Abstract
Anal squamous cell cancer is an uncommon malignancy caused by infection with oncogenic strains of Human papilloma virus. Anal cancer is much more common in immunocompromised persons, including those infected with Human immunodeficiency virus. High-grade anal intraepithelial neoplasia (HGAIN), the precursor of anal cancer, is identified by clinicians providing care for patients with anorectal disease, and is increasingly being identified during screening of immunosuppressed patients for anal dysplasia. The traditional treatment for HGAIN has been excision of macroscopic disease with margins. This approach is effective for patients with small unifocal HGAIN lesions. Patients with extensive multifocal HGAIN frequently have recurrence of HGAIN after excision, and may have postoperative complications of anal stenosis or fecal incontinence. This led to the suggestion by some that treatment for HGAIN should be delayed until patients developed anal cancer. Alternative approaches in identification and treatment have been developed to treat patients with multifocal or extensive HGAIN lesions. High-resolution anoscopy combines magnification with anoscopy and is being used to identify HGAIN and determine treatment margins. HGAIN can then be ablated with a number of modalities, including infrared coagulation, CO2 laser, and electrocautery. These methods for HGAIN ablation can be performed with local anesthesia on outpatients and are relatively well tolerated. High-resolution anoscopy-directed HGAIN ablation is evolving into a standard approach for initial treatment and then subsequent monitoring of a disease which should be expected to be recurrent. Another treatment approach for HGAIN is topical treatment, principally with 5-fluorouracil or imiquimod. Topical therapies have the advantage of being nonsurgical and are well suited for treating widespread multifocal disease. Topical treatments have the disadvantage of requiring extended treatment courses and causing a symptomatic inflammatory response. Successful treatment requires adherence to a regime that is uncomfortable at best and at worst painful. Topical treatments can be successful in motivated adherent patients willing to accept these side effects.
Introduction
Human papillomavirus (HPV) is a common viral infection. Studies, mostly of females with cervical infection, have shown that HPV infections are common and usually transient, and that most anogenital HPV infections resolve spontaneously in immunocompetent patients.Citation1,Citation2 HPV is even more prevalent in immunosuppressed patients than in immunocompetent patients.Citation3–Citation8 HPV infections are less likely to resolve, cause a variety of illnesses that progress more rapidly, and are more severe in Human immunodeficiency virus (HIV)-infected than in non-HIV-infected individuals. Persons who are coinfected with HIV and HPV also have higher levels of HPV, are often concurrently infected with multiple HPV types, and are more likely to have persistence of HPV infection.Citation4,Citation9–Citation14
Infection with specific oncogenic subtypes of HPV, particularly HPV 16 and 18, has been associated with development of anogenital neoplasia.Citation15,Citation16 HPV infection causes a precursor to cervical cancer, ie, cervical intraepithelial neoplasia.Citation17,Citation18 There is a similar causal association between HPV infection, which results in development of anal intraepithelial neoplasia (AIN), a precursor of anal squamous cell cancer.Citation19–Citation23 The incidence of anal cancer is increasing among men, particularly in HIV-infected men who have sex with men (MSM).Citation21–Citation24 While the frequency of progression of high-grade AIN to anal squamous cell cancer is uncertain, there are data suggesting that the long-term risk is in the range of 8.5%–13%.Citation23,Citation25,Citation26 A recent meta-analysis estimating the risk of anal cancer in HIV-infected MSM confirmed that the risk was substantial, and calculated an anal squamous cell cancer incidence in the highly active antiretroviral therapy era of 78 per 100,000 men.Citation27
Clinicians who provide care for patients with anorectal disease commonly encounter AIN in a number of clinical situations. Patients may present with symptomatic perianal or anal disease that on investigation is found to result from AIN. Some patients present with asymptomatic visible disease that is identified on physical examination. Others have AIN discovered after histopathological evaluation of tissue collected during surgical procedures for other anal diseases, eg, hemorrhoidectomy. However, increasingly, immunosuppressed persons who are at high risk for having persistent HPV infection and developing anal cancer are identified as having AIN after screening in primary care.Citation28 In the last decade, there have been developments in the medical and surgical options available for patients with AIN. These treatment options are continuing to evolve. For this review, studies concerning treatment of AIN published from 1992 to 2012 were sought using a combination of computerized and hand searches. In situations where results have been published sequentially from the same institution, the publication with the longest follow-up was used. This paper will review the currently available treatment strategies for AIN.
General principles of treatment
Clinicians treating AIN should be aware of the knowns and unknowns when they plan management of AIN with their patients. First, the primary goal when treating AIN is to prevent anal squamous cell cancer. It is well established that the prognosis of anal cancer is strongly associated with stage of the disease at diagnosis.Citation29,Citation30 Anal squamous cell cancer is often diagnosed at a late stage, and approximately 25% of newly diagnosed anal canal carcinomas are larger than 5 cm in diameter and clinically node-positive.Citation31 While it is accepted that high-grade intraepithelial neoplasia (HGAIN) is the anal squamous cell cancer precursor, the estimates of anal cancer risk are based on relatively small patient populations.Citation23,Citation25,Citation26 Therefore, with the risk of anal cancer being uncertain, some patients may choose the least aggressive treatment for AIN, which is periodic digital anal examination. This “watchful waiting treatment” enables detection and treatment of anal cancer at an earlier stage and is associated with an improved outcome.Citation29,Citation30
How best to treat HGAIN (AIN-2 or AIN-3) is controversial and cannot be answered directly with data at this time.Citation25,Citation32–Citation34 There are no randomized trials directly comparing different treatments.Citation32 Studies of AIN treatment are for the most part from single geographic locations, with patient populations that vary in degree of AIN and the proportion of the cohort that is immunosuppressed. Finally, the treatments reported for HGAIN, both medical and surgical, are not standardized. Reports of treatment outcome are mainly in the form of case series and open-label studies, with only a few randomized trials. Some treatments have been described only in case reports. With those caveats, there is general agreement that treatment of AIN depends on the size, number, location, and grade of lesions.Citation34 Other factors for clinicians to consider before recommending therapy are the duration of treatment, the expected treatment-related discomfort, the likelihood that the patient will adhere to treatment, and patient preference. Finally, available resources influence treatment as well as the experience of the clinicians treating the patient.
Despite the absence of complete data, clinicians and patients need to make informed treatment decisions. Patients can be confidently informed that condyloma acuminatum and low-grade intraepithelial neoplasia (LGAIN, AIN-1) have very low potential for malignancy.Citation34 Some, but not most, clinicians routinely treat LGAIN lesions, recognizing that LGAIN, especially in immunosuppressed populations, can progress to HGAIN. The rationale for treatment of LGAIN includes reducing the risk of progression to higher-grade AIN and to reduce patient anxiety. In our practice, we do not have the clinical capacity to treat patients with asymptomatic LGAIN routinely. Because LGAIN has low potential for malignancy, we recommend that patients with LGAIN attend for annual evaluation, including digital anal examination, anal cytology, and high-resolution anoscopy (HRA) for early detection of progression of HGAIN. We do treat patients with symptomatic LGAIN or LGAIN accompanying condyloma acuminata. We explain to patients that we treat their LGAIN lesions to reduce their symptoms and not to reduce the risk of cancer. We presently recommend some form of treatment to almost all patients who have HGAIN (AIN-2, AIN-3). We also recommend that patients with HGAIN who chose not to have treatment have periodic HRA evaluation for the purpose of early detection of progression to anal squamous cell cancer.
