Abstract
Pancreatic ductal adenocarcinoma is one of the most aggressive solid malignancies and is characterized by poor response to current therapy and a dismal survival rate. Recent insights regarding the role of cancer stem cells (CSCs) and epithelial–mesenchymal transition (EMT) in tumorigenesis have brought further understanding to the field and have highlighted new therapeutic targets. CSCs are a distinct subset of cancer cells, with the ability to differentiate into other cell types and self-renew in order to fuel the maintenance of tumor amplification. Transition of a cancer cell from an EMT leads to increased migratory and invasive properties, and thus facilitates initiation of metastasis. EMT is regulated by a complex network of factors that includes cytokines, growth factors, aberrant signaling pathways, transcription factors, and the tumor microenvironment. There is emerging evidence that the EMT process may give rise to CSCs, or at least cells with stem cell-like properties. We review the key pathways involved in both of these processes, the biomarkers used to identify CSCs, and new therapeutic approaches targeting CSCs and EMT in pancreatic ductal adenocarcinoma.
Background
Pancreatic ductal adenocarcinoma (PDAC) is one of the major causes of cancer death worldwide, responsible for an estimated 227,000 deaths each year. At the time of diagnosis, less than 20% of the patients diagnosed with PDAC present with localized disease amenable to surgical resection, while 40% present with locally advanced, unresectable disease; the remaining patients already suffer from distant metastases.Citation1,Citation2 Treatment strategies have not succeeded in significantly extending patient survival, and clinical outcome has not improved substantially over the past 35 years; the overall 5-year survival rate remains dismal, at around 5%.Citation3 The ability of PDAC to metastasize in early stages is a primary reason for its lethality. Evidence suggests that this process may be mediated by cancer stem cells (CSCs) as well as the ability of cells to undergo epithelial–mesenchymal transition (EMT).Citation4 Recent insights regarding the role of CSCs and EMT have brought further understanding to the field by identifying novel signaling pathways involved in tumorigenesis and tumor progression and have highlighted new potential therapeutic targets.
Cancer stem cells
CSCs have been identified as a distinct subset of cancer cells, with unique properties that differentiate them from the majority of cancer cells comprising a tumor. While unrestrained proliferation and resistance to apoptosis are hallmarks of cancer cells, CSCs are distinct in their ability to self-renew, differentiate into other cell types, and form tumors in immunodeficient mice.Citation5 These properties of CSCs fuel the maintenance of tumor amplification and tumor mass.Citation6,Citation7 This distinct population was initially identified in leukemias, with subsequent identification in solid malignancies of the breast, lung, prostate, colon, brain, head and neck, and liver, as well as in PDAC.Citation8–Citation13
Stem cell subpopulations in pancreatic cancer
Identification of CSCs is based upon the expression of cell surface molecules. These molecules, however, are not uniform across tumor types and are the topic of much debate. The first subpopulation of pancreatic CSCs was identified using knowledge of surface markers based on studies of tumorigenic breast cancer cells.Citation14 Li et al identified a subpopulation of cells derived from human tumors that had cell surface expression of CD44, CD24, and epithelial-specific antigen (ESA) ().Citation15 They demonstrated that while only 0.2%–0.8% of tumor cells had the CD44+CD24+ESA+ phenotype, injection of as few as 500 of these cells formed tumors that recapitulated the architecture of human PDAC from which they were derived.Citation15 While this phenotype has clearly been demonstrated to identify a CSC subpopulation in PDAC, the functional significance of these markers is not entirely clear. These proteins may facilitate cell–cell interactions, modulate signaling pathways (CD44 promotes c-MET activity and inhibits Hippo signaling; ESA upregulates c-myc and cyclin A/E) (), or they may be a byproduct of transcriptional networks regulating the stem-cell properties of the cells.Citation16–Citation21
Figure 1 EMT signaling pathways and CSC markers in pancreatic cancer.
Abbreviations: ALDH, aldehyde dehydrogenase 1; CSC, cancer stem cell; EMT, epithelial–mesenchymal transition; ESA, epithelial-specific antigen; HGF, hepatocyte growth factor.
