTravinsJDeLaBarreBTargeted inhibition of mutant IDH2 in leukemia cells induces cellular differentiationScience2013340613262262623558173
- KohnoTIchikawaHTotokiYKIF5B-RET fusions in lung adenocarcinomaNat Med201218337537722327624