Finally, the goals of treating patients with HGAIN are preventing morbidity and mortality from anal cancer without causing disturbances of anal function, ie, continence of stools and flatus. These goals imply that patients with HGAIN would need to have at least several years of expected lifespan to benefit from HGAIN treatment. Therefore, we do not recommend treatment for patients with HGAIN and comorbid illnesses that predict a short-term survival.
Surgical excision for HGAIN
A survey in 2000 of 663 members of the American Society of Colon and Rectal Surgeons found that 87% of respondents chose surgical excision with margins as the treatment of choice for AIN-3.Citation36 However, the surgical treatment of HGAIN has subsequently become controversial because of reports of incomplete efficacy, frequent recurrence, and complications associated with excisional treatments.Citation23,Citation25,Citation26,Citation33,Citation35–Citation38 These reports started to appear in the 1990s when medical immunosuppression for patients with immunologically-based disease and organ transplants became more common, as well as increasing numbers of persons with immunosuppression resulting from HIV. The survey findings reflected surgical opinion at a time when the epidemiology of HGAIN was changing. Back then, HGAIN was most commonly identified in non-immunosuppressed patients, compared with the current situation where HGAIN is commonly identified in immunosuppressed patients.
The outcome of surgical treatment for HGAIN has been consistent from a number of institutions in several countries, and has resulted in a re-examination of treatment options and the development of alternative treatments (see and ).Citation23,Citation25,Citation26,Citation33,Citation35–Citation38 Understanding of these evolving data provided an important background for informed treatment planning in patients with HGAIN. Brown et al reported 34 patients with HGAIN who were treated surgically and followed for a median of 41 months from a single hospital in the UK.Citation33 Four of the patients were medically immunosuppressed. Patients with lesions smaller than 1 cm (15 of 34) treated with simple excision had no disturbance of anal function. HGAIN extended to the margins in 19 of 34 specimens. The margin of excision for AIN was difficult to determine for small and larger lesions, and the excision was frequently not curative. Macroscopic recurrences occurred in 14 of 34 patients, and 12 of these recurrences were at the resection margin and four of 14 patients required more than one excision for macroscopic disease. Five of 19 patients with more extensive disease had postoperative disturbance of anal function. Two had fecal incontinence requiring use of pads and two had fecal incontinence and anal stenosis of a severity requiring permanent colostomy. The authors commented that three patients whose initial histology showed HGAIN were found to have clinically unsuspected invasive anal cancer in the excised specimen. None of the patients with excisions progressed to invasive anal squamous carcinoma.Citation33 This patient cohort included patients with extensive disease that were treated aggressively with excisions. While no patient developed cancer, approximately 25% of patients with extensive disease had anal function defects postoperatively.Citation33
Table 1 Cohort characteristics of HGAIN treatment trialsTable Footnote*
Table 2 HGAIN, outcomes by treatment type
Scholefield et al reported on their experience caring for a patient population that included 35 patients with perineal or perianal AIN-3 who were followed for a median of 63 months.Citation26 As a result of “significant associated morbidity” from their earlier more aggressive surgical approaches to AIN-3, the authors, whose surgical practice was located at a university hospital in the UK, changed their approach to a more conservative surgical approach for AIN-3.Citation26,Citation37 Only patients who had AIN-3 limited to less than 30% of anal circumference were offered excision. None of the patients in their cohort was known to be HIV-infected, but six of 35 were being treated in the long term with immunosuppressants.Citation37 In 12 of 28 patients with localized disease treated by excision, at least one margin was not clear. Macroscopic recurrence of AIN-3 occurred in four patients. All six patients with known immunosuppression had multifocal AIN-3. Three of these six patients developed invasive anal squamous cell carcinoma during follow-up. None of the 28 patients with focal disease treated with excision developed anal squamous cell carcinoma or were reported to have postoperative disturbances of anal function.Citation37 The three patients who developed anal squamous cell carcinoma all had multifocal AIN-3 and were not offered excision. These data indicate that limiting surgery to patients with less extensive disease reduces disturbances in anal function that seriously affect quality of life.Citation26,Citation37 The data also imply that not treating immunosuppressed patients with extensive disease puts them at high risk for progression to anal squamous cell carcinoma.
The most recent study on excision of AIN is consistent with prior reports of excision treatment of HGAIN. Watson et al from New Zealand reported the outcome of 72 patients with AIN who were observed for a median follow-up of 60 months.Citation23 Approximately 30% of the patients were immunosuppressed, with 17 receiving immunosuppressants and five being HIV-positive. Postoperative disturbances of anal function were common, and nine patients developed fecal incontinence; of these, four required colostomy. Despite this aggressive treatment, eight of 72 patients (11%) progressed to invasive anal squamous cell carcinoma.Citation23 These studies, when taken together, have changed the view of excision therapy for HGAIN. It is now considered by many not to be an ideal treatment for patients with extensive or multifocal HGAIN. The findings leading to this change include incomplete excisions that leave clinically inapparent HGAIN at surgical margins that subsequently recur, frequent HGAIN recurrences even in patients whose surgical sites indicate that the HGAIN has been completely excised, and patients who have excisions for more than minimal disease often have clinically important post-procedure morbidity.Citation23,Citation26,Citation33,Citation37
As a result of these data, some colorectal surgeons have suggested that treatment of HGAIN, especially in immunocompromised persons, is futile because recurrences are frequent, and progression to anal cancer occurs despite excision.Citation25,Citation26,Citation37 It was further suggested that, because chemoradiation for small invasive anal carcinomas is effective and that colostomy can usually be avoided if lesions are identified early, persons with HGAIN should not be treated in an attempt to prevent progression of HGAIN to squamous cell carcinoma, but could instead be monitored regularly for progression and only treated if squamous cell carcinoma develops.Citation25,Citation37 A report of the outcome of “expectant management of dysplasia” from a practice located at a US University and Veterans Administration practice followed 40 HIV-infected patients with AIN for a mean of 32 months.Citation25 Patients were recommended to have a clinical examination every 6 months, with biopsies of new macroscopic or symptomatic disease. Of these 40 patients, 28 had AIN-3. During follow-up, three of the patients with AIN-3 developed squamous cell carcinoma 10, 16, and 84 months after their diagnosis of AIN-3. All three cancers were less than 2.5 cm in diameter.Citation25
Comparisons between these studies that used excision for treatment of HGAIN and observation must be interpreted cautiously because each study included different populations with varying extents of disease and proportions of immunosuppressed patients. The reports were from different practice settings using different surgical approaches and variable follow-up methodology. Despite these differences, it is clear that the patient population with HGAIN has changed in the last two decades. Depending on practice location and the local prevalence of HIV, in most areas, the majority of patients with HGAIN are now immunosuppressed and more likely to have multifocal disease, where examination limited to visible macroscopic disease underestimates the AIN disease burden.
High-resolution anoscopy
HRA is a relatively recently described technique for identifying AIN that combines magnification with anoscopy.Citation39 HRA offers patients the advantages of initial diagnosis and surveillance of AIN. HRA uses acetowhitening to identify areas of dysplastic tissue for closer evaluation. After short-duration exposure to 3%–5% acetic acid (vinegar), dysplastic epithelium demonstrates relatively greater acetowhitening relative to normal epithelium. This allows the clinician to identify and focus a magnified examination on the acetowhite areas. Magnification is provided by a standard colposcope and allows inspection of the entire circumference of the distal rectum, transformation zone, anal canal, and perianal region. Acetowhite areas when examined with magnification demonstrate characteristic vascular and surface changes of AIN (). These findings are associated with HGAIN, allowing for directed biopsies.Citation39 HRA requires no anorectal preparation and is performed as an outpatient procedure. After HRA-directed biopsies, patients can be discharged with no treatment or simple analgesics, and can continue their usual activities and diet. As a result, HRA with biopsy can be used to identify and map the area of the anus with AIN and can be performed periodically as part of surveillance to identify AIN before it becomes macroscopic.
