Abstract
Objective
To study outcomes of concurrent chemoradiotherapy (CCRT) or radiotherapy (RT) alone followed by radical surgery in patients with local advanced cervical cancer.
Methods
A retrospective approach was carried out in 174 Chinese patients with International Federation of Obstetricians and Gynaecologists stage IB2–IIIB cervical carcinoma. A total of 121 patients were treated with CCRT, while the remaining 53 patients received RT alone, and the regimen of chemotherapy was weekly cisplatin (40 mg/mCitation2). Pathological response, overall survival (OS), progression-free survival (PFS), and complications were analyzed.
Results
The median age was 45 years and the mean primary tumor diameter was 4.8 ± 1.0 cm. Pathological complete response (CR) was achieved in 53 patients (30.5%). The CR rate was relatively higher in the CCRT group (31.4% vs 28.3%, P = 0.724), particularly when tumor diameter was less than 5 cm (38.2% vs 30.8%, P = 0.623). With median follow-up of 24 months, patients with CR had improved 3-year OS (100% vs 83.6%, P = 0.018) and 3-year PFS (93.1% vs 83.2%, P = 0.035) compared to patients with residual disease. CCRT was associated with significantly improved 3-year PFS (92.0% vs 76.5%, P = 0.032) compared to RT alone in patients with tumor diameter less than 5 cm. Thirty-seven patients (21.3%) experienced more than grade 2 toxicity, and one patient (0.6%) developed grade 3 uronephrosis. Data thus indicated that pathologic response, tumor size, and lymph-node involvement were highly correlated with clinical outcomes of the local advanced cervical disease.
Conclusion
Preoperative CCRT achieved outcomes superior to RT alone, depending on the pathologic response, tumor size and lymph-node involvement as major prognostic factors.
Introduction
Cervical carcinoma represents the second-leading cancer affecting women’s health worldwide, and is more serious in China, especially in the northwest regions. Although concomitant chemoradiotherapy (CCRT) has been recommended as standard treatment for local advanced cervical carcinoma (LACC) since 1999, 5-year overall survival (OS) of LACC patients is not satisfactory presently, and it is still crucial to explore a more effective therapeutic strategy for further OS improvement of LACC.Citation1–Citation6 The treatment strategy of cervical carcinoma has been changed significantly in the past two decades. Cisplatin-based CCRT has become the standard of care for LACC patients in most developed counties in the world.Citation7 CCRT can reduce tumor recurrence and metastasis by 30%–50% and improve OS by 9%–18% as well.Citation8 Nevertheless, it is still not clear whether CCRT can provide a significant advantage for LACCs, eg, stages III–IVA, in comparison with radiotherapy (RT) alone or in combination following radical surgery.Citation3,Citation9
Several previous observations focusing on neoadjuvant CCRT followed by surgery were reported, with findings that the extent of postoperative pathological response impacted on OS and progression-free survival (PFS) of patients with cervical cancer.Citation2,Citation10,Citation11 A meta-analysis study also demonstrated that survival benefit of CCRT might be restricted to lower-stage patients with International Federation of Obstetricians and Gynaecologists (FIGO) stage IB–IIA, IIB having an increase in OS of 10% and 7%, respectively, by CCRT.Citation12 However, these previous studies did not define effects of primary tumor size, treatment modality, and preoperative radiation dose on pathological response and survival of patients. Therefore, the aim of this study is to observe if any different outcomes may occur between CCRT and RT alone followed by radical surgery in patients with FIGO stage IB2– IIIB cervical cancer by analysis of postoperative pathologic response, tumor diameter, and lymph-node involvement as major prognostic factors.
Materials and methods
Patient population
From April 2006 to June 2011, 174 patients with cervical carcinoma (FIGO stage IB2–IIIB, according to the pelvic examination) were treated in the Department of Radiation Oncology, Xijing Hospital, Fourth Military Medical University, China. Pretreatment evaluations were included as follows: patient’s disease history, blood counts, liver and renal function tests, gynecological examination, tumor biopsy, chest X-ray, transvaginal ultrasound (TVS), transabdominal ultrasonography, and/or pelvic magnetic resonance imaging (MRI). Patient characteristics are shown in detail in . Tumor sizes were basically determined by TVS examination. All patients had Karnofsky performance status ≥ 70 and had no history of other malignancy or cancer therapy.
