Abstract
The chemokine CXCL12 (SDF-1) and its cell surface receptor CXCR4 were first identified as regulators of lymphocyte trafficking to the bone marrow. Soon after, the CXCL12/CXCR4 axis was proposed to regulate the trafficking of breast cancer cells to sites of metastasis. More recently, it was established that CXCR4 plays a central role in cancer cell proliferation, invasion, and dissemination in the majority of malignant diseases. The stem cell concept of cancer has revolutionized the understanding of tumorigenesis and cancer treatment. A growing body of evidence indicates that a subset of cancer cells, referred to as cancer stem cells (CSCs), plays a critical role in tumor initiation, metastatic colonization, and resistance to therapy. Although the signals generated by the metastatic niche that regulate CSCs are not yet fully understood, accumulating evidence suggests a key role of the CXCL12/CXCR4 axis. In this review we focus on physiological functions of the CXCL12/CXCR4 signaling pathway and its role in cancer and CSCs, and we discuss the potential for targeting this pathway in cancer management.
Introduction to the CXCL12/CXCR4 axis
Chemokines are a class of small (8–10 kDa) inflammatory or homeostatic cytokines sharing a common biological activity in stimulating the migration of different types of cells including lymphocytes, monocytes, neutrophils, endothelial cells, mesenchymal stem cells, and malignant epithelial cells.Citation1,Citation2 Chemokines are classified into four conserved groups – CXC, CC, C, and CX3C – based on the number and spacing of their N-terminal cysteine residues: CXC chemokines have a single nonconserved amino acid residue (X) between the first N-terminal cysteine residues (C); CC chemokines have these two cysteine residues adjacent; C chemokines have only one N-terminal cysteine; whereas CX3C chemokines contain three nonconserved amino acid residues separating the N-terminal cysteine pair. More than 50 chemokines have been discovered so far.Citation1,Citation3 The classification of the chemokine receptors is based on the type of their ligands. For example, CXC receptors bind CXC ligands, while CC receptors bind CC ligands, etc.Citation3
To date, over 20 chemokine receptors have been identified.Citation1,Citation3–Citation5 Chemokine receptors belong to a family of G protein-coupled receptors (GPCRs) containing seven transmembrane-spanning α-helix domains. One of the intracellular loops of the chemokine receptors couples with heterotrimeric G proteins that mediate a cascade of intracellular signaling following ligand binding.Citation4 The heterotrimeric G protein is composed of the Gα, Gβ, and Gγ subunits. Both Gα and Gβ subunits have covalently attached lipid tails that anchor G proteins to the plasma membrane. In the inactive or basal state, the Gα subunit contains the guanine nucleotide diphosphate (GDP).
Upon activation, GPCR acts as a GEF (guanine nucleotide exchange factor) and promotes the conformational change of the Gα subunit and replacement of the bound GDP by guanine nucleotide triphosphate (GTP). This exchange triggers the further conformation changes within the Gα subunit, which allows the trimeric G protein to be released from the receptor, and to dissociate into the GTP-bound Gα subunit and Gβ/Gγ dimer. Both the activated components interact with various effector proteins and initiate unique intracellular signaling cascades, such as activation of phospholipase C (PLC), regulation of adenylate cyclase, triggering of different kinase cascades including mitogen-activated protein kinase (MAPK), c-Jun N-terminal kinase (JNK), p38, and the phosphoinositide 3-kinase (PI3K) routes ().Citation5–Citation9
Figure 1 A schematic of the CXCL12/CXCR4 signaling pathways.
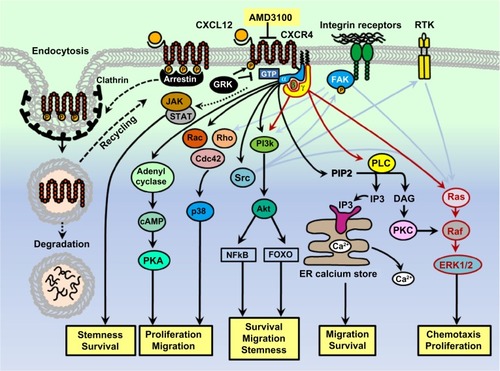
The distinct routes of the GPCRs signaling depend on the coupled Gα subunits, which are classified into four families; Gαs, Gαi, Gαq and Gα12. GPCRs coupled to the Gαs stimulate adenylyl cyclase whereas Gαi bound GPCRs inhibit it. The adenylyl cyclase serves as an effector enzyme that catalyzes 5′adenosine triphosphate into cyclic adenosine monophosphate (cAMP) and thereby activates cAMP-dependent protein kinase, which regulates a host of other downstream effectors including MAPK signaling pathwayCitation5,Citation10 Activated Gαi is also able to activate the Src family of tyrosine kinases, which play an important role in signal integration.Citation3,Citation6 GPCRs coupled to Gαq act through PLCβ, which cleaves phosphatidylinositol 4,5-bisphosphate to form the second messenger molecules called diacylglycerol and inositol-1,4,5-trisphosphate (IP3). Diacylglycerol activates another enzyme called protein kinase C (PKC), whereas IP3 diffuses to the endoplasmic reticulum where it opens calcium channels and triggers the release of calcium from intracellular stores into the cytoplasm. This intracellular calcium mobilization is frequently used for analysis of chemokine receptor activityCitation11 GPCRs coupling to Gα12 alternatively act via the Rho-GEF, which in turn activates the small G protein RhoA ().Citation5,Citation12
Activation of PI3K by GPCRs is thought to be dependent on the direct binding of Gβγ subunits.Citation13 PI3K activation triggers a signaling cascade leading to the activation of AKT (also called protein kinase B) and its downstream targets including phosphoinositide-dependent kinase 1 (PDK1), glycogen synthase kinase 3 (GSK3), mammalian target of rapamycin (mTOR), p70 ribosomal protein S6 kinase (p70S6K), forkhead family transcription factors (FOXO), and other signaling proteins. Notably PI3K activation in response to the GPCR-mediated signaling results in the activation of focal adhesion kinase (FAK), which induces migratory activity in different types of cells, including tumor cells.Citation6,Citation14,Citation15
The duration of the GPCR signaling depends on the Gα subunit lifespan in the GTP-bound state. Hydrolysis of the GTP of Gα-GTP to GDP leads to the inactivation of the Gα subunit and to its reassociation with the Gβ/Gγ dimer, which terminates all effector interactions.Citation16,Citation17 In addition, chemokine receptor signaling is tightly regulated by the process of internalization and lysosomal degradation. Upon GPCR signaling activation, intracellular domains of receptors are phosphorylated by the second messenger kinases such as G protein-coupled receptor kinases (GRKs), followed by the binding of the phosphorylated receptors with regulatory proteins called arrestins. Arrestins impair communication of GPCRs with the G proteins and target them for lysosomal degradation following protein internalization and trafficking ().Citation18,Citation19
Interestingly, it has been reported that several chemokine receptors including CCR2, CCR5, CXCR1, CXCR2, CXCR4, and CXCR7 can undergo homo- or hetero-dimerization upon ligand binding; a process that was proposed to regulate distinct intracellular signaling pathways.Citation7,Citation20,Citation21 Chemokines and their receptors display a high degree of redundancy in that most chemokines bind to multiple receptors and vice versa. The chemokine stromal cell-derived growth factor 1 (SDF-1), also known as CXCL12, binds primarily to its cognate receptor CXCR4, which is also a coreceptor for the entry of the Human immunodeficiency virus (HIV) into the target immune cells (T helper cells) besides the CD4 receptor.Citation22
The assumption that CXCR4 is the only receptor for CXCL12 was recently challenged since it was demonstrated that this chemokine also binds to the orphan receptor called CXCR7, which is a receptor for the interferon-inducible T cell chemoattractant CXCL11/I-TAC. Moreover, CXCR7 constitutively forms heterodimers with the CXCR4 receptor. Growing evidence indicates that binding of CXCL12 to CXCR7 does not result in activation of signaling pathways typical of G proteins. It has been proposed that CXCR7 serves as a ligand scavenger or acts as a “decoy” receptor.Citation23,Citation24 Very recently, this receptor has been described as an activator of various signaling pathways in a CXCL12-dependent manner.Citation23,Citation24 CXCR7 is broadly expressed in normal tissues including the heart, brain, spleen, kidney, lung, testis, ovary, thyroid and human placenta.Citation23 A germline deletion of CXCR7 resulted in perinatal lethality, and expression of CXCR7 was associated with cardiac development.Citation25 Moreover, CXCR7 is upregulated in many malignant cells, including breast, lung, cervical, pancreatic, and prostate cancer cells, and found to be involved in tumor cell growth, survival, and metastasis.Citation21,Citation22,Citation24
Physiological function of the CXCL12/CXCR4 signaling
CXCL12 is a small (8 kDa) homeostatic chemokine that was originally described as an efficacious lymphocyte chemoattractant and regulator of hematopoiesis, and was soon after also characterized as a modulator of multiple physiological processes.Citation26–Citation29 CXCL12 is a pleiotropic chemokine that is widely expressed in different organs including the brain, lung, colon, heart, kidney, and liver where it acts as a chemoattractant for immature and mature hematopoietic cells; it thus plays an important role in inflammation and immune surveillance of tissues. Additionally, CXCL12 serves as an emergent salvage signal for initiating tissue regeneration and repair. Of note, various tissues respond to the chemical or physical insults such as toxic agents, hypoxia, and irradiation by increasing expression and secretion of CXCL12, which is important for recruitment of CXCR4 positive stem and progenitor cells to a site requiring tissue regeneration.Citation29 CXCL12 is expressed from a single gene in six splice variant isoforms known as SDF-1α, SDF-1β, SDF-1γ, SDF-1δ, SDF-1ε, and SDF-1φ.Citation30–Citation32 These CXCL12 isoforms share the same first three exons but contain different fourth exons. Different splice variants are characterized by distinct properties such as stability and tissue of origin. SDF-1α is constitutively produced in many organs but tends to undergo rapid degradation in the blood. In contrast, SDF-1β displays high proteolytic stability and is expressed in highly vascularized organs such as the liver, spleen, and kidney. SDF-1γ is present in less vascularized organs such as the heart and brain.Citation30–Citation32 Dissection of the functional differences between the CXCL12 isoforms warrants further investigation.