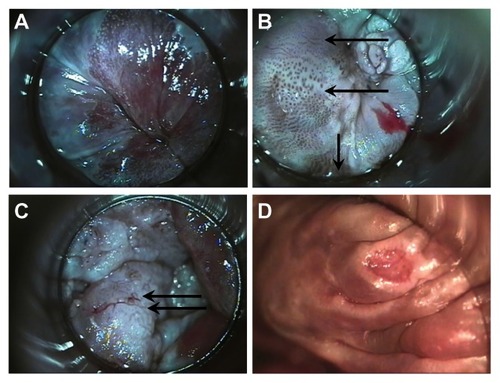
Figure 1 High-resolution anoscopy appearance of anal epithelium. (A) Normal and (B) vascular patterns of high-grade dysplasia; upper arrow points to fine irregular punctation, middle arrow points to coarse irregular punctation, lower arrow points to prominent and dilated linear vessels. Biopsies of all three areas were AIN-3. (C) Vascular patterns of high-grade dysplasia. Arrows point to mosaic pattern with punctation in the middle of the mosaic tiles. Biopsy showed squamous cell cancer in situ. (D) Surface findings of high-grade dysplasia include ulceration. Biopsy showed AIN-3.
HRA is a relatively simple procedure for the patient and clinician.Citation39 The pre-HRA patient visit includes an evaluation for risk of bleeding, medical risks, and patient education. We do not recommend any patient preparation and actively encourage patients not to take enemas or to try and cleanse themselves prior to the procedure. Such preparation can potentially affect cytology and the appearance of the anoderm. We occasionally prescribe benzodiazepines before the procedure for situational anxiety. Perioperative antibiotics are not recommended unless patients meet the recommendations for endocarditis or joint prophylaxis.Citation40,Citation41 HRA is most comfortable for the patient when performed in the lateral decubitus position or dorsal recumbent position. Once positioned, anal cytology is collected and 5% lidocaine is applied liberally to the anal canal during digital anal examination.Citation39 An anoscope coated with a mixture of surgical lubricant and 5% lidocaine cream is then used to introduce an applicator stick wrapped in gauze soaked in 5% acetic acid into the anal canal. After this has been in place for at least one minute, a colposcope is used to view the walls of the anal canal under magnification. Additional 5% acetic acid is applied to the areas being examined during the procedure, and the most abnormal acetowhite areas that are suspicious for AIN are biopsied with Baby Tischler Forceps. Homeostasis is achieved with Monsel’s solution.Citation39
Infrared coagulation of AIN
Infrared coagulation, which has been used successfully for treatment of internal hemorrhoids and condyloma, has recently been described as an outpatient technique for treating AIN.Citation42 Goldstone recognized that the standard surgical techniques had excessive morbidity and were unsuitable for treating multifocal disease, which is increasingly being identified in immunosuppressed patients. He theorized that intraepithelial AIN, which by definition is confined to the epidermis, would be amenable to infrared coagulation ablative therapy. He felt that infrared coagulation ablation, because of its limited depth of destruction and hemostatic effects, could be safely performed on outpatients by nonsurgeons (personal communication, Stephen E Goldstone). Subsequent to his initial description, there have been many reports of infrared coagulation ablation of AIN.Citation42–Citation47
Infrared coagulation ablation offers important advantages for patients being treated for AIN. Like HRA, infrared coagulation requires no anorectal preparation, and is performed as an outpatient procedure under local anesthesia. Infrared coagulation provides homeostasis simultaneously with tissue coagulation. Also, it does not create the vapor plume created by electrocautery or laser surgery, so does not require use of a smoke evacuator, which is rarely found in nonsurgical offices. The depth of tissue coagulation (in millimeters) is approximately equal to the length of the pulse applied. For example, a 1.5 second pulse penetrates the tissue to a depth of approximately 1.5 mm. This allows for local treatment without damaging deeper tissues. These characteristics have allowed infrared coagulation to be used successfully by nonsurgeons.Citation43–Citation45,Citation47 After infrared coagulation, most patients are able to continue their usual activities with simple analgesics and a high fiber diet after 1–2 days of oral narcotics and tub soaks. Infrared coagulation has a much lower frequency of functional adverse outcomes, eg, anal stenosis and fecal incontinence, than does excision.Citation43,Citation46 In additional, once healed from the treatment, infrared coagulation does not affect sexual function. As a result, infrared coagulation treatment is ideal for a disease that is often multifocal and recurrent.
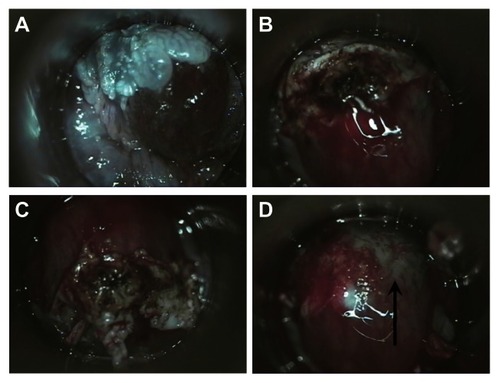
The first step in treating AIN with infrared coagulation is localization by HRA. Unlike the cervix, which remains stationary during colposcopy, the anal canal differs in appearance based on how and at what angle the anoscope is held. Therefore, AIN must be reidentified after biopsy confirmation. There are different methods used to aid in identification of AIN for treatment after biopsy. We document lesions suspicious for HGAIN to be biopsied in two ways. They are photographed and the appearance of lesions and biopsy sites on HRA are described in the medical record using a clock-face diagram, with the coccyx representing 12 o’clock. After the biopsy results are available, HRA is repeated and, using photographs, medical records, and biopsy reports, the areas with HGAIN are again identified and infiltrated with 1% lidocaine combined with 1:100,000 epinephrine for local anesthesia. Lesions are coagulated repeatedly with infrared coagulation in 1.6 second pulses until the entire surface and an approximately 3 mm surrounding border are coagulated. The coagulated tissue can then be removed with downward pressure combined with a rotary motion on the eschar using a large Scopette or small cotton Q-tip (). If the tissue remains adherent after repeating this maneuver several times, it can then be removed by curettage with a small Fox Dermal curette or with Baby Tichler forceps. Infrared coagulation is then repeated and the process continued until the submucosal vessels are identified and coagulated (). The number of HGAIN lesions that can be treated in a single procedure is dependent on the extent of disease and patient tolerance. All patients are given verbal and written post-procedure instructions. These include advice to consume a high-fiber diet (our instructions recommend consuming one cup of All Bran cereal daily), to avoid anal sexual activity until one week after last bleeding or anal pain, and sitting and soaking in a tub of hot water frequently for the first few days after infrared coagulation to reduce pain. We prescribe hydrocodone plus acetaminophen as needed for pain and before bowel movements to reduce pain for the first few days after the procedure.
Figure 2 (A) High-resolution anoscopic appearance of previously biopsied AIN-2 lesion. (B) Appearance of the lesion after IRC. Lesion has been twisted with a Scopette and the coagulated tissue is buckled up. (C) Eschar is completely loosened by additional pressure with twisting of Scopette. (D) Eschar removed, note coagulated (arrow) and noncoagulated submucosal vessels.