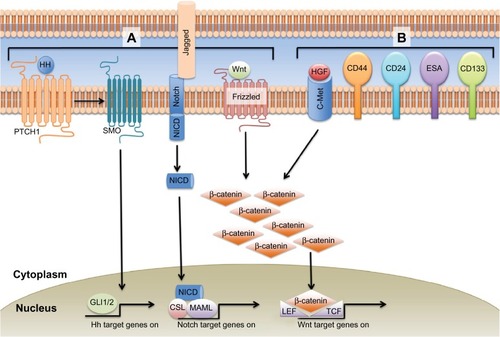
Subsequent studies have demonstrated that CD133+ cells isolated from human PDAC tumors were also highly tumorigenic and resistant to gemcitabine treatment.Citation22 Analysis of resected PDAC specimens from 80 patients revealed that CD133 expression was associated with a significantly lower 5-year survival rate (P = 0.0002) compared to tumor specimens that lacked CD133.Citation23 The function of CD133 is not known, but it is expressed in non-CSCs as well as CSCs in other malignancies.Citation24–Citation27 In normal pancreatic tissue, CXCR4 and its ligand stromal-derived factor-1 (SDF-1) are necessary for the maintenance of pancreatic ductal cell survival, proliferation, and migration during pancreatic organogenesis and regeneration.Citation28 In the CSC population, SDF-1 expressed on CD133+ cells with high metastatic potential, and it is also expressed at the invasive edge of tumors.Citation22 This suggests the existence of two distinct phenotypes of CSCs: stationary (eg, residing in the tumor bulk) and migratory (eg, metastatic cells).
Aldehyde dehydrogenase 1 (ALDH) expression has also been shown to identify PDAC cells with stem cell-like properties – high tumorigenic potential and characteristics of EMT.Citation28 Additionally, ALDH+ cells were identified in metastatic lesions arising from primary tumors without ALDH expression, and ALDH+ staining of the primary tumor was associated with a decreased median survival (14 versus 18 months, P = 0.05). In vitro studies have since demonstrated that ALDH+ cell populations have significantly enhanced tumorigenicity while ALDH+CD44+CD24+ cells did not exhibit this property, highlighting the fact that there is currently no universal marker that identifies pancreatic CSCs.Citation29 The function of ALDH in regulating CSCs is not known, but in normal tissues it plays a central role in ethanol and cyclophosphamide metabolism as well as in retinoic acid biosynthesis.Citation30
Another potential marker of pancreatic CSCs is c-Met, a receptor tyrosine kinase for hepatocyte growth factor (HGF) that has been shown to promote chemoresistance and malignancy ().Citation31 Initial work by Li et al based on the observation that c-Met is expressed in normal mouse pancreatic stem cells led to the discovery that c-Met-expressing cells were as tumorigenic as CD44+CD24+ESA+ cells, and also more tumorigenic than CD133+ cells.Citation32 Of the various subpopulations of cells, they found that CD44+ cells with high expression of c-Met had the highest tumorigenic potential and formed tumors that recapitulated the histology of the tumors that they were derived from.
While much attention has been drawn to the markers that identify CSCs, the role of signaling pathway alterations and the tumor microenvironment in CSC differentiation and function are also being actively explored. Pathways that mediate EMT, such as Notch, Wnt and Hedgehog, also may play a role in CSC function and maintenance in PDAC (), although the mechanisms of these interactions are not entirely clear.Citation33–Citation36 Signal transducer and activator of transcription 3 (STAT3) is a transcription factor that regulates many cellular processes, and has been shown to be required for maintenance of stem cell-like characteristics in other cancers, such as glioblastoma.Citation37–Citation39 The interactions that occur within the tumor microenvironment, however, also have an important role in promoting and maintaining tumorigenesis and the CSC population. The tumor microenvironment is comprised of many different cell compartments, including stellate cells, inflammatory cells, endothelial cells, and tumor cells, which are themselves a heterogenous population comprising CSCs and more differentiated lineages derived from them. One example of the interaction between CSCs and stellate cells, also important in embryogenesis, is through Activin and Nodal, members of the transforming growth factor β (TGFβ) family. CD133+ CSCs express high levels of Activin and Nodal, while blockade of the Nodal/Activin receptor Alk4/7 reverses the intrinsic chemoresistance to gemcitabine observed in this cell population.Citation40 Stellate cells have also been demonstrated to produce Activin and Nodal, which results in increased invasiveness of CSCs.Citation41 There is clear evidence, then, that the CSC niche is but one factor driving treatment resistance that characterizes PDAC.