Table 1 Characteristics of 174 patients with iB2–iiiB cervical carcinoma
Neoadjuvant chemoradiotherapy or radiotherapy alone
Preoperative pelvic RT was delivered using three-dimensional conformal radiation techniques and 6 or 15 MV photons using a linear accelerator (Clinac 23EX or 600 C/D; Varian Medical Systems, Palo Alto, CA, USA). Patients were immobilized with a custom vacuum mattress in the supine position and underwent a computed tomography (CT) simulation scan (PQS + AcQSim; Philips, Amsterdam, Netherlands) with intravenous contrast, using 5 mm slice thickness. Simulation images extended from L1 to 5 cm below the ischial tuberosities.
The clinical target volume (CTV) included the gross tumor, cervix, uterus, parametria, the upper part of the vagina to 3 cm below the tumor invasion (according to T2-weighted MRI image), and regional lymph nodes (common, external, internal iliac lymph nodes, obturator and presacral lymph nodes). The lymph nodes were delineated according to guidelines for delineation in pelvic intensity-modulated radiotherapy (IMRT).Citation13 The planning target volume (PTV) was defined by a uniform three-dimensional expansion around the CTV, using 7 mm margins around the lymph nodes; 10 mm around the vagina and, parametria; and 15 mm around the cervix and gross disease. The treatment planning was designed and computed using the Plato system version 2.7.5 (Varian). Pelvic irradiation dose was 40–50 Gy in 20–25 fractions.
The CT-based image-guided brachytherapy was applied to treat 16 patients with large and poorly responding tumors at the conclusion of pelvic external beam radiotherapy (EBRT). High-dose-rate brachytherapy was delivered with 5–22 Gy in 1–3 fractions to 90% of the high-risk CTV (D90 for HR-CTV), using interstitial implantation or an intracavity applicator (microSelectron-HDR Ir-192 set or the Fletcher applicator set; Nucletron, Veenendaal, Netherlands). The CCRT was administered to 121 patients by an intravenous infusion of weekly cisplatin (40 mg/mCitation2) during pelvic EBRT. Patients received either three (n = 30, 25%), four (n = 46, 38%), or five (n = 45, 37%) cycles of cisplatin. Chemotherapy was held under the following conditions: white blood cell count < 2.0 × 1 0 Citation9/L, absolute neutrophil count , 1.0 × 10 Citation9/L, platelet count < 50 × 10Citation9/L or grade 3–4 radiation enteritis or cystitis.
Surgery and histopathological examination
All patients underwent radical abdominal hysterectomy and pelvic lymphadenectomy, with the median number of lymph nodes removed being 18 (range 6–35). The interval between preoperative RT and radical surgery was 2–3 weeks. The resected primary and lymph-node specimens were prepared on hematoxylin and eosin stain slides. Pathological response to neoadjuvant therapy was evaluated based on the histo-pathological examination of resected specimens (ie, uterus, vaginal cuff, parametria, pelvic lymph nodes). Pathological response of primary tumor was classed as pathological complete response (CR), partial response (PR), or residual carcinoma (RC). Pathological response of resected lymph nodes was described in terms of lymph-node involvement (involved or not involved).
Follow-up observations
Toxicity assessment was performed according to the Radiation Therapy Oncology Group/European Organization for Research and Treatment for Cancer late-radiation morbidity-scoring scheme.Citation14 Surgical complications were classified by the Chassagne grading system.Citation15,Citation16 After completion of treatment, patients were followed at 3-month intervals for the first 12 months, and at 6-month intervals thereafter. Patients were followed up regularly with gynecological examination, laboratory studies (blood counts, liver and renal function tests), TVS, transabdominal ultrasonography, superficial lymph-node examination, and radiographic studies, such as chest CT and/or pelvic MRI.
Data collection and statistical analysis
The pelvic tumor control, OS, and PFS were calculated from the date of surgery to the last date of follow-up. Death in the absence of progression was censored in the calculation of PFS. The Kaplan–Meier method and log-rank test were used to estimate outcomes and effects on OS and PFS, respectively. Multivariate analysis of prognostic factors was performed with Cox proportional hazards regression. The chi-squared test was used to compare proportions between different groups. SPSS (IBM, Armonk, NY, USA) software was used for all statistical analyses, and P-values less than 0.05 were considered statistically significant.