Comparisons of studies using infrared coagulation for the treatment of HGAIN must be interpreted cautiously because each study included different populations, was carried out in different practice settings, used slightly different infrared coagulation methods, and had different follow-up methodology. Some studies using infrared coagulation ablation for treatment included only persons with limited disease, others included patients with generalized disease, and no study as yet has correlated the extent of HGAIN disease with response to infrared coagulation.Citation41–Citation46 There were variable numbers of immunocompromised patients, and the studies included different proportions of HIV-infected MSM, non-MSM men, and women.Citation42–Citation47 The definition of HGAIN was also study-specific, with some authors using HGAIN histology as an endpoint and other studies using a histology-cytology composite endpoint for HGAIN.Citation42–Citation47 Some authors analyzed their study data by “HGAIN lesion” as the unit of observation while others used “patient”. HRA-guided identification of lesions was also not standardized, with some programs using 3.5% acetic acid and others using 5%.Citation42–Citation47 In addition, ablative infrared coagulation treatment was not standardized. One group used 1.6 second treatment pulses and treated a margin around the lesion.Citation47 Other studies reported using a 1.5 second treatment pulse and did not treat margins.Citation42–Citation47 Despite these differences, there was similar efficacy in patients treated with infrared coagulation, that was consistently in the range of 64%–87%.Citation42–Citation47 Postoperative disturbances of anal function were uncommon, with less than 1% of patients developing anal stenosis and no patients having fecal incontinence.Citation43,Citation46 The only study of infrared coagulation treatment that included a comparison group comprised patients who either delayed treatment or did not have treatment and were compared with treated patients. The authors found significantly more untreated than treated patients who had HGAIN on re-evaluation (93% versus 13%). Two of the untreated patients and none of the treated patients developed squamous cell carcinoma.Citation47 These data on infrared coagulation treatment, when viewed together across multiple study sites, different populations, and nonstandardized methods, demonstrate that infrared coagulation ablation is an effective treatment for HGAIN in HIV-infected patients.
HRA-guided electrocautery ablation
Another alternative ablative technique is HRA-guided electrocautery ablation, which has been reported by two US centers.Citation48,Citation49 HRA-guided electrocautery was initially described as an operating room technique for extensive HGAIN disease in 2002.Citation48 It has recently been reported again as a potential HGAIN treatment in a larger group with longer follow-up, including 132 HIV-positive MSM followed for a median of 19 months and 100 HIV-negative MSM followed for 17.5 months.Citation49 In the most recent study, all patients were treated with electrocautery ablation for HGAIN at the beginning of the observation period. The practice location was a single surgical practice in New York City specializing in the treatment of HPV-related anorectal disease.Citation49 Recurrence was defined as HGAIN confirmed by either cytologic or biopsy occurring after treatment. The frequency of recurrence was higher in patients who had multiple HGAIN lesions at presentation, ie, a higher disease burden. At final evaluation of the cohort, 83% of HIV-negative patients and 69% of HIV-positive patients were free of HGAIN. Only one patient (0.4%) developed squamous cell carcinoma during the follow-up period. Unlike infrared coagulation, hyfrecator electrocautery ablation creates a smoke plume that must be removed with a smoke evacuator to prevent aerosol transmission of HPV. The author, who has extensive experience in identifying and treating AIN, reported that no patients developed anal stenosis or incontinence.Citation49 The author compared his results using HRA-guided electrocautery ablation with infrared coagulation of HRA, and concluded that the outcome of electrocautery ablation was similar and that the choice of modality for ablation should be based on clinician preference. He further concluded that electrocautery ablation is an effective treatment for HGAIN and, like infrared coagulation, can be used safely in the office.Citation49
CO2 laser fulguration
CO2 laser fulguration of anal canal tumors has been used successfully to treat patients at high operative risk.Citation50 One study reviewed the experience of a single institution using CO2 laser, alone or in combination with surgical excision and/or imiquimod, to treat HGAIN and LGAIN.Citation51 The 141 subjects included primarily MSM. They found that 63% of patients were disease-free at 12 months. However, because the patients in this report were treated with multiple modalities, it is difficult to determine the independent effect of CO2 laser treatment.Citation51
Medical treatment options for HGAIN
Topical 5-fluorouracil
Use of medical treatments for AIN has gained increasing recognition. Topical fluorouracil (5-FU) and imiquimod have both been used successfully for treatment of a wide range of mucosal diseases, including actinic cheilitis, Bowen’s disease of the anal and vulvar mucosa, and genital and perianal condyloma.Citation52–Citation56 Topical 5-FU has a well established role in the treatment of actinic keratoses and nonmelanoma skin cancers.Citation53 Fluorouracil 5% cream or solution is a treatment approved by the United States Food and Drug Administration (FDA) for actinic keratosis and superficial basal cell carcinoma. 5-FU is an antimetabolite that interferes with the synthesis of DNA and to a lesser extent inhibits the formation of RNA. While there have been a number of trials of topical 5-FU in the prevention of progression of lower genital tract neoplasia to invasive carcinoma in HIV-positive and HIV-negative women, mucosal use of topical 5-FU remains off-label.Citation52–Citation56 Topical treatment potentially has substantial advantages for treating AIN because it can be applied by the patient and is a superficial treatment with minimal risk of long-term loss of anal function, particularly incontinence of stool and flatus or anal stenosis. Topical therapies are well suited for treating widespread multifocal disease. However, topical treatments have the disadvantage of requiring extended treatment courses and causing an inflammatory response that is at best uncomfortable and can be so painful as to be treatment-limiting.
Treatment of anogenital mucosal disease with 5-FU is not standardized, and the amount and frequency of 5-FU used are variable. There have been several case series specifically reporting the outcome of 5-FU treatment for AIN. In a private practice setting in Kansas, Graham et al prospectively treated 11 patients with histologically proven perianal Bowen’s disease using 5-FU 5% twice daily for 16 weeks.Citation57 Only one patient was immunosuppressed and had HIV. In addition, some of the patients had excision of focal macroscopic disease. Most patients had to interrupt 5-FU for 4–7 days and several had repeated interruptions. No patient missed more than 3 weeks of treatment. The patients had a mean follow-up of 39 months. The HIV-infected patient was the only nonresponder and the other 10 remained without evidence of AIN at follow-up.Citation57 In a retrospective study of the efficacy of 5-FU done at a university medical center, the patient population included 11 HIV-infected MSM with AIN.Citation58 Treatment-related discomfort resulted in one patient stopping therapy and seven reducing the frequency of treatment. Treatment duration was variable, from 7 to 36 weeks.Citation58 After treatment, the area of AIN was reduced in 55% of patients (six of 11). Further, the grade of dysplasia was reduced in 27% of patients (three of 11).Citation58
In a prospective trial from two European academic centers, 46 HIV-infected MSM were enrolled,Citation59 approximately 75% of whom had multifocal HGAIN. The purpose of this pilot study was to evaluate the safety and efficacy of treatment with 5% 5-FU for all grades of AIN. The treatment was one gram of 5-FU cream applied intra-anally twice weekly at night with a “standard anal applicator for cream”.Citation59 Patients were allowed to interrupt treatment for up to a week for severe symptoms or reduce the frequency to once weekly for mild symptoms. Side effects were frequent, and occurred in 85% of participants; 37% had mild side effects, and 48% had moderate to severe side effects. Five of 46 participants had treatment interruptions or reduced frequency of treatment because of side effects. Eighteen participants (39%) had complete clearance of AIN and eight (17%) had a partial response. Seventeen participants (37%) did not respond.Citation59 These data taken together demonstrate the efficacy of 5-FU, but also demonstrate that the optimal dose, frequency of administration, and duration of treatment are uncertain. In our practice, we use topical 5-FU for patients with extensive disease, including those with combined perianal and anal HGAIN. Our clinical impression is that 5-FU works well, but only for patients who are motivated enough to tolerate the side effects for treatment of extensive and often severely symptomatic AIN.