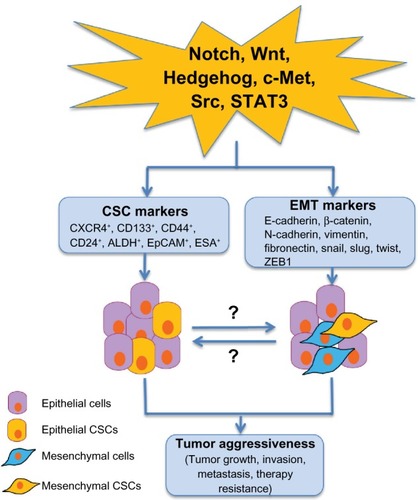
Figure 2 Potential cellular signaling pathways linking to pancreatic CSCs and EMT in tumor aggressiveness.
Abbreviations: CSC, cancer stem cell; EMT, epithelial–mesenchymal transition; STAT3, signal transducer and activator of transcription 3; PDAC, pancreatic ductal adenocarcinoma.
EMT in pancreatic cancer
Transition of a cancer cell from an epithelial to mesenchymal morphology leads to increased migratory and invasive properties, and thus facilitates initiation of metastasis.Citation42 This process is utilized in embryonic processes, such as gastrulation, in order to allow partial or complete transition between epithelial and mesenchymal phenotypes. A key feature of EMT is decreased expression of E-cadherin, a transmembrane cell adhesion molecule necessary for maintenance of intercellular contacts and cellular polarity in epithelial tissue, and increased expression of one or more of the mesenchymal markers vimentin, fibronectin, and N-cadherin. Additionally, the loss of E-cadherin expression in PDAC tumors is associated with larger tumors, distant metastases, and increased stage.Citation43
We have previously characterized PDAC cell lines as sensitive or chemoresistant based on IC50 values to chemotherapeutics, and also characterized the expression of EMT markers within these cell lines.Citation44 BxPC3 cells, which are treatment sensitive, express high levels of E-cadherin and β-catenin, but have reduced expression of N-cadherin and vimentin. MIApaca2 and PANC1, which are more resistant cell lines, have increased expression of N-cadherin and vimentin and diminished expression of E-cadherin and β-catenin, indicating that a mesenchymal phenotype may be associated with chemoresistance.