Results
Patient characteristics and postoperative pathologic response
Median age of 174 patients was 45 years (range 25–66). The mean tumor diameter was 4.8 ± 1.0 cm (range 4.1–8 cm), as measured by TVS. Pathologic subtypes were predominantly squamous cell carcinoma (n = 163, 93.7%), with a minority having adenocarcinoma (n = 8, 4.6%) or adenosquamous carcinoma (n = 3, 1.7%). The characteristics and postoperative pathological response of 174 Chinese patients with LACC are summarized in .
Survival outcomes of CCRT in comparison with RT alone
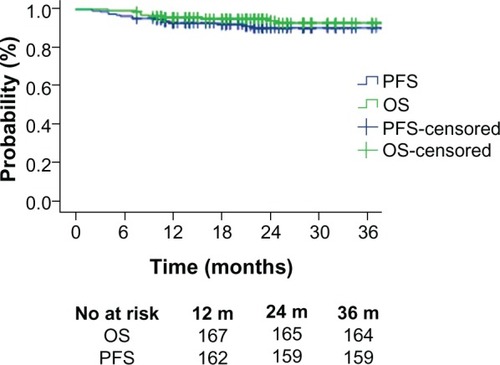
Median follow-up from the date of surgery was 24 months (range 4–68 months). The 3-year PFS and OS were 90.6% and 93.1%, respectively (). The 3-year local pelvic control was 97.1% (n = 169). Death was reported in eleven patients (11/174, 6.3%), from distant metastasis (7/174, 4.0%), pelvic recurrence (2/174, 1.1%), severe complication of renal failure (1/174, 0.6%), and second primary cancer (1/174, 0.6%). There was no statistical significance between CCRT and RT alone in metastasis rate (3/121, 2.5% vs 4/53, 7.5%; P = 0.202) and local recurrence rate (2/121, 1.7% vs 0/53, 0%; P = 1.000), but the tendency showed CCRT could possibly decrease the metastasis rate. In order to exclude the confounding factor, subgroup analysis classified by tumor size and postoperative pathologic response were performed.
Figure 1 Kaplan–Meier survival curves of 174 patients treated with concurrent chemoradiotherapy or radiotherapy alone followed by a radical hysterectomy.
Abbreviations: OS, overall survival; PFS, progression-free survival.
On multivariate analysis, the following prognostic factors were highly correlated with survival: primary tumor diameter, age, postoperative pathologic response, and pelvic lymph-node involvement status ( and ). Positive lymph-node involvements were confirmed in 15 patients (twelve patients in the CCRT and three patients in the RT alone group). There was no statistically significant difference in lymph-node involvement status between CCRT and RT alone (12/121, 9.9% vs 3/53, 5.7%; P = 0.058).
Figure 2 (A–C) Three major pathological responses, ie, complete response (CR), partial response (PR), and residual carcinoma (RC) in patients treated with preoperative concurrent chemoradiotherapy or radiotherapy alone. CR (A) shows mainly inflammatory cell infiltration, PR (B) shows presence of persistent atypical cells or cervical intraepithelial neoplasia, and RC (C) shows residual tumor tissue or tumor cells in cervical tissue.
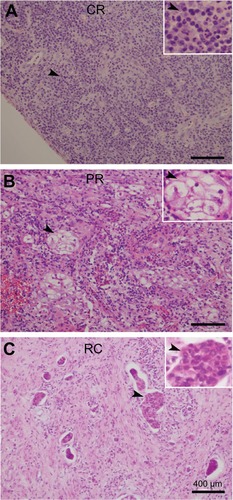
Table 2 Correlations of major clinical/pathological factors and overall survival
OS was not significantly associated with pathologic subtype (squamous cell carcinoma vs nonsquamous cell carcinoma, 3-year PFS, 93.1% vs 91.7%, P = 0.527; 3-year OS, 93.3% vs 91.7%, P = 0.588) or FIGO stage (IB2–IIB vs IIIB, 3-year PFS, 89.9% vs 100%, P = 0.461; 3-year OS, 92.5% vs 100%, P = 0.517). The treatment modality (CCRT vs RT alone, 3-year PFS, 92.0% vs 86.7%, P = 0.069; 3-year OS, 95.0% vs 88.1%, P = 0.124) and preoperative EBRT dose (40–45 Gy vs 46–50 Gy, 3-year PFS, 93.2% vs 89.0%, P = 0.094; 3-year OS, 94.6% vs 91.9%, P = 0.177) were not significantly associated with OS and PFS of patients with LACC ().