We spend a much greater amount of time in patient education when treating AIN with topical treatments than for other treatments, because we expect every patient to have clinically important treatment-related side effects and because all of the medications are used off-label. We discuss that AIN is not an FDA-approved indication for treatment with 5-FU. We discuss further that there are no FDA-approved medical treatments for AIN, and any proposed topical medical treatment would be off-label. We explain that the rationale for use of 5-FU comes from a variety of sources, including case series, open-label studies, and randomized controlled trials of other skin and mucosal diseases that support using topical 5-FU in the treatment of AIN. We also explain that the different reports using 5-FU for AIN have used different schedules of treatment and different concentrations and forms of 5-FU, and have had different outcomes.
At our clinic, we use an intermittent 6-month 5-FU treatment regimen following a one-month treatment run-in period. Our regime is designed to be intermittent, given that all published 5-FU treatment schedules used in HGAIN have required interruptions because of patient discomfort. Our regime generally allows treatment to occur without interruption. The one-month treatment run-in period is designed to give the patient experience with the discomfort of treatment and for the clinician to be able to assess the patient’s pain response and determine the need for analgesics prior to initiating the regime. There are two treatment cycles per month, on days 1–5 and days 15–20. These cycles allow the treatment days to be independent of the number of days in the month. During the first run-in cycle, 5-FU is used at bedtime on the first, third, and fifth day. The second cycle is once nightly for 5 nights. The patients are reassessed after the second run-in cycle to determine the extent of discomfort resulting from their treatment, to reassure them that the pain is not, as is usually interpreted a symptom of a problem, but instead is a sign of therapeutic efficacy of 5-FU. If the patient has severe discomfort, we give analgesics and if necessary treat with once-nightly cycles on the same schedule. If the patient is able to tolerate once nightly 5-day cycles, we increase the frequency of topical 5-FU to twice daily. The goal is 12 five-day cycles of treatment with twice daily 5-FU ().
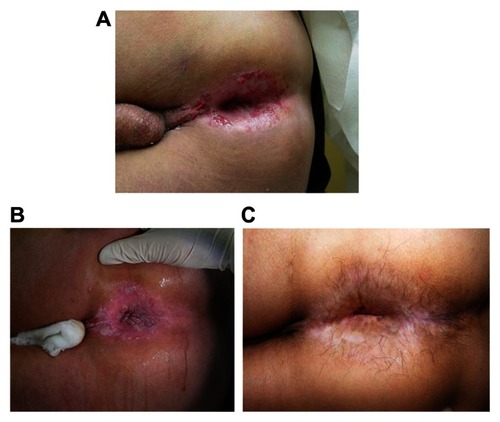
Figure 3 Medical treatment of intraepithelial neoplasia. (A) Appearance of perianal high-grade anal intraepithelial neoplasia. Multiple biopsies all showing either AIN-3 or squamous cell cancer in situ. (B) Perianal appearance after 5% acetic acid. (C) The patient was treated with topical 5-fluorouracil. This is the appearance of perianus after 2 years of observation.
Abbreviation: AIN, anal intraepithelial neoplasia.
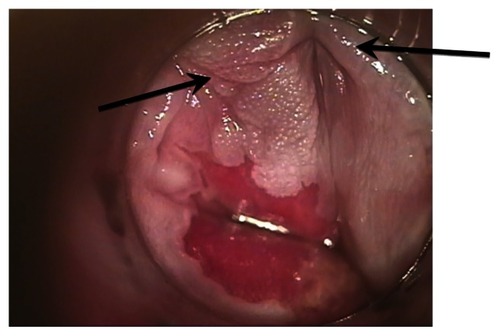
Applying medication to the entire anal canal is not simple for patients because it is applied blindly. The patient or their partner is instructed to put on latex gloves, put one quarter of a fingertip unit of 5-FU on the index finger, and rub the medication into the reachable anal canal. A fingertip unit of medication is the amount of ointment expressed from a tube from the distal interphalangeal joint to tip of the finger. Patients are advised to massage this medication into an approximate anal depth of between the first and second crease of their finger, ie, midway between the distal and proximal interphalangeal joints. The procedure is then repeated with the other hand so that the medication can be applied to the full anal canal. If there is perianal involvement, the same process is performed on the skin of the perianus. We carefully explain why using both hands is necessary, because the wrist cannot distribute the medication through 360 degrees. We have had several patients who have had diffuse disease prior to treatment who had an excellent response, but with focal failure of treatment. After questioning these patients on how they applied the medication, failure was attributed to applying the medication using only one hand and missing an area (). All patients receive printed instructions that repeat the education given verbally.
Figure 4 This figure illustrates the effect of incorrectly applying topical 5-fluorouracil. The patient applied 5-fluorouracil with one hand only and left one area of HGAIN incompletely treated. The arrows point to the HGAIN that was incompletely treated.
Patients are seen at least once a month and re-educated on application technique, and that signs of inflammation, including itching, burning, and pain, are evidence that the 5-FU treatment is working as expected, and should be interpreted positively as a sign of response and not negatively as a sign of an adverse reaction. We believe that the run-in period gives the patient the opportunity to experience less intense inflammation and tolerate twice-daily treatment better. It also allows the caregiver at one month follow-up evaluation an opportunity to prescribe analgesics or, if a very intense reaction occurs, to continue treatment with once daily 5-FU rather than increasing the treatment to twice daily. Most patients experience some irritation and pain after several days of treatment, that peaks after treatment day 5 and then resolves or subsides prior to the next treatment cycle. Examination during periods of irritation will reveal multiple superficial erosions. If the discomfort is still too severe when the next cycle is due to resume, then that cycle is skipped and resumed again at the next scheduled time. The missed cycle is added to the end of treatment. This simplifies the treatment regimen and reduces unintended nonadherence.
Topical imiquimod
Imiquimod is a topically applied agent with antiviral and antitumor activity, and works through an immunomodulator mechanism by increasing the activity of Toll-like receptors.Citation53 Imiquimod is approved by the FDA for the treatment of external genital and perianal warts, actinic keratoses, and superficial basal cell carcinoma, and is widely used.Citation53 In addition, imiquimod has been used off-label for treatment of a number of diseases. A study of reported off-label imiquimod use in 2009 found case reports, letters, and small trials documenting imiquimod use in over 60 conditions.Citation60 There are unique concerns about using imiquimod in the anal canal where, because of its proximity to the rectum, imiquimod could be more readily systemically absorbed. Unlike 5-FU that is routinely used intravenously and has systemic toxicity which is well known, the systemic toxicity of higher concentrations of imiquimod that could occur from rectal absorption is unknown.