The EMT process is regulated by a complex network of factors that includes cytokines, growth factors, aberrant signaling pathways, transcription factors, and the tumor microenvironment. TGFβ expression downregulates E-cadherin, and platelet-derived TGFβ has been demonstrated to cause transition to an invasive mesenchmyal phenotype with enhanced metastatic capability in vivo.Citation45,Citation46
The Src family of tyrosine kinases plays a key role in the development of PDAC. Src is overexpressed in up to 70% of pancreatic cancers.Citation47–Citation49 Src has been shown to play a role in E-cadherin regulation and EMT ().Citation50 Src activates STAT3 signaling and promotes STAT3 mediated tumor progression and communication within the tumor microenvironment. Constitutively active STAT3 activates the Twist promoter through epidermal growth factor receptor activation and is an important inducer of EMT in cancer cells.Citation51 Activation of the Notch pathway decreases E-cadherin expression through upregulation of Slug, while Wnt pathway activation induces EMT either directly or through TGFβ and other pathways ().Citation52–Citation54 The Hedgehog pathway is an important embryonic pathway that is associated with many malignancies, including PDAC.Citation55 Interaction between the Hedgehog and EMT pathways results in increased invasion, proliferation, and tumor progression, as well as in the promotion of CSCs ().Citation53,Citation56–Citation58
The transcription factors Snail, Slug, Twist and ZEB1 play a central role in the induction of EMT and downregulation of E-cadherin.Citation59 Upregulation of Snail in highly metastatic PDAC cell lines mediates inactivation of E-cadherin and induces EMT through a transcriptional repressor complex containing Snail and histone deacetylase 1 and 2.Citation60 Snail, which has high to moderate expression in 78% of PDAC specimens, appears to be a highly relevant mediator of E-cadherin repression, metastasis promotion, and chemoresistance in PDAC.Citation61,Citation62 ZEB1 is a target gene of Snail that represses the expression of E-cadherin through binding of E-boxes in the promoter region, and also through regulation of microRNAs specific for genes relevant in metastasis and migration of CSCs.Citation63 Twist1 is an inducer of EMT, and Tsai et al demonstrated that while activation of Twist1 increases the number of circulating tumor cells, downregulation of Twist1 led to increased proliferation and a higher number of metastases in an in vivo mouse model of skin cancer.Citation64 These results indicate that while EMT promotes stem cell-like behavior and dissemination, reversion to an epithelial cell-type may be required to form metastases. Ocana et al identified homeobox factor Prrx1 as an EMT activator that is coexpressed and cooperative with Twist1, but that also suppresses stemness. The loss of Prrx1 was required for metastases in vivo, but in this model EMT and stemness were uncoupled, thus illustrating the complexity of the relationship between EMT and CSCs.Citation65
The tumor microenvironment of PDAC is characterized by a dense desmoplastic reaction, also known as stroma, which leads to hypoxia and poor tissue perfusion. Hypoxia leads to upregulation of hypoxia inducible factor 1α, which leads to increased expression of Twist.Citation66,Citation67 Additionally, the matrix proteins secreted by pancreatic stellate cells, which regulate the fibrotic response, lead to the promotion of EMT and may contribute significantly to metastases.Citation68–Citation71 Rather than passively laying down stromal proteins, stellate cells are thought to play an active role in establishing the metastatic site, promoting angiogenesis through secretion of vascular endothelial growth factor, and promoting self-renewal of CSC through secretion of HGF, as well as Activin and Nodal.Citation72,Citation73
EMT-induced CSCs in pancreatic cancer