To observe any complications with the median follow-up period of 24 months, 37 of the patients experienced late grade ≥ 2 complications. Seven patients (7/174, 4.0%) experienced grade 3 complications, without significant differences between the CCRT and RT-alone groups (21.5% vs 20.8%, P = 0.782). Among these, one patient developed renal failure due to severe uronephrosis, six patients experienced two complications, and one patient experienced three complications ().
Table 3 Late complications in patients treated with preoperative CCRT or RT alone
Pathologic response as one key prognostic factor in CCRT and RT alone
The postoperative pathologic response included CR, PR, and RC. CR was defined as a complete disappearance of all macroscopic and microscopic diseases and mainly showing inflammatory cell infiltration, PR defined as presence of persistent atypical cells or cervical intraepithelial neoplasia, and RC defined as macroscopic and/or microscopic residual disease (). The CR rate was 30.5% (53/174), PR rate was 31.6% (55/174), and RC rate was 37.9% (66/174). Among patients with RC, 18 had greater than one-third cervical stromal invasion, four developed capillary-like space involvement, and one had ovarian invasion. Survival status was benefited in the CCRT group in comparison with that of the RC-alone group (P = 0.035, 94.9% vs 84.0% for 3-year PFS; P = 0.018, 100% vs 87.3% for 3-year OS) (). The data of this study did not show obvious differences in CR rate between CCRT and RT alone in all 174 cases (38/121, 31.4% vs 15/53, 28.3%; P = 0.724); however, CCRT showed potential benefit for CR rate enhancement (CCRT, 21/55, 38.2% vs RT alone, 8/26, 30.8%; P = 0.623) when tumor size was less than 5 cm.
Figure 3 Survival curves of progression-free survival (PFS) (A) and overall survival (OS) (B) of local advanced cervical carcinoma patients with pathological complete response, partial response, and residual carcinoma after preoperative concurrent-chemoradiotherapy (CCRT) or radiotherapy (RT)-alone modalities.
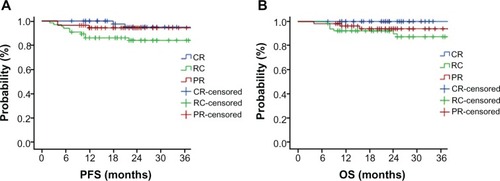
Treatment failures occurred in 15 patients (8.6%) and consisted of local-regional recurrence in five patients (2.9%) and distant metastasis in ten patients (5.7%), mainly in the RC group (three patients with regional recurrences and seven patients with metastases). None of the regional recurrences occurred in the CR group, except for one patient with metastasis. Two patients with distant metastases and two patients with regional recurrence were observed in the PR group.
Tumor size as another prognostic factor in CCRT or RT alone
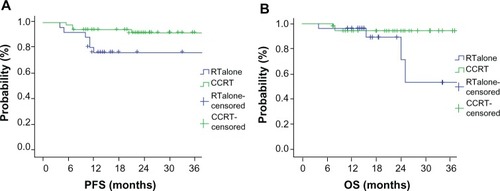
According to Cox regression analysis, there was no statistical difference in survival between preoperative CCRT and RT alone (3-year PFS, 92.0% vs 86.7%, P = 0.069; 3-year OS, 95.0% vs 88.1%, P = 0.124) for all 174 patients. Nevertheless, preoperative CCRT did have significantly improved 3-year PFS (P = 0.032, 92.0% CCRT vs 76.5% RT alone), but not OS (P = 0.055, 94.4% CCRT vs 53.6% RT alone) compared with RT alone when tumor size was less than 5 cm. Statistical data analysis for those 81 patients with tumor size less than 5 cm treated with CCRT or RT alone is shown in .
Figure 4 Survival curves of 3-year progression-free survival (PFS) (A) and overall survival (OS) (B) in 81 local advanced cervical carcinoma patients with tumor size less than 5 cm and treated with preoperative concurrent-chemoradiotherapy (CCRT) or radiotherapy (RT) alone.