The expression of CXCL12 cognate receptor CXCR4 has been shown to be highest in hematopoietic cells but it is also widely and constitutively expressed by numerous cells types including hematopoietic stem cells, endothelial stem cells, liver oval stem cells, neural stem cells, skeletal muscle satellite cells, primordial germ cells, retina pigment epithelium stem cells, and embryonic stem cells. All of these cells not only express functional CXCR4 on their surface but also follow a CXCL12 gradient.Citation26–Citation29,Citation33–Citation39 A growing body of evidence suggests that the CXCL12/CXCR4 axis is essential for the migration of progenitor cells during embryonic hematopoiesis and organogenesis as well as for organ homeostasis, vascularization, and tissue regeneration.Citation38–Citation51 Due to the apparent redundancy within the chemokine system, knockout of one of the chemokine/GPCR axes could be compensated to a large extend by other GPCR routes. However, mice lacking the CXCR4 or CXCL12 genes exhibit a significant defect in the colonization of embryonic bone marrow (BM) by hematopoietic stem cells (HSC), and show defects in the development of other organs including the heart, brain, and blood vessels. In the case of CXCL12 and CXCR4, ablation of either gene is lethal and these embryos die in utero. Thus, the CXCL12/CXCR4 axis appears to have a fundamental physiological role in normal tissue development.Citation26,Citation27,Citation49–Citation52
Role of the CXCL12/CXCR4 axis in cancer and cancer stem cells
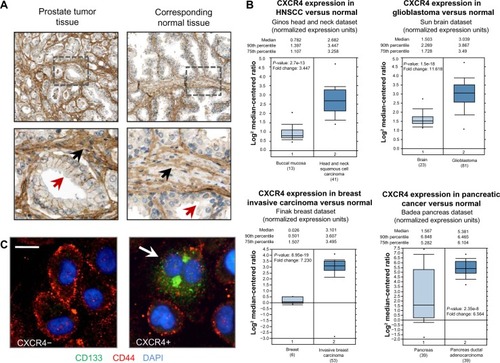
The chemokine CXCL12 and its cognate receptor CXCR4 were first identified as regulators of trafficking and tissue localization of B cells from patients with chronic lymphocytic leukemia.Citation53,Citation54 Shortly thereafter CXCR4 was proposed to regulate the trafficking and invasion of breast cancer cells to sites of metastases.Citation55 More recently, it has been established that CXCR4 plays a central role in tumor cell dissemination and metastasis development in more than 75% of all cancers including breast, ovarian, lung, colon, prostate, kidney, melanoma, brain, esophageal, pancreatic, and many forms of leukemia.Citation56–Citation60 Whereas CXCR4 expression is low or absent in many normal tissues, it is highly expressed in more than 23 different types of tumors including prostate, brain, breast, kidney, pancreas, ovarian cancer, and melanoma ().Citation56 CXCL12 protein levels are highest in organs that are known to be the common sites of metastasis including the liver, bone marrow, and lungs, suggesting that tumor cells may use chemokine-mediated trafficking patterns, which are normally utilized during organogenesis, vascularization, and tissue regeneration.Citation30–Citation32,Citation55
Figure 2 CXCR4 expression in cancer cells and tumor stroma.
Abbreviations: CXCR, CXC chemokine receptor; CD, cluster of differentiation; DAPI, 4’,6-diamidino-2-phenylindole; HNSCC, head and neck squamous cell carcinoma; FACS, fluorescence-activated cell sorting.
The CXCR4/CXCL12 axis also indirectly promotes tumor metastasis by mediating invasion and proliferation of tumor cells, and enhancing tumor-associated neoangiogenesis.Citation61–Citation63 The stromal fibroblasts found within carcinomas and termed carcinoma-associated fibroblasts (CAFs) constitute the major cellular component of the tumor microenvironment. For a long period of time, neither the source of CAFs nor the differences between CAFs and fibroblasts from nonneoplastic tissue have been defined. Recent studies have shown that CAFs can arise from multiple origins including resident tissue fibroblasts, epithelial cells, human mesenchymal stem cells, and HSC.Citation64–Citation66,Citation67,Citation68
Analogous to their normal counterparts, which are present in inflammatory environments and aid in wound healing through tissue remodeling and repair, CAFs promote angiogenesis and matrix degradation through elevated secretion of CXCL12 and matrix metalloproteinase-1 (MMP-1).Citation66,Citation69 During the past 25 years, the accumulation of evidence supports a critical and complex role of MMPs in tumor development. For example, MMPs can increase genomic instability in normal epithelium, which may result in tumor formation. MMPs are the key enzymes responsible for degradation of the extracellular matrix, which is the physical barrier for cancer cell invasion.Citation70 A recent study has identified CXCR4 as a CAF-associated gene, implying an existence of an autocrine feedback loop ().Citation71 The CXCR4/CXCL12 signaling in CAFs results in a multitude of cellular functions, including migration within the tumor microenvironment, adhesion, proliferation, and secretion of MMPs.Citation72–Citation75
A large number of studies demonstrated that CAF-derived CXCL12 not only stimulates carcinoma cell growth directly through the CXCR4 receptor displayed on tumor cells, or indirectly through MMP-mediated tissue remodeling, but also serves to recruit endothelial progenitor cells into tumors and thereby promotes neoangiogenesis.Citation64,Citation65
Moreover, CAF-mediated CXCL12 promotes an epithelial-to-mesenchymal transition (EMT) in primary tumors.Citation76 The acquisition of the EMT program is a critical process for the progression of cancers from local carcinomas to invasive malignancies, which is often associated with the loss of epithelial differentiation and gain of mesenchymal phenotype. Recent studies have shown a molecular link between EMT and self-renewal and demonstrated that cancer cells undergoing EMT gain the cancer stem cell (CSC) phenotype and tumorigenicity.Citation77–Citation80
A growing body of evidence indicates that a subset of cancer cells referred to as CSCs play a critical role in tumor initiation and resistance to anticancer therapy.Citation77 Similarly to normal stem cells, CSCs possess the ability to self-renew and to differentiate into all cell populations within the tumor mass.Citation81 This stem cell concept of tumorigenesis was proven the first time in 1994 by John Dick et al who demonstrated that CD34+CD38− acute myeloid leukemia cell subset is a cell population initiating the malignant disease in immunodeficient mice.Citation82 Clarke et al introduced the CSC concept in solid tumors in 2003.Citation83 In this study they identified CD44+CD24−/low breast cancer CSCs. As few as 100 cells with the CD44+CD24−/low phenotype were able to form tumors in mice, whereas tens of thousands of cells with alternate phenotypes were unable to initiate tumor growth.Citation83 During the last few years, similar discoveries were made in other tumor types. Moreover, this population has been implicated to therapy resistance and tumor recurrence.Citation84–Citation86
Several lines of evidence suggest a link between the EMT and CSC characteristics in various types of cancer. Alterations in genes associated with developmental pathways such as Wnt, SHH and Notch are common in CSC populations, and could facilitate the EMT process. Analogous to differentiated somatic cells, which can be reset to a pluripotent stage by the process of induced dedifferentiation, non-CSC tumor cells can be reprogrammed by the activation of developmental pathways and EMT programs that change their self-renewal and differentiation potency.Citation79,Citation80,Citation85,Citation87,Citation88
The positive correlation between EMT and CSC properties could lead to the concept of “migrating cancer stem cells” as the basis of metastatic colonization.Citation89,Citation90 Recent findings demonstrated that a distinct subpopulation of CSCs can initiate tumor growth at secondary sites. The features of CSCs such as invasion, attachment-independent survival, and the ability to interact with micromilieu at the extravasation site support their involvement in metastatic dissemination.Citation84,Citation91 Moreover, recent studies have revealed striking similarities of the signaling pathways regulating CSC and driving metastasis formation. For example, Liu and colleagues reported a 186-gene “invasiveness” gene signature (IGS) that was identified by comparing the gene expression profiles of normal breast epithelium and breast CSCs with CD44+/CD24−/low phenotype. Among 295 breast cancer patients, there was a significant association between the IGS signature and metastasis-free survival. The IGS was also applied to discriminate low- and high-risk patients with medulloblastoma, lung, and prostate cancers, demonstrating the prognostic value of markers that define CSCs.Citation92 More specifically, several reports demonstrated that CSCs are involved in metastatic dissemination in xenograft models of breast, pancreatic, and colorectal carcinoma.Citation83,Citation93,Citation94
In support of the hypothesis that metastatic tumor cells could have both EMT and CSC phenotypes, clinical studies have shown that the majority (>80%) of the circulating tumor cells (CTCs) in patients with metastatic castration-resistant prostate cancer coexpress stem cell marker CD133 and mesenchymal proteins including vimentin, N-cadherin, and O-cadherin.Citation95 Study of CTCs from metastatic breast cancer patients revealed that 62% of the cells were positive for at least one of three EMT markers – Twist1, AKT2, PI3Kα – and 69% of the total CTC population were positive for the CSC marker aldehyde dehydrogenase (ALDH).Citation96
Although the signals generated by the metastatic niche that regulate CSCs are not fully understood, recent studies provide strong evidence for the important role of the chemokine receptor CXCR4 for CSC maintenance, dissemination, and consequent metastatic colonization. The role of CXCR4 has been examined in the CSC context in various types of cancer including pancreatic, colon, renal, brain, lung, and prostate cancer ( and ). Moreover, Fusi et al showed that CTCs from patients with metastatic carcinoma or melanoma are positive for CXCR4 expression.Citation97
Table 1 CXCR4 as a marker for putative cancer stem cell populations in solid tumors
All together, these findings demonstrate that activation of the CXCL12/CXCR4 signaling can be indicative of the metastatic CSC population and suggest that therapeutic modulation of the CXCR4/CXCL12 axis may be essential for inhibition of metastatic and tumorigenic potential of CSCs.