There is emerging evidence that the EMT program may give rise to CSCs, or at least cells with stem cell-like properties. As outlined above, CSCs exhibit activation of many pathways involved in EMT. In breast cancer, activation of these EMT-related pathways results in increased invasiveness and metastatic potential.Citation74 Mani et al were able to induce expression of stem cell markers by either conditionally overexpressing the EMT-inducing transcription factors Snail or Twist, or by exposing the cells to TGF-β1.Citation75 In addition to expression of stem cell markers, these cells also adopted a mesenchymal phenotype and grew into mammospheres more effectively, suggesting that EMT is able to induce the characteristics associated with stem cells. This work identified the possibility that EMT also may have a direct relationship with CSCs, and may induce the CSC phenotype and enhance metastatic ability. Rhim et al have since demonstrated that circulating tumor cells, which are thought to require EMT to extravasate into the blood stream, have a 100-fold increase in CD24+CD44+ expression compared to the source pancreas.Citation76
Targeting CSCs and EMT in pancreatic cancer
Targeting CSCs has been shown to be difficult in other malignancies. To evaluate treatment response in chronic myeloid leukemia, Graham et al showed in peripheral blood samples that even when a significant proportion of dividing cells were killed with chemotherapy, CSCs were able to survive and remain viable in a quiescent state to potentially cause relapse at a later date.Citation77 Therapeutic strategies do not specifically target populations of cancer cells, but rather aim to debulk tumors either through cytotoxicity or through targeted inhibition of key signaling pathways. Due to the intrinsic chemoresistance of CSCs, monotherapy with cytotoxic agents, such as gemcitabine, has actually been demonstrated to increase the relative proportion of c-Met+ CSCs.Citation32 Newer agents that target pathways or genes that are upregulated specifically in CSCs, such as c-MET or Alk-4/7, have been shown to reduce the number of CSCs and tumor burden in vivo.Citation32,Citation40 Targeting of the stroma with a hedgehog pathway inhibitor in addition to cytotoxic gemcitabine and targeted inhibition of Alk4/7 resulted in a synergistic response in vivo, as defined by progression-free survival and enhanced drug delivery.Citation40
Other potential targets include the pathways mediating EMT, such as Notch, Wnt, Hedgehog, Src, and TGFβ. Other compounds, such as Silibinin, target EMT-inducing transcription factors, such as Zeb1, directly.Citation78 While there are many clinical trials exploring the possibility of targeting CSCs and/or EMT-related pathways, this treatment strategy is in early stages of development. Other intriguing possibilities include immunotherapy directed against CSC markers, such as ALDH, but this is complicated by the fact that many of these markers can be found on normal stem cells in the hematopoietic system.Citation79
Conclusion
The discovery and characterization of CSCs in PDAC and recent advances in understanding the role of EMT and the tumor microenvironment have led to a greater understanding of tumor behavior and prognosis. Beyond characterization of cell surface markers, however, there is still much that remains unknown about CSCs, including the mechanisms they utilize to maintain their innate chemoresistance. If targeting CSCs is to become a viable strategy, then an understanding of the key signaling pathways involved in CSC maintenance and activity is required. Additionally, understanding the role of EMT in maintaining this niche could aid in the design of therapeutic strategies. While there is currently no effective treatment for PDAC, our increasing understanding of the disease components brings hope for new strategies to target the cells and processes that make PDAC such a difficult disease to treat.
Acknowledgments
This work was supported by the National Institutes of Health Grants CA161976, Digestive Disease Research Center (DDRC) Translational Award 5P30DK058404-08, and Vanderbilt-Ingram Cancer Center Support Grant 5P30 CA068485-1 (all to Nipun B Merchant), and DDRC Grant P30 DK058404 (to Nagaraj S Nagathihalli).
Disclosure
The authors report no conflict of interest in this work.