Preoperative EBRT dose analysis
Patients with a higher preoperative EBRT dose had an improved pathological CR (40–45 Gy/20–25 f vs 46–50 Gy/23–25 f, 15% vs 35.6%; P = 0.002), even though EBRT dose did not influence OS and PFS. Relationships between EBRT dose and local control (P = 0.704), lymph-node involvement (P = 0.521), and complications (P = 0.667) were all analyzed, but none of these results showed statistical significance.
Discussion
In this retrospective approach, CCRT achieved an outcome superior to RT alone in 174 Chinese patients with FIGO stage IB2–IIIB cervical carcinoma. A new finding of this study is that preoperative CCRT was associated with significantly improved PFS and OS compared with RT alone when the tumor size was less than 5 cm. Although CCRT has become the standard of care for LACC and systemic surgery was not recommended in a routine procedure, 3-year OS (74%) or 5-year OS or PFS (50%–63%) of the standard CCRT alone were still not satisfactory.Citation17 On the other hand, a clinical trial showed a contribution of surgery to the patients with bulky residual disease after CCRT for cervical carcinoma by increasing OS and local control rate.Citation18 We applied preoperative CCRT/RT by pelvic radiation of 40–50 Gy instead of radical radiation for the patients with LACC in this study. Interestingly, our data showed preoperative CCRT achieved better outcome in comparison to RT alone for LACC with acceptable low toxicity and complications. This study suggests that a combination of preoperative CCRT and radical surgery may provide a feasible and effective treatment for patients with LACC, though further comprehensive investigation is needed for modified concurrent chemotherapy in improved treatment of patients with late-stage or bulky tumor size.
Postoperative pathological response was highly related with OS and PFS. RC after surgery was identified as a high-risk factor for local failure.Citation11,Citation19,Citation20 So far, there are no consensus criteria for postoperative pathological response classification after neoadjuvant treatment for LACC. Huguet et al found that PFS was significantly different when the size of histological residual tumor in uterine cervix was more or less than 5 mm.Citation21 Ferrandina et al observed clinical results of 161 patients with LACC treated with neoadjuvant chemo-radiotherapy and radical surgery.Citation22 PFS and OS were lower for patients with macroscopic residual disease compared to patients with histological CR and microscopic residual disease. Classe et al gave a similar conclusion in their analysis of 175 patients with FIGO stage IB2–IVA cervical cancer.Citation20 In our study, patients with pathological CR had better PFS and OS compared to patients with pathological residual disease. Besides, we found that 55 patients (31.6% of all patients) with pathological atypical cells and cervical intraepithelial neoplasia had different outcomes compared to patients with pathological CR and residual disease. The clinical significance and histological significance of atypical cells and cervical intraepithelial neoplasia after CCRT should require longer follow-up and identification of specific biomarkers. As for the dose delivered by pelvic EBRT, 40–45 Gy/20–25 f was reported in some previous studies,Citation20,Citation21 whereas we further demonstrated that a dose of 46–50 Gy/23–25 f could increase pathological CR without increasing severe complications in this study.
The precise relationship between tumor size and progression is still not clearly understood in LACC, though primary tumor diameter was regarded as one of the important prognostic factors.Citation19,Citation23–Citation25 While it was shown that 5-year local control and DFS were 96% and 95%, respectively, when tumor size was less than 5.2 cm, while 5-year local control and DFS were 67% and 66% when the tumor diameter was more than 5.2 cm, Hirakawa et al reported that tumor size was an independent prognostic factor for locoregional failure and survival on multivariate analysis.Citation19 Data from our study revealed that the OS and PFS were significantly different when the tumor size was less than 5 cm or ≥5 cm. The results of our study are consistent with an observation by Baiocchi et al.Citation26 They found that tumor size larger than 5 cm did not correlate with the risk of recurrence and death from cancer, and we further showed preoperative CCRT improved PFS and OS in comparison with that of RT alone when patients had a tumor diameter less than 5 cm, indicating that beneficial effect of CCRT on PFS and OS of patients might be limited or depend on tumor size of cervical cancer.