Regulation and biological effect of CXCR4
The CXCR4/CXCL12 signaling in tumor cells is regulated at several levels.Citation49 First, expression of the CXCR4 and CXCL12 genes are regulated at the transcriptional level by hypoxia. Several reports suggest that low tumor oxygenation and other signals from the tumor microenvironment such as growth factors collaborate to promote EMT associated with high invasiveness and resistance to chemo- and radiotherapy.Citation98 In hypoxia, the lack of oxygen leads to the hypoxia inducible factor 1α (HIF1α)-dependent activation of the CXCR4 and CXCL12 expression.Citation99,Citation100 In addition, tumor fibroblasts and macrophages within the irradiated tumor niche start to produce growth factors and cytokines including CXCL12 which may lead to the invasive behavior of CSCs.Citation101,Citation102 The overlapping signaling mechanisms that govern tumor resistance to conventional treatment and invasiveness could explain why, in some cases, cancer, which relapses after treatment, can develop into more aggressive metastatic disease, which is difficult to treat, and is associated with poor clinical prognosis.Citation103–Citation106
Studies of the molecular interaction between stroma and tumor cells suggest that CSCs can acquire resistance to chemo- and radiotherapy by adhesion to extracellular matrix or accessory cells via integrin signaling and, thus, may be responsible for residual disease and relapses. At the molecular level, CXCR4 is an important mediator of the interaction of tumor cells with extracellular matrix proteins such as laminin, fibronectin, and collagen, which contributes to metastatic spread.Citation107–Citation109 Our recent study demonstrated that CXCL12/CXCR4 signaling pathway regulates the adhesion of CD133+/CD44+ prostate cancer progenitors to the extracellular protein fibronectin that is important for metastatic process. Moreover, the expression of α5 and β3 integrin subunits, which form receptors for fibronectin, are strongly upregulated in CD133+/CD44+ progenitor cells compared to CD133−/CD44− prostate cancer cells.Citation110 Despite the fact that CXCR4 does not directly modulate cell attachment, CXCR4 receptor engagement by CXCL12 plays an essential role in managing cell adhesion by modulation of integrin expression, FAK phosphorylation, and activation of p38 MAPK and ROCK kinases.Citation108,Citation111 The disruption of the interaction of cancer cells with their microenvironmental milieu by CXCR4 inhibition leads to their sensitization to the cytotoxic therapeutic agents.Citation112,Citation113 These findings are consistent with data of high CXCR4 expression by nasopharyngeal carcinoma cells in postradiotherapy patients.Citation114 Moreover, elevated CXCR4 expression shows prognostic value for patients with renal, colorectal, and breast carcinoma.Citation115–Citation119
In addition to HIF-1α, some other transcription factors can influence CXCR4 transcription, including v-ets erythroblastosis virus E26 oncogene homolog 1 and NF-kB nuclear factor kappa-light-chain enhancer of activated B cells, which mediate CXCR4-dependent tumor invasion upon stimulation with hepatocyte growth factor.Citation37,Citation120,Citation121 Furthermore, a novel vesnarinone-responsive molecule Krüppel-like factor 2 and histone deacetylase 3-interacting protein CREB3 were also shown to activate the transcription of the CXCR4 and, therefore, contribute to cell migration.Citation122,Citation123 CXCR4 expression and function are positively regulated by the developmental signaling pathways Wnt, SHH and Notch and the oncogenic pathways PI3K/AKT, NF-kB, and JAK/STAT that are also strongly implicated as CSC regulators.Citation124–Citation128 In turn, activation of the CXCL12/CXCR4 signaling may affect these pathways, suggesting a positive feedback loop between CXCR4 and the signaling routes regulating self-renewal capacity and tumorigenicity of cancer cells.Citation126,Citation127,Citation129–Citation133 At the intracellular level, CXCL12/CXCR4 signaling triggers several phosphorylation cascades controlled by Src and AKT.
The PI3K/AKT axis serves as the central route in the CXCL12/CXCR4 signaling cascade.Citation134–Citation135 Recently, we showed that activation of the CXCL12/CXCR4 and PI3K/AKT signaling pathways is important for self-renewal and tumorigenicity of prostate cancer cells with stem cell characteristics.Citation136,Citation137 PI3K/AKT signaling regulates transcription through the FOXO by phosphorylating conserved serine/threonine residues. Transcriptionally active FOXOs affect a wide range of biological processes, including cell survival, DNA repair, oxidative stress response, and longevity.Citation138 Among the members of the FOXO family, FOXO3A has been shown to be important for the maintenance of neural, hematopoietic, endothelial stem cells,Citation139–Citation141 and cancer stemlike cell populations.Citation136,Citation137 The chromatin immunoprecipitation assay demonstrated that FOXO3A binds to the CXCR4 promoter.Citation110 These data suggested that the CXCR4/AKT positive feedback system may play a role in the maintenance and dissemination of the prostate cancer progenitors.
In addition, CXCL12/CXCR4 signaling may promote tumor growth through transactivation of receptor tyrosine kinases such as EGFR, IGF-1R, and FGFR, which contributes to enhanced invasive signals and metastatic growth of breast, prostate, and ovarian tumors.Citation142–Citation144
CXCR4 has also been demonstrated to elicit intracellular signals through interaction with the scaffolding proteins independently of heterotrimeric G-protein coupling.Citation145 For example, CXCR4 signaling can be modulated by β-arrestin that induces CXCR4 internalization and attenuates CXCR4-mediated G protein activation. β-arrestin can be recruited to the CXCR4/CXCR7 heterodimeric complex resulting in potentiation of downstream β-arrestin-dependent cell signaling pathways, including ERK1/2, p38 MAPK, and SAPK/JNK, which enhances cell migration in response to CXCL12 stimulation.Citation21,Citation24,Citation25,Citation146–Citation148
Finally, recent studies support a new view of CXCR7 as a signaling receptor independent of G proteins.Citation149 CXCL12 binding to CXCR7 activates the PI3K/AKT, PLC/MAPK, and protein kinase C pathways and promotes tumor growth, neovascularization, and dissemination.Citation24,Citation25,Citation146
In summary, activation of CXCL12/CXCR4 axis may be critical for different aspects of tumor initiation, progression, metastasis, and therapy resistance, and targeting CXCR4 signaling might be beneficial in cancer treatment.