Imiquimod is the best studied of the off-label medical treatments for AIN, and has been shown in multiple small studies to be an effective treatment for AIN.Citation61–Citation64 In a study from Germany, 10 HIV-positive MSM with different grades of AIN were treated with imiquimod 5% cream three times a week for a maximum of 16 weeks.Citation61 All patients showed regression of AIN by at least two grades. HPV 16 was not detectable by polymerase chain reaction in any of the patients at follow-up.Citation61 In a prospective study of 28 HIV-infected MSM with AIN from Germany who were treated with anal imiquimod suppositories, 17 (77%) of 22 compliant patients and 17 (61%) of all study patients were free of disease by clinical and histologic criteria after 16 weeks of therapy.Citation63 The authors also found the numbers of HPV copies and types were significantly reduced in adherent patients.Citation63 The significant reduction in HPV copies persisted during a median follow-up of 7 months. The authors theorized that the persistent reduction in HPV copies might have been due to induction of HPV-specific T cell immunity.Citation63 In a prospective, randomized, double-blind study of imiquimod versus placebo from the UK, the placebo group after re-evaluation was given the opportunity to use open-label imiquimod therapy.Citation64 The authors found that 61% (29 of 47) of the patients remained free of HGAIN after a median follow-up of 33 months.Citation64 These studies demonstrate that imiquimod was useful for treating patients with widespread multifocal AIN, who are difficult to treat.Citation61–Citation64 Imiquimod can be used as primary therapy or as an adjunct to destructive therapy.Citation51
Studies of imiquimod treatment for HGAIN share the same limitations, as do reports of other treatments for this condition. Studies have used different definitions of AIN, with some using histology as an endpoint and others using a combined endpoint of cytology plus histology.Citation61–Citation64 All have included only MSM, and extrapolating the outcomes of these studies to the broader AIN population which includes women, and non-MSM, non-HIV-infected, and non-immunosuppressed patients is not ideal.Citation62–Citation64 Different forms of treatment were used, with some using imiquimod suppositories that are not widely available and others using cream. Different amounts of imiquimod cream were used, with some studies using a half sachet of cream and other studies using a full sachet.Citation61–Citation64
We give patients similar detailed instructions about imiquimod treatment of HGAIN as described above for 5-FU treatment. Again, patient education is essential because we expect every patient to have clinically important treatment-related side effects to imiquimod, and its use is off-label. We discuss that treatment of AIN using imiquimod is not an FDA-approved use. We further discuss that there are no FDA-approved medical treatments for AIN, and any proposed topical medical treatment would be off-label. The patient or their partner is instructed to put on latex gloves, put one quarter of a sachet of imiquimod on the index finger, and rub the medication into the anal canal at bedtime on Monday, Wednesday, and Friday. The patients are advised to massage this medication into an approximate depth of between the first and second crease of their finger, ie, midway between the distal and proximal interphalangeal joints. The procedure is then repeated with the other hand so that the medication is applied to the entire anal canal. The dose we use per treatment is one half sachet of cream. If there is perianal involvement, the same process is performed on the skin of the perianus. Patients also receive printed instructions in addition to these verbal instructions.
Similar to the pretreatment education process used for patients treated with 5-FU, we educate patients that signs of inflammation, including itching, burning, and pain, are evidence that the imiquimod treatment is working, and are expected if treatment is successful. We discuss with them that these symptoms should be interpreted positively as a sign of response and not negatively as a sign of an adverse reaction. Further, we discuss that imiquimod can lead to transient flu-like symptoms on the day following treatment. We see patients after 2 weeks of treatment, and if they do not have symptoms or signs (erythema or erosions) of inflammation, we increase the imiquimod frequency to nightly Monday through Friday. We see patients monthly during the 16-week treatment course to treat discomfort and encourage adherence. If inflammatory symptoms cannot be controlled with simple analgesics, we advise the patients to hold the imiquimod until symptoms improve and then resume treatment.
Adherence with treatment is difficult to achieve with uncomfortable treatments. Typical levels of adherence are illustrated by 28 patients who were treated with imiquimod, of whom six (21%) applied the imiquimod occasionally or not at all.Citation61 Not surprisingly, those who did not use imiquimod regularly did not show any clinical response. Nonadherence with treatment recommendations for imiquimod is frequent and is likely related to the duration of treatment and treatment-related discomfort. Adherence is likely better with destructive surgical treatments or excisions than with topical treatments.
Trichloroacetic acid and bichloroacetic acid
Topical 85% trichloroacetic acid (TCA) or bichloroacetic acid are directly cytotoxic therapies that destroy tissue by chemical coagulation of proteins. They are recommended by the US Centers for Disease Control and Prevention as first-line treatments for condyloma acuminatum.Citation65 As a result, many clinicians are experienced in using TCA to treat anogenital condyloma acuminatum and TCA is readily available. TCA treatment has other advantages, including being inexpensive, not requiring anesthesia, being without systemic side effects, requiring minimal clinician training, and being safe for use in pregnancy.Citation65,Citation66 Treatment with TCA involves first identifying AIN lesions by HRA, and then a small amount of TCA is applied directly and repeatedly to the AIN lesion until it turns a dense white color.Citation66 The endpoint of treatment is when the entire lesion “frosts” and appears white. The procedure we use is to first pour several mL of TCA into a small cup. We then tilt the cup and dip the wooden stick end of a cotton swab and let it become saturated with TCA. We then apply the stick saturated with TCA to the AIN lesion, being careful to not get the TCA on adjacent normal tissues. Because TCA has low viscosity, it can spread easily to normal tissues that can also become chemically coagulated.Citation65 If this occurs, the TCA can be blotted up with the cotton end of the cotton swab. In addition, the TCA can, if necessary, be neutralized with sodium bicarbonate (ie, baking soda). The TCA should be allowed to dry before the patient sits or stands.
There has been a single report of TCA treatment in 54 patients with AIN. The study included a mixture of immunosuppressed patients, 65% of whom were HIV-infected. Patients were treated with topical TCA at intervals of 1–2 months for up to four applications. A median of two treatments was required for patients who had no evidence of AIN at follow-up HRA examination. When analyzed on a per lesion basis, 73% of AIN-1 patients and 71% of AIN-2/3 patients had no evidence of AIN on follow-up HRA examination. The response was better for patients with two or fewer lesions. Patients were not prescribed analgesics and reported that treatment-related side effects were minimal.Citation66 The authors concluded that TCA, because of its ease of use, low cost, and good safety profile, was a reasonable first-line therapy for patients with two or fewer AIN lesions.Citation66
Completing treatment of AIN
AIN does not occur in isolation. Anogenital HPV infection causes other intraepithelial neoplasias that are named for the affected site, eg, penile and vulvar intraepithelial neoplasia. These lesions are the precursors of other anogenital malignancies, including invasive anal, penile, vulvar, and cervical carcinoma. All of these conditions have an increased prevalence in immunosuppressed patients. Therefore, treatment of AIN cannot be considered complete until the patient with AIN has been evaluated for these associated lesions.
Preventing and treating AIN by vaccination
Preventing HPV infection, would be expected to prevent HGAIN and anal cancer. There are two HPV vaccines available that prevent infection with HPV 16 and HPV 18, which are the HPV strains responsible for the majority of cervical and anal cancers.Citation15–Citation20 One of the vaccines, in addition to preventing HPV 16 and HPV 18 infection, also prevents HPV 6 and HPV 11 infection, which cause the majority of genital warts. Both HPV vaccines have been shown to be effective in preventing cervical intraepithelial neoplasia, the precursor lesion of cervical cancer.Citation67,Citation68 These vaccines are widely recommended for girls and women, but are not routinely recommended for administration to boys and men.
There are recent data showing that HPV vaccination can reduce persistent anal HPV infection and HGAIN among immunized MSM. In a randomized, placebo-controlled, double-blind primary prevention study, the quadrivalent HPV vaccine or placebo was given to 4065 healthy men and boys.Citation69 Of these, 602 had sex with male partners. Analysis of this cohort demonstrated that persistent anal infection with HPV 6, 11, 16, or 18 was reduced by 60% in the intention-to-treat population and 95% in the per protocol cohort. HGAIN related to infection with HPV 6, 11, and 16 was reduced by 54% in the intention-to-treat population and 75% in the per protocol cohort.Citation69 In a retrospective secondary prevention study from a single anorectal disease practice in New York City, rates of recurrent HGAIN in non-HIV-infected MSM who accepted or declined the quadrivalent HPV vaccine as part of routine medical care were compared.Citation70 MSM who chose to receive the quadrivalent HPV vaccine were significantly less likely to have HGAIN or be infected with oncogenic HPV two years after vaccination.Citation70 There were no immunocompromised subjects enrolled in either of these studies, and the role of vaccination in preventing HGAIN in immunocompromised individuals is uncertain.