In Huang et al’s research, the prognostic factors contained incomplete tumor regression, a low hemoglobin level, and positive lymph-node metastasis.Citation27 The survival state might depend on the number of lymph nodes involved: those with four or more involved lymph nodes had worse cause-specific survival compared with patients with one to three involved lymph nodes.Citation28 Most research has focused on how to reduce the rate of pelvic lymph-node metastasis rate recently. In Huguet et al’s report, pelvic lymph-node metastasis rate was reduced to 7.8% after CCRT based on cisplatin and 5-FU in IB–IIB stage cervical carcinoma.Citation29 As a contrast, in Kirova et al and Colombo et al, after CCRT based on weekly cisplatin, the pelvic lymph-node metastasis rate was 20.0% and 22.5%, respectively.Citation30,Citation31 For stage IB–IVA, Classee et al reported that the rate was 32.5% after CCRT based on weekly cisplatin, and this rate could be reduced to 16.1% by CCRT based on cisplatin and 5-FU in Macchia et al’s study.Citation32,Citation33 Taken together with our observation in this study, postoperative pathologic response, tumor diameter, and lymph-node involvement might be highly correlated with clinical outcomes of patients with LACC.
In conclusion, this retrospective study has shown that CCRT followed by radical surgery achieved a better outcome compared with RT alone in LACC patients, based on pathologic response, tumor diameter, and lymph-node involvement. The novelty of this study is that preoperative CCRT was associated with significantly improved PFS and OS compared with RT alone when tumor size was less than 5 cm. We should indicate that this observation is still retrospective and from a pilot study, which might be a limitation along with a lack of sufficiently balanced numbers of patients. Nevertheless, these findings may deserve further comprehensive investigation in a randomized clinical trial.
Acknowledgments
This work was supported in part by a grant from the National Science Foundation of China (81272346 and 30970862). Authors also thank Dr Loren K Mell, Center for Advanced Radiotherapy Technologies, Department of Radiation Oncology, University of California San Diego, CA, USA, for his critical reading and valuable suggestions on this manuscript revision.
Disclosure
The authors report no conflicts of interest in this work.
References
- PetersWA3rdLiuPYBarrettRJ2ndConcurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervixJ Clin Oncol2000181606161310764420
- KeysHMBundyBNStehmanFBCisplatin, radiation, and adjuvant hysterectomy compared with radiation and adjuvant hysterectomy for bulky stage IB cervical carcinomaN Engl J Med19993401154116110202166
- MorrisMEifelPJLuJPelvic radiation with concurrent chemotherapy compared with pelvic and para-aortic radiation for high-risk cervical cancerN Engl J Med19993401137114310202164
- ThomasGMImproved treatment for cervical cancer: concurrent chemotherapy and radiotherapyN Engl J Med19993401198120010202172
- National Cancer InstituteClinical announcement on cervical cancerBethesda (MD)National Institutes of Health1999
- JemalAMurrayTWardECancer statistics, 2005CA Cancer J Clin200555103015661684
- KatoSOhnoTThephamongkholKMulti-institutional phase II clinical study of concurrent chemoradiotherapy for locally advanced cervical cancer in East and Southeast AsiaInt J Radiat Oncol Biol Phys20107775175719836154
- PótiZPatyánikMNemeskériCRadiochemotherapy of cervical carcinoma. (Necessity of dose reduction in chemotherapy)Orv Hetil200614713151320 Hungarian16999017
- TabataTTakeshimaNNishidaHHiraiYHasumiKA randomized study of primary bleomycin, vincristine, mitomycin and cisplatin (BOMP) chemotherapy followed by radiotherapy versus radiotherapy alone in stage IIIB and I VA squamous cell carcinoma of the cervixAnticancer Res2003232885289012926129
- FerrandinaGLeggeFFagottiAPreoperative concomitant chemoradiotherapy in locally advanced cervical cancer: safety, outcome, and prognostic measuresGynecol Oncol2007107S127S13217727936
- MariagraziaDAnnaFGabriellaFPreoperative chemoradio-therapy in locally advanced cervical cancer: long-term outcome and complicationsGynecol Oncol200599S166S17016150482
- Chemoradiotherapy for Cervical Cancer Meta-Analysis CollaborationReducing uncertainties about the effects of chemoradiotherapy for cervical cancer: a systematic review and meta-analysis of individual patient data from 18 randomized trialsJ Clin Oncol2008265802581219001332