Critical analysis of the potential for targeting the CXCL12/CXCR4 axis in cancer management
Multiple agents are currently being developed to target CXCL12/CXCR4 signaling in cancer. Among these inhibitors is anti-CXCR4 drug AMD3100, also known as plerixafor (Mozobil; Sanofi SA, Paris, France), which is approved for stem cell mobilization in patients with non-Hodgkin’s lymphoma and multiple myeloma, while the CXCL12 analog CTCE-9908 (Chemokine Therapeutics Corp, Vancouver, BC, Canada) is approved for clinical use in patients with osteosarcoma. Novel CXCR4 antagonists BKT140 (Emory University), POL6326 (Polyphor Ltd, Allschwill, Switzerland), and TG-0054 (ChemoCentryx, Inc, Mountain View, CA, USA), which were characterized as powerful human stem cell mobilizers, are currently in clinical trials for multiple myeloma, leukemia, and lymphoma. NOX-A12 (Noxxon Pharma AG, Berlin, Germany) is the only anticancer agent in active clinical development that neutralizes CXCL12 resulting in a complete block of CXCL12 signaling through its two receptors, CXCR4 and CXCR7. The anti-CXCL12 aptamer NOX-A12 is in clinical trial for the treatment of chronic lymphocytic leukemia and multiple myeloma. CXCR4 inhibitor MSX-122 (Altiris Therapeutics Inc, Tucker, GA, USA) is in Phase I trials for advanced malignant diseases that are metastatic or unresectable and that are resistant to standard therapy, while CXCR7-specific inhibitor CCX2066 (ChemoCentryx, Inc) is in preclinical studies.Citation134,Citation150
CXCR4 antagonist AMD3100 is the most studied among the agents that inhibit CXCL12/CXCR4 signaling. AMD3100 was initially studied as an anti-HIV agent and then it was discovered that this compound increases white blood cell counts in the blood and is able to mobilize stem cells from the bone marrow. This observation led to the examination of its anticancer activity.Citation151 AMD3100 has already been shown to decrease metastatic potential in animal models for different types of tumors, including breast, ovarian and colorectal cancer, melanoma, and oral squamous cell carcinoma.Citation152–Citation158 Similarly, blocking CXCR4 receptor function by a monoclonal antibody or polypeptide inhibits cancer cell proliferation, motility, and invasion in multiple preclinical models both in vitro and in vivo.Citation110,Citation113,Citation159,Citation160
The fact that CXCR4 is present in normal and cancer stem-like cells in various tissues suggests that this molecule could be essential for maintenance and viability of tumor progenitor cell population. Indeed, recent data suggest that inactivation of the CXCL12/CXCR4 axis by neutralizing antibody or with the CXCR4-specific small molecule antagonist AMD3100 inhibits glioma, renal, colon, pancreas, and prostate cancer progenitors as well as tumor initiating population within gefitinib-resistant lung cancer and tamoxifen-resistant breast cancer cells in vitro and in animal models ().Citation107,Citation161–Citation163
Preclinical and clinical data demonstrated that tumor cells can be protected from the effect of ionizing radiation by hypoxia, and determination of microenvironmental parameters such as tumor hypoxic fraction, vasculature, and perfusion may have a prognostic value for the response to radiotherapy.Citation164–Citation166 Moreover, low oxygen tension is a critical microenvironmental factor in regulating tumor initiating cells.Citation98,Citation167 Hypoxia promotes expansion of glioma and colon CSCs and converts non-stem cancer cells into CSC populations with increased self-renewal capacity.Citation168,Citation169 The effects of reduced oxygen tension on CSCs are mediated at least in part through the activation of the HIF signaling pathway.Citation167 As described above, CXCR4 expression is also induced under hypoxic stress via activation of the HIF pathway.Citation99,Citation100
Pharmacologic inhibition of the CXCL12/CXCR4 interaction by AMD3100 or neutralizing antibody prevents the recurrence of glioblastoma after irradiation in mice by inhibition of vasculogenesis.Citation170 Preclinical studies have shown that radiation upregulates HIF-1 expression level and activity in vivo.Citation171,Citation172 This induces the CXCL12 gene expression and promotes the mobilization of CD11b+ monocytes from the BM, recruitment of these BM derived cells into the tumors, and development of functional tumor vasculature,Citation170,Citation173,Citation174 thereby supporting all remaining viable tumor cells. Similarly, concomitant treatment with local irradiation and AMD3100 induced a significant tumor growth delay and increased radiocurability in lung tumors by retention of BM derived cells.Citation175
Another study demonstrated the role of CXCR4 in tumor radioresistance more definitively and showed that activation of CXCR4-mediated STAT3 signaling in non-small cell lung cancer cells (NSCLC) is functionally crucial for the maintenance of stemness and resistance to radiotherapy.Citation176 Another molecular route that can underlie the CXCR4-mediated radioprotection is the integrin signaling pathway. Accumulating evidence suggests that CXCR4 route enhances integrin-mediated adhesion and cooperates with integrin signaling in mediating chemoresistance.Citation177–Citation179 In fact, CXCR4 engagement by CXCL12 induces expression of the integrin receptors such as α3, α5, β1, and β3 subunits, activation of FAK, and integrin-linked kinase, which is accompanied by the up-regulation of ERK1/2, JNK, and p38 phosphorylation.Citation108,Citation179–Citation182 Previous findings showed that integrins might induce tumor radioresistance via activating SAPK/JNK, MEK1/2, PI3K/AKT, and NF-kB signaling pathways.Citation183–Citation185
Collectively, these clinical and preclinical data are consistent with the CXCL12/CXCR4 pathway being a potential target to inhibit tumor growth and neovascularization, metastatic dissemination, and therapy resistance. Furthermore, the availability of pharmacologic inhibitors impinging CXCL12/CXCR4 signaling pathway opens novel opportunities for translational and clinical studies. However, important challenges remain prior to the clinical use of CXCR4 inhibitors in patients with solid tumors. First, CXCR4 is expressed by numerous types of healthy tissues.Citation26–Citation29,Citation33–Citation39,Citation183–Citation188 Interference with CXCL12/CXCR4 signaling leads to deficiencies in hematopoiesis and organ homeostatic functions as well as in tissue repair after various stresses and insults, including cytotoxic drugs and radiation injury. Given the ubiquitous expression of CXCR4 and the functional importance of the CXCL12/CXCR4 signaling axis, this may impede the use of the CXCR4-targeted therapeutic tools in the clinic. Clinical studies of AMD3100 as a mobilizer of HSC in non-Hodgkin’s lymphoma and multiple myeloma patients have demonstrated that AMD3100 has minimal side effects. However, in the clinic, AMD3100 is given to the patients as a daily subcutaneous injection with granulocyte colony-stimulating factor for a limited time (1–7 days),Citation189 whereas in preclinical studies for evaluating the antitumor efficacy of AMD3100, it is often delivered continuously via a subcutaneous osmotic infusion pump.Citation110,Citation162,Citation190,Citation191 In clinical study for HIV treatment, AMD3100 was administered as a daily intravenous infusion or subcutaneous injection for a period of 11–14 days and, despite its efficacy for the treatments for HIV patients, the trials were discontinued due to cardiac toxicity.Citation192,Citation193
Accumulating experimental evidence suggests that combinatorial strategies based on bulk tumor reduction and CSC-specific pathway inhibition offer a promising treatment modality and are predicted to have a greater efficacy in tumor reduction and prevention of relapse than monotherapies.Citation88,Citation110,Citation137 If these conditions can be met, the use of two types of therapy in low-dose combination can provide better therapeutic effects with less side-effect toxicity. Recent prostate tumor xenograft studies in mice showed that a combination of AMD3100, which targets prostate cancer stem-like cells, and the conventional chemotherapeutic drug Taxotere (Sanofi SA), which targets the bulk tumor, is significantly more effective in eradicating tumors as compared to monotherapy.Citation110,Citation136,Citation137,Citation194 However, efficacy of CXCR4 inhibitors against CSC function in cancer patients remains to be determined. The ongoing clinical trials for CXCR4 inhibitors as chemosensitizers in acute myeloid leukemia and other hematological malignancies will help to elucidate this question.
Conclusion
Although the collective evidence from the preclinical and clinical studies support the potential efficacy of CXCR4 inhibitors for development of innovative approaches to cancer treatment, several significant challenges remain before translation of these inhibitors into the clinic. A major factor that can prevent a successful clinical use of the CXCR4-targeting anticancer therapy is a potential side effect on the stem cell compartment in normal tissues. This may be especially important when this treatment is combined with radiotherapy and other cytotoxic therapy associated with depletion of normal tissue progenitors. Thus, biology-driven rational design of novel combination therapies will be critical for the development of low-side-effect cancer treatment and can be based on the synergistic antitumor effect of CXCR4 inhibition and conventional therapy. There is also a need for the evaluation of the relationship between CXCR4 and tumor initiating cells in cancer patients. In fact, evaluation of the CXCR4 and CXCL12 expression level may have significant prognostic value in various types of cancer, including glioma, prostate, breast, colon, ovarian, pancreatic, and lung cancer where high expression of CXCR4 or CXCL12 predicts poor patient outcome.Citation195–Citation203 However, direct proof for stemness of CXCR4+ cells from primary human tumor tissues is still missing. A better understanding of the role of CXCR4 pathway for the maintenance of tumor progenitor population may be necessary for the development of screening tests to identify the patients who are likely to respond to CXCR4 inhibition. In addition, recent discovery of the cancer stem cell plasticity and heterogeneity can make the CXCR4+ tumor cell population a moving target that could be hard to track and eradicate.Citation85 Nevertheless, the key role of CXCR4 in tumor initiation, vascularization, dissemination, and therapy resistance underscore the importance of CXCR4 inhibition for the optimization of current anticancer treatment strategies.
Acknowledgment
The authors wish to acknowledge funding from the German Federal Ministry of Education and Research (Grant-No 03Z1NN11).
Disclosure
The authors report no conflicts of interest in this work.