Summary
In the last two decades, the patient population with HGAIN has changed and the majority of those with HGAIN are now immunosuppressed. HRA mapping followed by ablation, either with infrared coagulation or cautery, overcomes some of the concerns associated with surgical excision of HGAIN. Ablation of AIN is a well tolerated destructive surgical procedure performed with local anesthesia on an outpatient basis, and is a useful treatment for a disease that should be expected to be recurrent. There are developing data suggesting that ablative treatment may change the natural history of HGAIN. Although there are many unanswered questions concerning ablative treatment in HIV-infected persons, the most important is whether successful ablative treatment of HGAIN reduces the incidence of anal squamous cell cancer and mortality in immunocompromised persons. The weakness of ablative treatment in HGAIN is that it treats the complications of HPV infection and does not directly treat the HPV infection itself. Determining the ideal method for destruction of HGAIN will require standardizing the treatment and then testing that treatment with carefully designed, multisite, randomized, clinical trials.
Available topical therapies have the advantage of being suitable for outpatient use and are well suited for treating widespread multifocal disease. Additionally, they offer the advantage of treating condyloma acuminatum, LGAIN, and HGAIN simultaneously. The principal disadvantage of the currently available topical therapies is they are difficult to apply and require adherence to a regime that is uncomfortable at best and at worst painful for months. Successful medical treatment of patients with HGAIN requires the treating clinician to work as an educator and a cheerleader to assist the patient in completing what is at best a long uncomfortable treatment. The goals of screening and treatment for HGAIN are reduction of morbidity, and treating patients when they have small, superficial, discrete premalignant lesions rather than when they progress to anal squamous cell carcinoma.
Acknowledgments
S Robert Harla and Jotam Pasipanodya are thanked for providing critical reviews of the manuscript. Dr Pasipanodya is also acknowledged for help with construction of the tables.
Disclosure
The author reports no conflicts of interest in this work.
References
- HoGYBiermanRBeardsleyLChangCJBurkRDNatural history of cervicovaginal papillomavirus infection in young womenN Engl J Med19983384234289459645
- WoodmanCBCollinsSWinterHNatural history of cervical human papillomavirus infection in young women: a longitudinal cohort studyLancet20013571831183611410191
- PalefskyJMMinkoffHKalishLACervicovaginal human papillomavirus infection in human immunodeficiency virus-1 (HIV)-positive and high-risk HIV-negative womenJ Natl Cancer Inst19999122623610037100
- CritchlowCWSurawiczCMHolmesKKProspective study of high grade anal squamous intraepithelial neoplasia in a cohort of homosexual men: influence of HIV infection, immunosuppression and human papillomavirus infectionAIDS19959125512628561979
- KiviatNRompaloABowdenRAnal human papillomavirus infection among human immunodeficiency virus-seropositive and -seronegative menJ Infect Dis19901623583611973695
- SunXWKuhnLEllerbrockTVChiassonMABushTJWrightTCJrHuman papillomavirus infection in women infected with the human immunodeficiency virusN Engl J Med1997337134313499358128
- BirkelandSAStormHHLammLUCancer risk after renal transplantation in the Nordic countries, 1964–1986Int J Cancer1995601831897829213
- AdamiJGabelHLindelofBCancer risk following organ transplantation: a nationwide cohort study in SwedenBr J Cancer2003891221122714520450
- DelMAInsaccoECinelABonaldiLMinucciDChieco-BianchiLHuman papillomavirus infections of the genital region in human immunodeficiency virus seropositive women: integration of type 16 correlates with rapid progressionEur J Gynaecol Oncol19941550588206072
- MayaudPGillDKWeissHAThe interrelation of HIV, cervical human papillomavirus, and neoplasia among antenatal clinic attenders in TanzaniaSex Transm Infect20017724825411463923
- JamiesonDJDuerrABurkRCharacterization of genital human papillomavirus infection in women who have or who are at risk of having HIV infectionAm J Obstet Gynecol2002186212711810079
- LeviJEKleterBQuintWGHigh prevalence of human papillomavirus (HPV) infections and high frequency of multiple HPV genotypes in human immunodeficiency virus-infected women in BrazilJ Clin Microbiol2002403341334512202576
- PalefskyJMHollyEARalstonMLJayNPrevalence and risk factors for human papillomavirus infection of the anal canal in human immunodeficiency virus (HIV)-positive and HIV-negative homosexual menJ Infect Dis19981773613679466522
- AhdiehLKleinRSBurkRPrevalence, incidence, and type-specific persistence of human papillomavirus in human immunodeficiency virus (HIV)-positive and HIV-negative womenJ Infect Dis200118468269011517428
- EgelkroutEMGallowayDBiology of genital human papillomavirusesHolmesKSparlingPStammWSexually Transmitted Diseases4th edNew York, NYMcGraw-Hill2008
- KhannaNBrooksSEChenTTSimsirAGordonNJTaylorGHuman papillomavirus absence predicts normal cervical histopathologic findings with abnormal Papanicolaou smears: a study of a university-based inner city populationJ Hum Virol2001428328711907386
- BoschFXLorinczAMunozNMeijerCJShahKVThe causal relation between human papillomavirus and cervical cancerJ Clin Pathol20025524426511919208
- WalboomersJMJacobsMVManosMMHuman papillomavirus is a necessary cause of invasive cervical cancer worldwideJ Pathol1999189121910451482
- HootsBEPalefskyJMPimentaJMSmithJSHuman papillomavirus type distribution in anal cancer and anal intraepithelial lesionsInt J Cancer20091242375238319189402
- AbramowitzLJacquardACJaroudFHuman papillomavirus genotype distribution in anal cancer in France: the EDiTH V studyInt J Cancer201112943343920839262
- FrischMGlimeliusBvan den BruleAJSexually transmitted infection as a cause of anal cancerN Engl J Med1997337135013589358129
- ZbarAPFengerCEfronJBeer-GabelMWexnerSDThe pathology and molecular biology of anal intraepithelial neoplasia: comparisons with cervical and vulvar intraepithelial carcinomaInt J Colorectal Dis20021720321512073068
- WatsonAJSmithBBWhiteheadMRSykesPHFrizelleFAMalignant progression of anal intra-epithelial neoplasiaANZ J Surg20067671571716916390
- D’SouzaGWileyDJLiXIncidence and epidemiology of anal cancer in the Multicenter AIDS Cohort StudyJ Acquir Immune Defic Syndr20084849149918614927
- DevarajBCosmanBCExpectant management of anal squamous dysplasia in patients with HIVDis Colon Rectum200649364016283561
- ScholefieldJHCastleMTWatsonNFMalignant transformation of high-grade anal intraepithelial neoplasiaBr J Surg2005921133113616044425
- MachalekDAPoyntenMJinFAnal human papillomavirus infection and associated neoplastic lesions in men who have sex with men: a systematic review and meta-analysisLancet Oncol20121348750022445259
- WeisSEVecinoIPogodaJMPrevalence of anal intraepithelial neoplasia defined by anal cytology screening and high-resolution anoscopy in a primary care population of HIV-infected men and womenDis Colon Rectum20115443344121383563