References
- BarugolaGFalconiMBettiniRThe determinant factors of recurrence following resection for ductal pancreatic cancerJOP20078Suppl 113214017228145
- GriffinJFSmalleySRJewellWPatterns of failure after curative resection of pancreatic carcinomaCancer199066156612354408
- SiegelRNaishadhamDJemalACancer statistics, 2013CA Cancer J Clin12013631113023335087
- LiYKongDAhmadABaoBSarkarFHPancreatic cancer stem cells: Emerging target for designing novel therapyCancer Lett201233819410022445908
- HanahanDWeinbergRAThe hallmarks of cancerCell20001001577010647931
- CleversHThe cancer stem cell: premises, promises and challengesNat Med201117331331921386835
- ClarkeMFDickJEDirksPBCancer stem cells – perspectives on current status and future directions: AACR Workshop on cancer stem cellsCancer Res200666199339934416990346
- EppertKTakenakaKLechmanERStem cell gene expression programs influence clinical outcome in human leukemiaNat Med20111791086109321873988
- TangCAngBTPervaizSCancer stem cell: target for anti-cancer therapyFASEB J200721143777378517625071
- MaSChanKWHuLIdentification and characterization of tumorigenic liver cancer stem/progenitor cellsGastroenterology200713272542255617570225
- O’BrienCAPollettAGallingerSDickJEA human colon cancer cell capable of initiating tumour growth in immunodeficient miceNature2007445712310611017122772
- KimCFJacksonELWoolfendenAEIdentification of bronchioalveolar stem cells in normal lung and lung cancerCell2005121682383515960971
- LeeCJDoschJSimeoneDMPancreatic cancer stem cellsJ Clin Oncol200826172806281218539958
- Al-HajjMWichaMSBenito-HernandezAMorrisonSJClarkeMFProspective identification of tumorigenic breast cancer cellsProc Natl Acad Sci U S A200310073983398812629218
- LiCHeidtDGDalerbaPIdentification of pancreatic cancer stem cellsCancer Res20076731030103717283135
- AbelEVSimeoneDMBiology and clinical applications of pancreatic cancer stem cellsGastroenterology201314461241124823622133
- LitvinovSVVeldersMPBakkerHAFleurenGJWarnaarSOEp-CAM: a human epithelial antigen is a homophilic cell-cell adhesion moleculeJ Cell Biol199412524374468163559
- AignerSSthoegerZMFogelMCD24, a mucin-type glycoprotein, is a ligand for P-selectin on human tumor cellsBlood1997899338533959129046
- St JohnTMeyerJIdzerdaRGallatinWMExpression of CD44 confers a new adhesive phenotype on transfected cellsCell199060145522403843
- van der VoortRTaherTEWielengaVJHeparan sulfate-modified CD44 promotes hepatocyte growth factor/scatter factor-induced signal transduction through the receptor tyrosine kinase c-MetJ Biol Chem1999274106499650610037743
- MünzMKieuCMackBSchmittBZeidlerRGiresOThe carcinoma-associated antigen EpCAM upregulates c-myc and induces cell proliferationOncogene200423345748575815195135
- HermannPCHuberSLHerrlerTDistinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancerCell Stem Cell20071331332318371365
- MaedaSShinchiHKuraharaHCD133 expression is correlated with lymph node metastasis and vascular endothelial growth factor-C expression in pancreatic cancerBr J Cancer20089881389139718349830
- ShmelkovSVSt ClairRLydenDRafiiSAC133/CD133/Prominin-1Int J Biochem Cell Biol200537471571915694831
- SinghSKClarkeIDTerasakiMIdentification of a cancer stem cell in human brain tumorsCancer Res200363185821582814522905
- FrankNYMargaryanAHuangYABCB5-mediated doxorubicin transport and chemoresistance in human malignant melanomaCancer Res200565104320433315899824
- MikiJFurusatoBLiHIdentification of putative stem cell markers, CD133 and CXCR4, in hTERT-immortalized primary nonmalignant and malignant tumor-derived human prostate epithelial cell lines and in prostate cancer specimensCancer Res20076773153316117409422
- RasheedZAYangJWangQPrognostic significance of tumorigenic cells with mesenchymal features in pancreatic adenocarcinomaJ Natl Cancer Inst2010102534035120164446
- KimMPFlemingJBWangHALDH activity selectively defines an enhanced tumor-initiating cell population relative to CD133 expression in human pancreatic adenocarcinomaPLoS ONE201166e2063621695188
- RasheedZAMatsuiWBiological and clinical relevance of stem cells in pancreatic adenocarcinomaJ Gastroenterol Hepatol201227Suppl 2151822320910