References
- ViolaALusterADChemokines and their receptors: drug targets in immunity and inflammationAnnu Rev Pharmacol Toxicol20084817119717883327
- SmithHWhittallCWekslerBMiddletonJChemokines stimulate bidirectional migration of human mesenchymal stem cells across bone marrow endothelial cellsStem Cells Dev201221347648621513440
- BalkwillFCancer and the chemokine networkNat Rev Cancer20044754055015229479
- GilmanAGG proteins: transducers of receptor-generated signalsAnnu Rev Biochem1987566156493113327
- PierceKLPremontRTLefkowitzRJSeven-transmembrane receptorsNat Rev Mol Cell Biol20023963965012209124
- NewDCWongYHMolecular mechanisms mediating the G proteincoupled receptor regulation of cell cycle progressionJ Mol Signal20072217319972
- MelladoMRodríguez-FradeJMMañesSMartínez-ACChemokine signaling and functional responses: the role of receptor dimerization and TK pathway activationAnnu Rev Immunol20011939742111244042
- EpsteinRJThe CXCL12-CXCR4 chemotactic pathway as a target of adjuvant breast cancer therapiesNat Rev Cancer200441190190915516962
- LattinJZidarDASchroderKKellieSHumeDASweetMJG-protein-coupled receptor expression, function, and signaling in macrophagesJ Leukoc Biol2007821163217456803
- GeritsNKostenkoSShiryaevAJohannessenMMoensURelations between the mitogen-activated protein kinase and the cAMP-dependent protein kinase pathways: comradeship and hostilityCell Signal20082091592160718423978
- PrincenKHatseSVermeireKDe ClercqEScholsDEvaluation of SDF-1/CXCR4-induced Ca2+ signaling by fluorometric imaging plate reader (FLIPR) and flow cytometryCytometry A2003511354512500303
- ZhangRXieXTools for GPCR drug discoveryActa Pharmacol Sin201233337238422266728
- SchwindingerWFRobishawJDHeterotrimeric G-protein betagamma-dimers in growth and differentiationOncogene200120131653166011313913
- RozengurtESignal transduction pathways in the mitogenic response to G protein-coupled neuropeptide receptor agonistsJ Cell Physiol1998177450751710092204
- LiangJSlingerlandJMMultiple roles of the PI3K/PKB (Akt) pathway in cell cycle progressionCell Cycle20032433934512851486
- McCuddenCRHainsMDKimpleRJSiderovskiDPWillardFSG-protein signaling: back to the futureCell Mol Life Sci200562555157715747061
- TeicherBAFrickerSPCXCL12 (SDF-1)/CXCR4 pathway in cancerClin Cancer Res201016112927293120484021
- FergusonSSEvolving concepts in G protein-coupled receptor endocytosis: the role in receptor desensitization and signalingPharmacol Rev200153112411171937
- LuttrellLMGesty-PalmerDBeyond desensitization: physiological relevance of arrestin-dependent signalingPharmacol Rev201062230533020427692
- MelladoMRodríguez-FradeJMVila-CoroAJChemokine receptor homo- or heterodimerization activates distinct signaling pathwaysEMBO J200120102497250711350939
- DécaillotFMKazmiMALinYRay-SahaSSakmarTPSachdevPCXCR7/CXCR4 heterodimer constitutively recruits beta-arrestin to enhance cell migrationJ Biol Chem201128637321883219721730065
- BergerEAMurphyPMFarberJMChemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and diseaseAnnu Rev Immunol19991765770010358771
- SunXChengGHaoMCXCL12/CXCR4/CXCR7 chemokine axis and cancer progressionCancer Metastasis Rev201029470972220839032
- SinghAKAryaRKTrivediAKChemokine receptor trio: CXCR3, CXCR4 and CXCR7 crosstalk via CXCL11 and CXCL12Cytokine Growth Factor Rev2013241414922989616
- YuSCrawfordDTsuchihashiTBehrensTWSrivastavaDThe chemokine receptor CXCR7 functions to regulate cardiac valve remodelingDev Dyn2011240238439321246655
- AiutiAWebbIJBleulCSpringerTGutierrez-RamosJCThe chemokine SDF-1 is a chemoattractant for human CD34+ hematopoietic progenitor cells and provides a new mechanism to explain the mobilization of CD34+ progenitors to peripheral bloodJ Exp Med19971851111208996247
- MaQJonesDBorghesaniPRImpaired B-lymphopoiesis, myelopoiesis and derailed cerebellar neuron migration in CXCR4- and SDF-1-deficient miceProc Natl Acad Sci U S A199895944894539689100
- ZouYRKottmannAHKurodaMTaniuchiILittmanDRFunction of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar developmentNature199839366855955999634238
- KuciaMJankowskiKRecaRCXCR4-SDF-1 signalling, locomotion, chemotaxis and adhesionJ Mol Histol200435323324515339043
- JanowskiMFunctional diversity of SDF-1 splicing variantsCell Adh Migr20093324324919287206
- YuLCecilJPengSBIdentification and expression of novel isoforms of human stromal cell-derived factor 1Gene200637417417916626895
- HoTKShiwenXAbrahamDTsuiJBakerDStromal-cell-derived factor-1 (SDF-1)/CXCL12 as potential target of therapeutic angiogenesis in critical leg ischaemiaCardiol Res Pract2012201214320922462026
- RatajczakMZMajkaMKuciaMExpression of functional CXCR4 by muscle satellite cells and secretion of SDF-1 by muscle-derived fibroblasts is associated with the presence of both muscle progenitors in bone marrow and hematopoietic stem/progenitor cells in musclesStem Cells200321336337112743331
- KuciaMRecaRMiekusKTrafficking of normal stem cells and metastasis of cancer stem cells involve similar mechanisms: pivotal role of the SDF-1-CXCR4 axisStem Cells200523787989415888687
- DayCEGuillenCWillarsGBWardlawAJCharacterization of the migration of lung and blood T cells in response CXCL12 in a three-dimensional matrixImmunology2010130456457120331475
- AgleKAVongsaRADwinellMBCalcium mobilization triggered by the chemokine CXCL12 regulates migration in wounded intestinal epithelial monolayersJ Biol Chem201028521160661607520348095
- EsencayMNewcombEWZagzagDHGF upregulates CXCR4 expression in gliomas via NF-kappaB: implications for glioma cell migrationJ Neurooncol2010991334020157762
- BurgerJAKippsTJCXCR4: a key receptor in the crosstalk between tumor cells and their microenvironmentBlood200610751761176716269611
- TiveronMCCremerHCXCL12/CXCR4 signalling in neuronal cell migrationCurr Opin Neurobiol200818323724418644448
- SchoberAZerneckeAChemokines in vascular remodelingThromb Haemost200797573073717479183
- PetitIJinDRafiiSThe SDF-1-CXCR4 signaling pathway: a molecular hub modulating neo-angiogenesisTrends Immunol200728729930717560169
- KarshovskaEZagoracDZerneckeAWeberCSchoberAA small molecule CXCR4 antagonist inhibits neointima formation and smooth muscle progenitor cell mobilization after arterial injuryJ Thromb Haemost20086101812181518647221
- ZerneckeASchoberABotISDF-1alpha/CXCR4 axis is instrumental in neointimal hyperplasia and recruitment of smooth muscle progenitor cellsCirc Res200596778479115761195
- RehimiRKhalidaNYusufFMorosan-PuopoloGBrand-SaberiBA novel role of CXCR4 and SDF-1 during migration of cloacal muscle precursorsDev Dyn201023961622163120503359
- NerviBLinkDCDiPersioJFCytokines and hematopoietic stem cell mobilizationJ Cell Biochem200699369070516888804
- YuJLiMQuZYanDLiDRuanQSDF-1/CXCR4-mediated migration of transplanted bone marrow stromal cells toward areas of heart myocardial infarction through activation of PI3K/AktJ Cardiovasc Pharmacol201055549650520179608
- GambaryanNPerrosFMontaniDTargeting of c-kit+ haematopoietic progenitor cells prevents hypoxic pulmonary hypertensionEur Respir J20113761392139920884740
- YuLHalesCAEffect of chemokine receptor CXCR4 on hypoxia-induced pulmonary hypertension and vascular remodeling in ratsRespir Res2011122121294880
- RatajczakMZZuba-SurmaEKuciaMThe pleiotropic effects of the SDF-1-CXCR4 axis in organogenesis, regeneration and tumorigenesisLeukemia200620111915192416900209
- BleulCCFuhlbriggeRCCasasnovasJMAiutiASpringerTAA highly efficacious lymphocyte chemoattractant, stromal cell-derived factor 1 (SDF-1)J Exp Med19961843110111099064327
- LazariniFThamTNCasanovaPArenzana-SeisdedosFDubois-DalcqMRole of the alpha-chemokine stromal cell-derived factor (SDF-1) in the developing and mature central nervous systemGlia200342213914812655598
- VagimaYLapidKKolletOGoichbergPAlonRLapidotTPathways implicated in stem cell migration: the SDF-1/CXCR4 axisMethods Mol Biol201175027728921618098
- BurgerJABurgerMKippsTJChronic lymphocytic leukemia B cells express functional CXCR4 chemokine receptors that mediate spontaneous migration beneath bone marrow stromal cellsBlood199994113658366710572077