- TaylorARockallAGReznekRHPowellMEMapping pelvic lymph nodes: guidelines for delineation in intensity-modulated radiotherapyInt J Radiat Oncol Biol Phys2005631604161216198509
- CoxJDStetzJPajakTFToxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC)Int J Radiat Oncol Biol Phys199531134113467713792
- ChassagneDSismondiPHoriotJCA glossary for reporting complications of treatment in gynecological cancersRadiother Oncol1993261952028316648
- DindoDDemartinesNClavienPAClassification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a surveyAnn Surg200424020521315273542
- Dueñas-GonzálezAZarbáJJPatelFPhase III, open-label, randomized study comparing concurrent gemcitabine plus cisplatin and radiation followed by adjuvant gemcitabine and cisplatin versus concurrent cisplatin and radiation in patients with stage IIB to IVA carcinoma of the cervixJ Clin Oncol2011291678168521444871
- HouvenaeghelGLelievreLButtarelliMContribution of surgery in patients with bulky residual disease after chemoradiation for advanced cervical carcinomaEur J Surg Oncol20073349850317156969
- HirakawaMNagaiYToitaTHigh-risk group for locoregional recurrence in patients with stage IB-IIB squamous cell carcinoma of the cervix treated with concurrent chemoradiotherapyAnticancer Res2011311437144121508399
- ClasseJMRauchPRodierJFSurgery after concurrent chemoradiotherapy and brachytherapy for the treatment of advanced cervical cancer: morbidity and outcome: results of a multicenter study of the GCCLCC (Groupe des Chirurgiens de Centre de Lutte Contre le Cancer)Gynecol Oncol200610252352916504274
- HuguetFCojocariuOMLevyPPreoperative concurrent radiation therapy and chemotherapy for bulky stage IB2, IIA, and IIB carcinoma of the uterine cervix with proximal parametrial invasionInt J Radiat Oncol Biol Phys2008721508151518676093
- FerrandinaGLeggeFFagottiAPreoperative concomitant chemoradiotherapy in locally advanced cervical cancer: safety, outcome, and prognostic measuresGynecol Oncol2007107S127S13217727936
- LèguevaquePMottonSDelannesMCompletion surgery or not after concurrent chemoradiotherapy for locally advanced cervical cancer?Eur J Obstet Gynecol Reprod Biol201115518819221232839
- MottonSHouvenaeghelGDelannesMResults of surgery after concurrent chemoradiotherapy in advanced cervical cancer: comparison of extended hysterectomy and extrafascial hysterectomyInt J Gynecol Cancer20102026827520169670
- LiuMTHsuJCLiuWSPrognostic factors affecting the outcome of early cervical cancer treated with radical hysterectomy and post-operative adjuvant therapyEur J Cancer Care (Engl)20081717418118302655
- BaiocchiGGuimaraesGCRosa OliveiraRAPrognostic factors in pelvic exenteration for gynecological malignanciesEur J Surg Oncol20123894895422818842
- HuangYTWangCCTsaiCSLong-term outcome and prognostic factors for adenocarcinoma/adenosquamous carcinoma of cervix after definitive radiotherapyInt J Radiat Oncol Biol Phys20118042943620542643
- SongSSongCKimHJ20 year experience of postoperative radiotherapy in IB-IIA cervical cancer patients with intermediate risk factors: Impact of treatment period and concurrent chemotherapyGynecol Oncol2012124636722004904
- HuguetFCojocariuOMLevyPPreoperative concurrent radiation therapy and chemotherapy for bulky stage IB2, IIA, and IIB carcinoma of the uterine cervix with proximal parametrial invasionInt J Radiat Oncol Biol Phys2008721508151518676093
- KirovaYMBourhalebZAlranSPreoperative concomitant radiochemotherapy in bulky carcinoma of the cervix: Institut Gurie experienceCancer Radiother200913291297 French19524469
- ColomboPEBertrandMMGutowskiMTotal laparoscopic radical hysterectomy for locally advanced cervical carcinoma (stages IIB, IIA and bulky stages IB) after concurrent chemoradiation therapy: surgical morbidity and oncological resultsGynecol Oncol20091440440919555996
- ClasseJMRauchPRodierJFSurgery after concurrent chemoradiotherapy and brachytherapy for the treatment of advanced cervical cancer: morbidity and outcome: results of a multicenter study of the GCCLCCGynecol Oncol200610252352916504274
- MacchiaGFerrandinaGDeodatoFConcomitant boost dose escalation plus large-field preoperative chemoradiation in locally advanced carcinoma of the uterine cervix: results of a phase I study (LARA-CC-1)Gynecol Oncol201011812813320494419