- GherardiEBirchmeierWBirchmeierCVande WoudeGTargeting MET in cancer: rationale and progressNat Rev Cancer20121228910322270953
- LiCWuJJHynesMc-Met is a marker of pancreatic cancer stem cells and therapeutic targetGastroenterology2011141622182227. e521864475
- BrabletzSBajdakKMeidhofSThe ZEB1/miR-200 feedback loop controls Notch signalling in cancer cellsEMBO J201130477078221224848
- MizumaMRasheedZAYabuuchiSThe gamma secretase inhibitor MRK-003 attenuates pancreatic cancer growth in preclinical modelsMol Cancer Ther20121191999200922752426
- WangLHeidtDGLeeCJOncogenic function of ATDC in pancreatic cancer through Wnt pathway activation and beta-catenin stabilizationCancer Cell200915320721919249679
- MuellerMTHermannPCWitthauerJCombined targeted treatment to eliminate tumorigenic cancer stem cells in human pancreatic cancerGastroenterology200913731102111319501590
- GuryanovaOAWuQChengLNonreceptor tyrosine kinase BMX maintains self-renewal and tumorigenic potential of glioblastoma stem cells by activating STAT3Cancer Cell201119449851121481791
- SherryMMReevesAWuJKCochranBHSTAT3 is required for proliferation and maintenance of multipotency in glioblastoma stem cellsStem Cells200927102383239219658181
- KimEKimMWooDHPhosphorylation of EZH2 activates STAT3 signaling via STAT3 methylation and promotes tumorigenicity of glioblastoma stem-like cellsCancer Cell201323683985223684459
- LonardoEHermannPCMuellerMTNodal/Activin signaling drives self-renewal and tumorigenicity of pancreatic cancer stem cells and provides a target for combined drug therapyCell Stem Cell20119543344622056140
- LonardoEFrias-AldeguerJHermannPCHeeschenCPancreatic stellate cells form a niche for cancer stem cells and promote their self-renewal and invasivenessCell Cycle20121171282129022421149
- KaramitopoulouETumor budding cells, cancer stem cells and epithelial-mesenchymal transition-type cells in pancreatic cancerFront Oncol2012220923316479
- ShinSJKimKOKimMKExpression of E-cadherin and uPA and their association with the prognosis of pancreatic cancerJpn J Clin Oncol200535634234815937032
- NagarajNSWashingtonMKMerchantNBCombined blockade of Src kinase and epidermal growth factor receptor with gemcitabine overcomes STAT3-mediated resistance of inhibition of pancreatic tumor growthClin Cancer Res201117348349321266529
- LabelleMBegumSHynesRODirect signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasisCancer Cell201120557659022094253
- BardeesyNChengKHBergerJHSmad4 is dispensable for normal pancreas development yet critical in progression and tumor biology of pancreas cancerGenes Dev200620223130314617114584
- ShieldsDJMurphyEADesgrosellierJSOncogenic Ras/Src cooperativity in pancreatic neoplasiaOncogene201130182123213421242978
- LutzMPEsserIBFlossmann-KastBBOverexpression and activation of the tyrosine kinase Src in human pancreatic carcinomaBiochem Biophys Res Commun199824325035089480838
- NagarajNSSmithJJRevettaFWashingtonMKMerchantNBTargeted inhibition of SRC kinase signaling attenuates pancreatic tumorigenesisMol Cancer Ther2010982322233220682659
- NagathihalliNSMerchantNBSrc-mediated regulation of E-cadherin and EMT in pancreatic cancerFront Biosci (Landmark Ed)2012172059206922652764
- LoHWHsuSCXiaWEpidermal growth factor receptor cooperates with signal transducer and activator of transcription 3 to induce epithelial-mesenchymal transition in cancer cells via up-regulation of TWIST gene expressionCancer Res200767199066907617909010
- LeongKGNiessenKKulicIJagged1-mediated Notch activation induces epithelial-to-mesenchymal transition through Slug-induced repression of E-cadherinJ Exp Med2007204122935294817984306
- FuxeJVincentTGarcia de HerrerosATranscriptional crosstalk between TGF-β and stem cell pathways in tumor cell invasion: role of EMT promoting Smad complexesCell Cycle20109122363237420519943
- ZhouBPHungMCWnt, hedgehog and snail: sister pathways that control by GSK-3beta and beta-Trcp in the regulation of metastasisCell Cycle20054677277615917668
- ThayerSPdi MaglianoMPHeiserPWHedgehog is an early and late mediator of pancreatic cancer tumorigenesisNature2003425696085185614520413