- MöhleRFailenschmidCBautzFKanzLOverexpression of the chemokine receptor CXCR4 in B cell chronic lymphocytic leukemia is associated with increased functional response to stromal cell-derived factor-1 (SDF-1)Leukemia199913121954195910602415
- MüllerAHomeyBSotoHInvolvement of chemokine receptors in breast cancer metastasisNature20014106824505611242036
- BalkwillFThe significance of cancer cell expression of the chemokine receptor CXCR4Semin Cancer Biol200414317117915246052
- VandercappellenJVan DammeJStruyfSThe role of CXC chemokines and their receptors in cancerCancer Lett2008267222624418579287
- ZlotnikANew insights on the role of CXCR4 in cancer metastasisJ Pathol2008215321121318523970
- FurusatoBMohamedAUhlénMCXCR4 and cancerPathol Int201060749750520594270
- Darash-YahanaMPikarskyEAbramovitchRRole of high expression levels of CXCR4 in tumor growth, vascularization, and metastasisFASEB J200418111240124215180966
- SunXWeiLChenQTerekRMCXCR4/SDF1 mediate hypoxia induced chondrosarcoma cell invasion through ERK signaling and increased MMP1 expressionMol Cancer201091720102637
- EckSMBlackburnJSSchmuckerACBurragePSBrinckerhoffCEMatrix metalloproteinase and G protein coupled receptors: co-conspirators in the pathogenesis of autoimmune disease and cancerJ Autoimmun2009333–421422119800199
- OrimoAWeinbergRAStromal fibroblasts in cancer: a novel tumor-promoting cell typeCell Cycle20065151597160116880743
- XuJClarkRAExtracellular matrix alters PDGF regulation of fibroblast integrinsJ Cell Biol19961322392498567727
- DunphyJEThe Fibroblast – A Ubiquitous Ally for the SurgeonN Engl J Med196326813671377
- MishraPJMishraPJHumeniukRCarcinoma-associated fibroblast-like differentiation of human mesenchymal stem cellsCancer Res200868114331433918519693
- OrimoAGuptaPBSgroiDCStromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretionCell2005121333534815882617
- ShangguanLTiXKrauseUInhibition of TGF-β/Smad signaling by BAMBI blocks differentiation of human mesenchymal stem cells to carcinoma-associated fibroblasts and abolishes their protumor effectsStem Cells201230122810281923034983
- McDonaldLTLaRueACHematopoietic stem cell derived carcinoma-associated fibroblasts: a novel originInt J Clin Exp Pathol20125986387323119103
- Shuman MossLAJensen-TaubmanSStetler-StevensonWGMatrix metalloproteinases: changing roles in tumor progression and metastasisAm J Pathol201218161895189923063657
- EckSMCoteALWinkelmanWDCXCR4 and matrix metalloproteinase-1 are elevated in breast carcinoma-associated fibroblasts and in normal mammary fibroblasts exposed to factors secreted by breast cancer cellsMol Cancer Res2009771033104419584257
- AlsayedYNgoHRunnelsJMechanisms of regulation of CXCR4/SDF-1 (CXCL12)-dependent migration and homing in multiple myelomaBlood20071092708271717119115
- BusilloJMBenovicJLRegulation of CXCR4 signalingBiochim Biophys Acta20071768495296317169327
- ZhouYLarsenPHHaoCYongVWCXCR4 is a major chemokine receptor on glioma cells and mediates their survivalJ Biol Chem200227751494814948712388552
- LukerKELukerGDFunctions of CXCL12 and CXCR4 in breast cancerCancer Lett20062381304116046252
- JungYKimJKShiozawaYRecruitment of mesenchymal stem cells into prostate tumours promotes metastasisNat Commun20134179523653207
- ScheelCWeinbergRACancer stem cells and epithelial-mesenchymal transition: concepts and molecular linksSemin Cancer Biol2012225–639640322554795
- GuptaPBOnderTTJiangGIdentification of selective inhibitors of cancer stem cells by high-throughput screeningCell2009138464565919682730
- ManiSAGuoWLiaoMJThe epithelial-mesenchymal transition generates cells with properties of stem cellsCell2008133470471518485877
- AsieduMKIngleJNBehrensMDRadiskyDCKnutsonKLTGFβ/TNFα-mediated epithelial-mesenchymal transition generates breast cancer stem cells with a claudin-low phenotypeCancer Res201171134707471921555371
- ShackletonMQuintanaEFearonERMorrisonSJHeterogeneity in cancer: cancer stem cells versus clonal evolutionCell2009138582282919737509
- LapidotTSirardCVormoorJA cell initiating human acute myeloid leukaemia after transplantation into SCID miceNature199436764646456487509044
- Al-HajjMWichaMSBenito-HernandezAMorrisonSJClarkeMFProspective identification of tumorigenic breast cancer cellsProc Natl Acad Sci U S A200310073983398812629218
- BaccelliITrumppAThe evolving concept of cancer and metastasis stem cellsJ Cell Biol2012198328129322869594
- PeitzschCKurthIKunz-SchughartLBaumannMDubrovskaADiscovery of the cancer stem cell related determinants of radioresistanceRadiother Oncol EpubJuly32013
- BaumannMKrauseMHillRExploring the role of cancer stem cells in radioresistanceNat Rev Cancer20088754555418511937
- TangDGUnderstanding cancer stem cell heterogeneity and plasticityCell Res201222345747222357481
- ScaffidiPMisteliTIn vitro generation of human cells with cancer stem cell propertiesNat Cell Biol20111391051106121857669
- BrabletzTJungASpadernaSHlubekFKirchnerTOpinion: migrating cancer stem cells – an integrated concept of malignant tumour progressionNat Rev Cancer20055974474916148886
- HindriksenSBijlsmaMFCancer Stem Cells, EMT, and Developmental Pathway Activation in Pancreatic TumorsCancers2012449891035
- DalerbaPChoRWClarkeMFCancer stem cells: models and conceptsAnnu Rev Med20075826728417002552
- LiuRWangXChenGYThe prognostic role of a gene signature from tumorigenic breast-cancer cellsN Engl J Med2007356321722617229949
- LiuHPatelMRPrescherJACancer stem cells from human breast tumors are involved in spontaneous metastases in orthotopic mouse modelsProc Natl Acad Sci U S A201010742181151812020921380
- PangRLawWLChuACA subpopulation of CD26+ cancer stem cells with metastatic capacity in human colorectal cancerCell Stem Cell20106660361520569697
- ArmstrongAJMarengoMSOlteanSCirculating tumor cells from patients with advanced prostate and breast cancer display both epithelial and mesenchymal markersMol Cancer Res201198997100721665936
- AktasBTewesMFehmTHauchSKimmigRKasimir-BauerSStem cell and epithelial-mesenchymal transition markers are frequently overexpressed in circulating tumor cells of metastatic breast cancer patientsBreast Cancer Res200911R4619589136
- FusiALiuZKümmerlenVNonnemacherAJeskeJKeilholzUExpression of chemokine receptors on circulating tumor cells in patients with solid tumorsJ Transl Med2012105222433180
- HillRPMarie-EgyptienneDTHedleyDWCancer stem cells, hypoxia and metastasisSemin Radiat Oncol200919210611119249648
- IshikawaTNakashiroKKlosekSKHypoxia enhances CXCR4 expression by activating HIF-1 in oral squamous cell carcinomaOncol Rep200921370771219212630
- ZagzagDLukyanovYLanLHypoxia-inducible factor 1 and VEGF upregulate CXCR4 in glioblastoma: implications for angiogenesis and glioma cell invasionLab Invest200686121221123217075581
- NguyenDHOketch-RabahHAIlla-BochacaIRadiation acts on the microenvironment to affect breast carcinogenesis by distinct mechanisms that decrease cancer latency and affect tumor typeCancer Cell201119564065121575864
- Sofia ValaIMartinsLRImaizumiNLow doses of ionizing radiation promote tumor growth and metastasis by enhancing angiogenesisPLoS One20105e1122220574535
- FagundesHPerezCAGrigsbyPWLockettMADistant metastases after irradiation alone in carcinoma of the uterine cervixInt J Radiat Oncol Biol Phys19922421972041526855
- AndersonPDischeSLocal tumor control and the subsequent incidence of distant metastatic diseaseInt J Radiat Oncol Biol Phys1981712164516487037703
- FuksZLeibelSAWallnerKEThe effect of local control on metastatic dissemination in carcinoma of the prostate: long-term results in patients treated with 125I implantationInt J Radiat Oncol Biol Phys19912135375471869452
- von EssenCFRadiation enhancement of metastasis: a reviewClin Exp Metastasis199192771042032423
- EnglTReljaBMarianDCXCR4 chemokine receptor mediates prostate tumor cell adhesion through alpha5 and beta3 integrinsNeoplasia20068429030116756721
- SunYXFangMWangJCooperCRPientaKJTaichmanRSExpression and activation of alpha v beta 3 integrins by SDF-1/CXC12 increases the aggressiveness of prostate cancer cellsProstate2007671617317034033
- GoelHLLiJKoganSLanguinoLRIntegrins in prostate cancer progressionEndocr Relat Cancer200815365766418524948