- GoldmanSAuerGErhardtKSeligsonUPrognostic significance of clinical stage, histologic grade, and nuclear DNA content in squamous-cell carcinoma of the anusDis Colon Rectum1987304444483595363
- JohnsonLGMadeleineMMNewcomerLMSchwartzSMDalingJRAnal cancer incidence and survival: the surveillance, epidemiology, and end results experience, 1973–2000Cancer200410128128815241824
- AjaniJAWinterKAGundersonLLFluorouracil, mitomycin, and radiotherapy vs fluorouracil, cisplatin, and radiotherapy for carcinoma of the anal canal: a randomized controlled trialJAMA20082991914192118430910
- MacayaAMunoz-SantosCBalaguerABarberaMJInterventions for anal canal intraepithelial neoplasiaCochrane Database Syst Rev201212CD00924423235673
- BrownSRSkinnerPTidyJSmithJHSharpFHosieKBOutcome after surgical resection for high-grade anal intraepithelial neoplasia (Bowen’s disease)Br J Surg1999861063106610460644
- Chin-HongPVPalefskyJMHuman papillomavirus anogenital disease in HIV-infected individualsDermatol Ther200518677615842614
- GoldstoneSA stand against expectant management of anal dysplasiaDis Colon Rectum2006491648164916972138
- ClearyRKSchaldenbrandJDFowlerJJSchulerJMLampmanRMTreatment options for perianal Bowen’s disease: survery of American Society of Colon and Rectal Surgeons MembersAm Surg20006668668810917483
- ScholefieldJHOgunbiyiOASmithJHRogersKSharpFTreatment of anal intraepithelial neoplasiaBr J Surg199481123812407953374
- MarchesaPFazioVWOliartSGoldblumJRLaveryICPerianal Bowen’s disease: a clinicopathologic study of 47 patientsDis Colon Rectum199740128612939369101
- JayNBerryJMHogeboomCJHollyEADarraghTMPalefskyJMColposcopic appearance of anal squamous intraepithelial lesions: relationship to histopathologyDis Colon Rectum1997409199289269808
- WilsonWTaubertKAGewitzMPrevention of infective endocarditis: guidelines from the American Heart Association: a guideline from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working GroupCirculation20071161736175417446442
- American Academy of Orthopaedic SurgeonsAntibiotic prophylaxis for bacteremia in patients with joint replacements Available from: http://www.aaos.org/about/papers/advistmt/1033aspAccessed August 29, 2012
- GoldstoneSEKawalekAZHuyettJWInfrared coagulator: a useful tool for treating anal squamous intraepithelial lesionsDis Colon Rectum2005481042105415868241
- PinedaCEBerryJMJayNPalefskyJMWeltonMLHigh-resolution anoscopy targeted surgical destruction of anal high-grade squamous intraepithelial lesions: a ten-year experienceDis Colon Rectum20085182983518363070
- StierEAGoldstoneSEBerryJMInfrared coagulator treatment of high-grade anal dysplasia in HIV-infected individuals: an AIDS malignancy consortium pilot studyJ Acquir Immune Defic Syndr200847566118156992
- CranstonRDHirschowitzSLCortinaGMoeAAA retrospective clinical study of the treatment of high-grade anal dysplasia by infrared coagulation in a population of HIV-positive men who have sex with menInt J STD AIDS20081911812018334066
- GoldstoneRNGoldstoneABRussJGoldstoneSELong-term follow-up of infrared coagulator ablation of anal high-grade dysplasia in men who have sex with menDis Colon Rectum2011541284129221904144
- WeisSEVecinoIPogodaJMSusaJSTreatment of high-grade anal intraepithelial neoplasia with infrared coagulation in a primary care population of HIV-infected men and womenDis Colon Rectum2012551236124323135581
- ChangGJBerryJMJayNPalefskyJMWeltonMLSurgical treatment of high-grade anal squamous intraepithelial lesions: a prospective studyDis Colon Rectum20024545345812006924
- MarksDKGoldstoneSEElectrocautery ablation of high-grade anal squamous intraepithelial lesions in HIV-negative and HIV-positive men who have sex with menJ Acquir Immune Defic Syndr20125925926522134151
- WatembergSLandauOAvrahamiRKaplanIGilerSKottISuccessful treatment of anal tumors with CO2 laser in elderly, high-risk patientsJ Clin Laser Med Surg1996141151179484086
- NathanMHickeyNMayuranathanLVowlerSLSinghNTreatment of anal human papillomavirus-associated disease: a long term outcome studyInt J STD AIDS20081944544918574114
- SillmanFHSedlisABoyceJGA review of lower genital intraepithelial neoplasia and the use of topical 5-fluorouracilObstet Gynecol Surv1985401902202984615
- LoveWEBernhardJDBordeauxJSTopical imiquimod or fluorouracil therapy for basal and squamous cell carcinoma: a systematic reviewArch Dermatol20091451431143820026854
- MaimanMWattsDHAndersenJClaxPMerinoMKendallMAVaginal 5-fluorouracil for high-grade cervical dysplasia in human immunodeficiency virus infection: a randomized trialObstet Gynecol19999495496110576182
- DansoDLyonsFBradbeerCCervical screening and management of cervical intraepithelial neoplasia in HIV-positive womenInt J STD AIDS20061757958416942648
- DuongTHFlowersLCVulvo-vaginal cancers: risks, evaluation, prevention and early detectionObstet Gynecol Clin North Am20073478380218061869
- GrahamBDJetmoreABFooteJEArnoldLKTopical 5-fluorouracil in the management of extensive anal Bowen’s disease: a preferred approachDis Colon Rectum20054844445015747068
- SnyderSMSiekasLAboulafiaDMInitial experience with topical fluorouracil for treatment of HIV-associated anal intraepithelial neoplasiaJ Int Assoc Physicians AIDS Care (Chic)201110838821266323
- RichelOWielandUde VriesHJTopical 5-fluorouracil treatment of anal intraepithelial neoplasia in human immunodeficiency virus-positive menBr J Dermatol20101631301130720716208
- GanjianSOurianAJShamtoubGWuJJMuraseJEOff-label indications for imiquimodDermatol Online J200915419624982
- GutzmerRKaspariMVogelbruchMSuccessful treatment of anogenital Bowen’s disease with the immunomodulator imiquimod, and monitoring of therapy by DNA image cytometryBr J Dermatol200214716016512100202
- KreuterAHochdorferBStuckerMTreatment of anal intraepithelial neoplasia in patients with acquired HIV with imiquimod 5% creamJ Am Acad Dermatol20045098098115153912
- WielandUBrockmeyerNHWeissenbornSJImiquimod treatment of anal intraepithelial neoplasia in HIV-positive menArch Dermatol20061421438144417116834
- FoxPANathanMFrancisNA double-blind, randomized controlled trial of the use of imiquimod cream for the treatment of anal canal high-grade anal intraepithelial neoplasia in HIV-positive MSM on HAART, with long-term follow-up data including the use of open-label imiquimodAIDS2010242331233520729710
- WorkowskiKABermanSSexually transmitted diseases treatment guidelines, 2010MMWR Recomm Rep201059111021160459
- SinghJCKuohungVPalefskyJMEfficacy of trichloroacetic acid in the treatment of anal intraepithelial neoplasia in HIV-positive and HIV-negative men who have sex with menJ Acquir Immune Defic Syndr20095247447919779306
- GarlandSMHernandez-AvilaMWheelerCMQuadrivalent vaccine against human papillomavirus to prevent anogenital diseasesN Engl J Med20073561928194317494926
- The FUTURE II GroupQuadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesionsN Engl J Med20073561915192717494925
- PalefskyJMGiulianoARGoldstoneSHPV vaccine against anal HPV infection and anal intraepithelial neoplasiaN Engl J Med20113651576158522029979
- SwedishKAFactorSHGoldstoneSEPrevention of recurrent high-grade anal neoplasia with quadrivalent human papillomavirus vaccination of men who have sex with men: a nonconcurrent cohort studyClin Infect Dis20125489189822291111