- VarnatFDuquetAMalerbaMHuman colon cancer epithelial cells harbour active HEDGEHOG-GLI signalling that is essential for tumour growth, recurrence, metastasis and stem cell survival and expansionEMBO Mol Med200916–733835120049737
- FeldmannGDharaSFendrichVBlockade of hedgehog signaling inhibits pancreatic cancer invasion and metastases: a new paradigm for combination therapy in solid cancersCancer Res20076752187219617332349
- DembinskiJLKraussSCharacterization and functional analysis of a slow cycling stem cell-like subpopulation in pancreas adenocarcinomaClin Exp Metastasis200926761162319421880
- PeinadoHOlmedaDCanoASnail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype?Nat Rev Cancer20077641542817508028
- von BurstinJEserSPaulMCE-cadherin regulates metastasis of pancreatic cancer in vivo and is suppressed by a SNAIL/HDAC1/HDAC2 repressor complexGastroenterology20091371361371371. e119362090
- HotzBArndtMDullatSBhargavaSBuhrHJHotzHGEpithelial to mesenchymal transition: expression of the regulators snail, slug, and twist in pancreatic cancerClin Cancer Res200713164769477617699854
- YinTWangCLiuTZhaoGZhaYYangMExpression of snail in pancreatic cancer promotes metastasis and chemoresistanceJ Surg Res2007141219620317583745
- WellnerUSchubertJBurkUCThe EMT-activator ZEB1 promotes tumorigenicity by repressing stemness-inhibiting microRNAsNat Cell Biol200911121487149519935649
- TsaiJHDonaherJLMurphyDAChauSYangJSpatiotemporal regulation of epithelial-mesenchymal transition is essential for squamous cell carcinoma metastasisCancer Cell201222672573623201165
- OcañaOHCórcolesRFabraAMetastatic colonization requires the repression of the epithelial-mesenchymal transition inducer Prrx1Cancer Cell201222670972423201163
- SatohKHamadaSKimuraKUp-regulation of MSX2 enhances the malignant phenotype and is associated with twist 1 expression in human pancreatic cancer cellsAm J Pathol2008172492693918349132
- SunSNingXZhangYHypoxia-inducible factor-1alpha induces Twist expression in tubular epithelial cells subjected to hypoxia, leading to epithelial-to-mesenchymal transitionKidney Int200975121278128719279556
- MasamuneAWatanabeTKikutaKShimosegawaTRoles of pancreatic stellate cells in pancreatic inflammation and fibrosisClin Gastroenterol Hepatol20097Suppl 11S48S5419896099
- ErkanMAdlerGApteMVStellaTUM: current consensus and discussion on pancreatic stellate cell researchGut201261217217822115911
- BachemMGSchünemannMRamadaniMPancreatic carcinoma cells induce fibrosis by stimulating proliferation and matrix synthesis of stellate cellsGastroenterology2005128490792115825074
- KannoASatohKMasamuneAPeriostin, secreted from stromal cells, has biphasic effect on cell migration and correlates with the epithelial to mesenchymal transition of human pancreatic cancer cellsInt J Cancer2008122122707271818381746
- XuZVonlaufenAPhillipsPARole of pancreatic stellate cells in pancreatic cancer metastasisAm J Pathol201017752585259620934972
- MasamuneAKikutaKWatanabeTSatohKHirotaMShimosegawaTHypoxia stimulates pancreatic stellate cells to induce fibrosis and angiogenesis in pancreatic cancerAm J Physiol Gastrointest Liver Physiol20082954G709G71718669622
- HugoHAcklandMLBlickTEpithelial–mesenchymal and mesenchymal–epithelial transitions in carcinoma progressionJ Cell Physiol2007213237438317680632
- ManiSAGuoWLiaoMJThe epithelial-mesenchymal transition generates cells with properties of stem cellsCell2008133470471518485877
- RhimADMirekETAielloNMEMT and dissemination precede pancreatic tumor formationCell20121481–234936122265420
- GrahamSMJørgensenHGAllanEPrimitive, quiescent, Philadelphia-positive stem cells from patients with chronic myeloid leukemia are insensitive to STI571 in vitroBlood200299131932511756187
- WuKZengJLiLSilibinin reverses epithelial-to-mesenchymal transition in metastatic prostate cancer cells by targeting transcription factorsOncol Rep20102361545155220428808
- VisusCWangYLozano-LeonATargeting ALDH(bright) human carcinoma-initiating cells with ALDH1A1-specific CD8+ T cellsClin Cancer Res201117196174618421856769