- DubrovskaAElliottJSalamoneRJCXCR4 Expression in Prostate Cancer Progenitor CellsPLoS ONE201272e3122622359577
- StruckhoffAPVitkoJRRanaMKDynamic regulation of ROCK in tumor cells controls CXCR4-driven adhesion eventsJ Cell Sci2010123Pt 340141220053635
- AzabAKRunnelsJMPitsillidesCCXCR4 inhibitor AMD3100 disrupts the interaction of multiple myeloma cells with the bone marrow microenvironment and enhances their sensitivity to therapyBlood2009113184341435119139079
- ZengZSamudioIJMunsellMInhibition of CXCR4 with the novel RCP168 peptide overcomes stroma-mediated chemoresistance in chronic and acute leukemiasMol Cancer Ther20065123113312117172414
- OuDLChenCLLinSBHsuCHLinLIChemokine receptor expression profiles in nasopharyngeal carcinoma and their association with metastasis and radiotherapyJ Pathol2006210336337316955398
- ParkerCCKimRHLiBDChuQDThe chemokine receptor CXCR4 as a novel independent prognostic marker for node-positive breast cancer patientsJ Surg Oncol2012106439339822473623
- HillerDJMeschonatCKimRLiBDChuQDChemokine receptor CXCR4 level in primary tumors independently predicts outcome for patients with locally advanced breast cancerSurgery2011150345946521878231
- YoppACShiaJButteJMCXCR4 expression predicts patient outcome and recurrence patterns after hepatic resection for colorectal liver metastasesAnn Surg Oncol201219Suppl 3S339S34621584832
- D’AlterioCConsalesCPolimenoMConcomitant CXCR4 and CXCR7 expression predicts poor prognosis in renal cancerCurr Cancer Drug Targets201010777278120578990
- ChuQDPanuLHolmNTLiBDJohnsonLWZhangSHigh chemokine receptor CXCR4 level in triple negative breast cancer specimens predicts poor clinical outcomeJ Surg Res2010159268969519500800
- MaroniPBendinelliPMatteucciEDesiderioMAHGF induces CXCR4 and CXCL12-mediated tumor invasion through Ets1 and NF-kappaBCarcinogenesis200728226727916840440
- MatteucciERidolfiEMaroniPBendinelliPDesiderioMAc-Src/histone deacetylase 3 interaction is crucial for hepatocyte growth factor dependent decrease of CXCR4 expression in highly invasive breast tumor cellsMol Cancer Res20075883384517699109
- KimHCChoiKCChoiHKHDAC3 selectively represses CREB3-mediated transcription and migration of metastatic breast cancer cellsCell Mol Life Sci201067203499351020473547
- UchidaDOnoueTBegumNMVesnarinone downregulates CXCR4 expression via upregulation of Krüppel-like factor 2 in oral cancer cellsMol Cancer200986219671192
- JinZZhaoCHanXHanYWnt5a promotes Ewing sarcoma cell migration through upregulating CXCR4 expressionBMC Cancer20121248023075330
- TamuraMSatoMMNashimotoMRegulation of CXCL12 expression by canonical Wnt signaling in bone marrow stromal cellsInt J Biochem Cell Biol201143576076721296678
- HanYHeTHuangDRPardoCARansohoffRMTNF-alpha mediates SDF-1 alpha-induced NF-kappa B activation and cytotoxic effects in primary astrocytesJ Clin Invest2001108342543511489936
- KukrejaPAbdel-MageedABMondalDLiuKAgrawalKCUp-regulation of CXCR4 expression in PC-3 cells by stromal-derived factor-1alpha (CXCL12) increases endothelial adhesion and transendothelial migration: role of MEK/ERK signaling pathway-dependent NF-kB activationCancer Res200565219891989816267013
- SoldevilaGLiconaISalgadoARamírezMChávezRGarcía-ZepedaEImpaired chemokine-induced migration during T-cell development in the absence of Jak 3Immunology2004112219120015147562
- WangJCaiJHanFSilencing of CXCR4 blocks progression of ovarian cancer and depresses canonical Wnt signaling pathwayInt J Gynecol Cancer201121698198721738044
- FarehMTurchiLVirolleVThe miR 302–367 cluster drastically affects self-renewal and infiltration properties of glioma-initiating cells through CXCR4 repression and consequent disruption of the SHH-GLI-NANOG networkCell Death Differ201219223224421720384
- PengSBPeekVZhaiYAkt activation, but not extracellular signal-regulated kinase activation, is required for SDF-1alpha/CXCR4-mediated migration of epitheloid carcinoma cellsMol Cancer Res20053422723615831676
- HelbigGChristophersonKW2ndBhat-NakshatriPNF-kappaB promotes breast cancer cell migration and metastasis by inducing the expression of the chemokine receptor CXCR4J Biol Chem200327824216312163812690099
- Vila-CoroAJRodríguez-FradeJMMartín De AnaAMoreno-OrtízMCMartínez-ACMelladoMThe chemokine SDF-1alpha triggers CXCR4 receptor dimerization and activates the JAK/STAT pathwayFASEB J199913131699171010506573
- RamseyDMMcAlpineSRHalting metastasis through CXCR4 inhibitionBioorg Med Chem Lett2013231202523211868
- ChinniSRSivaloganSDongZCXCL12/CXCR4 signaling activates Akt-1 and MMP-9 expression in prostate cancer cells: the role of bone microenvironment-associated CXCL12Prostate2006661324816114056
- DubrovskaAKimSSalamoneRJThe role of PTEN/Akt/PI3K signaling in the maintenance and viability of prostate cancer stem-like cell populationsProc Natl Acad Sci U S A2009106126827319116269
- DubrovskaAElliottJSalamoneRJCombination therapy targeting both tumor-initiating and differentiated cell populations in prostate carcinomaClin Cancer Res201016235692570221138868
- HuangHTindallDJFOXO factors: a matter of life and deathFuture Oncol200621838916556075
- MiyamotoKArakiKYNakaKFoxo3a is essential for maintenance of the hematopoietic stem cell poolCell Stem Cell20071110111218371339
- ZhuSEvansSYanBTranscriptional regulation of Bim by FOXO3a and Akt mediates scleroderma serum-induced apoptosis in endothelial progenitor cellsCirculation2008118212156216518981303
- WuYPengHCuiMCXCL12 increases human neural progenitor cell proliferation through Akt-1/FOXO3a signaling pathwayJ Neurochem200910941157116719302476
- PorcileCBajettoABarbieriFStromal cell-derived factor-1alpha (SDF-1alpha/CXCL12) stimulates ovarian cancer cell growth through the EGF receptor transactivationExp Cell Res2005308224125315921680
- ChinniSRYamamotoHDongZSabbotaABonfilRDCherMLCXCL12/CXCR4 transactivates HER2 in lipid rafts of prostate cancer cells and promotes growth of metastatic deposits in boneMol Cancer Res20086344645718337451
- CabiogluNSummyJMillerCCXCL-12/stromal cell-derived factor-1alpha transactivates HER2-neu in breast cancer cells by a novel pathway involving Src kinase activationCancer Res200565156493649716061624
- DefeaKBeta-arrestins and heterotrimeric G-proteins: collaborators and competitors in signal transductionBr J Pharmacol2008153Suppl 1S298S30918037927
- HeinrichELLeeWLuJLowyAMKimJChemokine CXCL12 activates dual CXCR4 and CXCR7-mediated signaling pathways in pancreatic cancer cellsJ Transl Med2012106822472349
- SunYChengZMaLPeiGBeta-arrestin 2 is critically involved in CXCR4-mediated chemotaxis, and this is mediated by its enhancement of p38 MAPK activationJ Biol Chem200227751492124921912370187
- ChengZJZhaoJSunYBeta-arrestin differentially regulates the chemokine receptor CXCR4-mediated signaling and receptor internalization, and this implicates multiple interaction sites between beta-arrestin and CXCR4J Biol Chem200027542479248510644702
- Sánchez-MartínLSánchez-MateosPCabañasCCXCR7 impact on CXCL12 biology and diseaseTrends Mol Med2013191122223153575
- de NigrisFSchianoCInfanteTNapoliCCXCR4 inhibitors: tumor vasculature and therapeutic challengesRecent Pat Anticancer Drug Discov20127325126422376154
- PeledAWaldOBurgerJDevelopment of novel CXCR4-based therapeuticsExpert Opin Investig Drugs2012213341353
- SmithMCLukerKEGarbowJRCXCR4 regulates growth of both primary and metastatic breast cancerCancer Res200464238604861215574767
- KimMKohYJKimKECXCR4 signaling regulates metastasis of chemoresistant melanoma cells by a lymphatic metastatic nicheCancer Res20107024104111042121056990
- D’AlterioCBarbieriAPortellaLInhibition of stromal CXCR4 impairs development of lung metastasesCancer Immunol Immunother201261101713172022399057
- UchidaDOnoueTKuribayashiNBlockade of CXCR4 in oral squamous cell carcinoma inhibits lymph node metastasesEur J Cancer201147345245920965717
- KajiyamaHShibataKTerauchiMInoKNawaAKikkawaFInvolvement of SDF-1alpha/CXCR4 axis in the enhanced peritoneal metastasis of epithelial ovarian carcinomaInt J Cancer20081221919917893878
- MatsusueRKuboHHisamoriSHepatic stellate cells promote liver metastasis of colon cancer cells by the action of SDF-1/CXCR4 axisAnn Surg Oncol20091692645265319588204
- EhteshamMMinEIssarNMKaslRAKhanISThompsonRCThe role of the CXCR4 cell surface chemokine receptor in glioma biologyJ Neurooncol2013113215316223494875
- PanJMestasJBurdickMDStromal derived factor-1 (SDF-1/CXCL12) and CXCR4 in renal cell carcinoma metastasisMol Cancer200655617083723
- O’BoyleGSwidenbankIMarshallHBarkerCEArmstrongJWhiteSAFrickerSPPlummerRWrightMLovatPEInhibition of CXCR4-CXCL12 chemotaxis in melanoma by AMD11070Br J Cancer201310881634164023538388
- GassenmaierMChenDBuchnerACXC chemokine receptor 4 is essential for maintenance of renal cell carcinoma-initiating cells and predicts metastasisStem Cells Epub4302013
- DubrovskaAHartungABouchezLCCXCR4 activation maintains a stem cell population in tamoxifen-resistant breast cancer cells through AhR signallingBr J Cancer20121071435222644306
- RedjalNChanJASegalRAKungALCXCR4 inhibition synergizes with cytotoxic chemotherapy in gliomasClin Cancer Res200612226765677117121897
- OvergaardJHypoxic radiosensitization: adored and ignoredJ Clin Oncol200725264066407417827455
- YarominaAKrauseMThamesHPre-treatment number of clonogenic cells and their radiosensitivity are major determinants of local tumour control after fractionated irradiationRadiother Oncol200783330431017517444
- ZipsDBökeSKroeberTPrognostic value of radiobiological hypoxia during fractionated irradiation for local tumor controlStrahlenther Onkol2011187530631021533758
- LiZRichJNHypoxia and hypoxia inducible factors in cancer stem cell maintenanceCurr Top Microbiol Immunol2010345213020582533
- HeddlestonJMLiZMcLendonREHjelmelandABRichJNThe hypoxic microenvironment maintains glioblastoma stem cells and promotes reprogramming towards a cancer stem cell phenotypeCell Cycle20098203274328419770585
- SoedaAParkMLeeDHypoxia promotes expansion of the CD133-positive glioma stem cells through activation of HIF-1alphaOncogene200928453949395919718046
- KioiMVogelHSchultzGHoffmanRMHarshGRBrownJMInhibition of vasculogenesis, but not angiogenesis, prevents the recurrence of glioblastoma after irradiation in miceJ Clin Invest2010120369470520179352
- ChenFHChiangCSWangCCRadiotherapy decreases vascular density and causes hypoxia with macrophage aggregation in TRAMP-C1 prostate tumorsClin Cancer Res20091551721172919240176
- MoellerBJCaoYLiCYDewhirstMWRadiation activates HIF-1 to regulate vascular radiosensitivity in tumors: role of reoxygenation, free radicals, and stress granulesCancer Cell20045542944115144951
- LydenDHattoriKDiasSImpaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growthNat Med20017111194120111689883
- CeradiniDJKulkarniARCallaghanMJProgenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1Nat Med200410885886415235597
- KozinSVKamounWSHuangYDawsonMRJainRKDudaDGRecruitment of myeloid but not endothelial precursor cells facilitates tumor regrowth after local irradiationCancer Res201070145679568520631066
- JungMJRhoJKKimYMUpregulation of CXCR4 is functionally crucial for maintenance of stemness in drug-resistant non-small cell lung cancer cellsOncogene201332220922122370645
- ShenWBendallLJGottliebDJBradstockKFThe chemokine receptor CXCR4 enhances integrin-mediated in vitro adhesion and facilitates engraftment of leukemic precursor-B cells in the bone marrowExp Hematol200129121439144711750103
- HartmannTNBurgerJAGlodekAFujiiNBurgerMCXCR4 chemokine receptor and integrin signaling co-operate in mediating adhesion and chemoresistance in small cell lung cancer (SCLC) cellsOncogene200524274462447115806155
- JonesJMarianDWeichECXCR4 chemokine receptor engagement modifies integrin dependent adhesion of renal carcinoma cellsExp Cell Res2007313194051406517706641
- KuonenFSecondiniCRüeggCMolecular pathways: emerging pathways mediating growth, invasion, and metastasis of tumors progressing in an irradiated microenvironmentClin Cancer Res201218195196520222730447
- BettinkSIWernerCChenCHIntegrin-linked kinase is a central mediator in angiotensin II type 1- and chemokine receptor CXCR4 signaling in myocardial hypertrophyBiochem Biophys Res Commun2010397220821320493167
- KijowskiJBaj-KrzyworzekaMMajkaMThe SDF-1-CXCR4 axis stimulates VEGF secretion and activates integrins but does not affect proliferation and survival in lymphohematopoietic cellsStem Cells200119545346611553854
- EkeIKochUHehlgansSPINCH1 regulates Akt1 activation and enhances radioresistance by inhibiting PP1alphaJ Clin Invest201012072516252720530873
- AhmedKMZhangHParkCCNF-κB Regulates Radioresistance Mediated By β1-Integrin in Three-Dimensional Culture of Breast Cancer CellsCancer Res201373123737374823576567
- OuJLuanWDengJSaRLiangHαV integrin induces multicellular radioresistance in human nasopharyngeal carcinoma via activating SAPK/JNK pathwayPLoS One201276e3873722719931
- JordanNJKoliosGAbbotSEExpression of functional CXCR4 chemokine receptors on human colonic epithelial cellsJ Clin Invest199910481061106910525044
- ZhuBXuDDengXCXCL12 enhances human neural progenitor cell survival through a CXCR7- and CXCR4-mediated endocytotic signaling pathwayStem Cells201230112571258322987307
- DongFHarveyJFinanAWeberKAgarwalUPennMSMyocardial CXCR4 expression is required for mesenchymal stem cell mediated repair following acute myocardial infarctionCirculation2012126331432422685115
- De ClercqEThe bicyclam AMD3100 storyNat Rev Drug Discov20032758158712815382
- RighiEKashiwagiSYuanJCXCL12/CXCR4 blockade induces multimodal antitumor effects that prolong survival in an immunocompetent mouse model of ovarian cancerCancer Res201171165522553421742774
- RubinJBKungALKleinRSA small-molecule antagonist of CXCR4 inhibits intracranial growth of primary brain tumorsProc Natl Acad Sci U S A200310023135131351814595012
- ScholtenDJCanalsMMaussangDPharmacological modulation of chemokine receptor functionBr J Pharmacol201216561617164321699506
- HendrixCWFlexnerCMacFarlandRTPharmacokinetics and safety of AMD-3100, a novel antagonist of the CXCR-4 chemokine receptor, in human volunteersAntimicrob Agents Chemother20004461667167310817726
- DomanskaUMTimmer-BosschaHNagengastWBCXCR4 inhibition with AMD3100 sensitizes prostate cancer to docetaxel chemotherapyNeoplasia201214870971822952424
- RamosEAGrochoskiMBraun-PradoKEpigenetic changes of CXCR4 and its ligand CXCL12 as prognostic factors for sporadic breast cancerPLoS One2011612e2946122220212
- WuYJinMXuHClinicopathologic Significance of HIF-1α, CXCR4, and VEGF Expression in Colon CancerClin Dev Immunol2010201053753120953377
- PoppleADurrantLGSpendloveIThe chemokine, CXCL12, is an independent predictor of poor survival in ovarian cancerBr J Cancer201210671306131322415233
- MaréchalRDemetterPNagyNHigh expression of CXCR4 may predict poor survival in resected pancreatic adenocarcinomaBr J Cancer200910091444145119352387
- WangMChenGYSongHTHongxYangZYSuiGJSignificance of CXCR4, phosphorylated STAT3 and VEGF-A expression in resected non-small cell lung cancerExp Ther Med20112351752222977534
- ZhangNHLiJLiYCo-expression of CXCR4 and CD133 proteins is associated with poor prognosis in stage II–III colon cancer patientsExp Ther Med20123697398222970002
- JungSJKimCParkCHCorrelation between Chemokine Receptor CXCR4 Expression and Prognostic Factors in Patients with Prostate CancerKorean J Urol201152960761122025955
- ZhangLYeSBMaGThe expressions of MIF and CXCR4 protein in tumor microenvironment are adverse prognostic factors in patients with esophageal squamous cell carcinomaJ Transl Med2013116023497377
- Bennani-BaitiIMCooperALawlorERIntercohort Gene Expression Co-analysis Reveals Chemokine Receptors as Prognostic Indicators in Ewing’s SarcomaClin Cancer Res201016143769377820525755
- ZhangSSHanZPJingYYCD133(+)CXCR4(+) colon cancer cells exhibit metastatic potential and predict poor prognosis of patientsBMC Med2012108522871210
- HermannPCHuberSLHerrlerTDistinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancerCell Stem Cell20071331332318371365
- ZhengXXieQLiSZhangWCXCR4-positive subset of glioma is enriched for cancer stem cellsOncol Res2011191255556122812188