Abstract
Objective
The goal of this study was to investigate the between cluster of differentiation 109 (CD109) expression and tumor diameter, invasion depth, tumor grade, presence of lymph-node metastasis, and overall survival in patients with vulvar squamous cell carcinoma, which is the most common type of vulvar cancer.
Method
Twenty-six patients who underwent an operation for vulvar cancer between 1999 and 2009 in our clinic were included in this study. Immunohistochemical staining was performed on formalin-fixed and paraffin-embedded tissue.
Result
Tumor diameter was not found to be significantly associated with CD109 expression, intensity of cytoplasmic staining, or combined score (P = 0.325, P = 0.169, P = 0.352, respectively). Invasion depth and combined score were also not significantly correlated with CD109 expression (P = 0.324 and P = 0.174 respectively). There was a negative correlation between invasion depth and the intensity of cytoplasmic staining (P = 0.042). There was no significant correlation between tumor stage and CD109 expression, the intensity of cytoplasmic staining, and the combined score (P = 0.574, P = 0.389, P = 0.605, respectively). A significant positive correlation was observed between tumor grade and CD109 expression, the intensity of cytoplasmic staining, and the combined score (P = 0.003, P = 0.018, P = 0.008, respectively). No significant difference was found between the percentages of CD109 expression in patients with positive (48%) and negative (11%) lymph nodes (P = 0.058). The percentage of CD109 expression did not significantly differ in relation to overall survival (P = 0.483).
Conclusion
Comprehensive and more extensive studies are needed to examine the relationship between CD109 expression and vulvar malignant lesions.
Introduction
Vulvar cancer (VC) is a rare neoplasm. It accounts for about 2.5%–5.0% of all gynecological malignancies.Citation1 This malignancy most frequently affects postmenopausal women aged over 60 years, and it is commonly complicated by other diseases such as coronary disease, hypertension, and diabetes.Citation2 Squamous cell carcinoma (SCC) is the most common type among the other vulvar malignancies, accounting for more than 90% of all vulvar malignancies.Citation1,Citation2 There is a very low incidence of VC in Turkey. Turgut et al investigated the gynecological malignancies between 2001 and 2011 in a tertiary center located at the southeastern region of Turkey.Citation3 Of the 231 patients operated on, only four (1.8%) had VC.Citation3 The Turkish Ministry of Health declared that there were 103 patients with VC in the period 2004–2006 in Turkey.Citation4 There have been few controlled trials conducted on VC in Turkey. Ayhan et al reported the largest retrospective evaluation of demographic, pathologic, and follow-up data on 91 patients with VC obtained from hospital records and some private gynecologic oncology files.Citation5
No established specific etiological factors have been established for VC. Keratinized SCC unrelated to human papillomavirus (HPV) is common in elderly women. This type of carcinoma is associated with vulvar dystrophy, such as hypertrophic dystrophy, or inflammatory dermatosis, such as lichen sclerosus.Citation6 In contrast, HPV-related bowenoid (warty) carcinoma and basaloid carcinoma, which is particularly associated with vulvar intraepithelial neoplasia, are common in young women.Citation7 It has been estimated that 40.4% of VCs studied were related to HPV.Citation7,Citation8 In Spain, a case series found an HPV association of 17.4% for condylomatous-basaloid tumors and 12.3% for squamous cell tumors, which represent estimations lower than those published for Europe.Citation7,Citation9 The Centers for Disease Control and Prevention reported that 51% of VCs in patients aged 37–65 years were attributable to HPV between 2004 and 2008 in the USA.Citation10
Transforming growth factor beta (TGF-β) plays an important role in cell proliferation, differentiation, synthesis of matrix proteins, embryogenesis, and tissue regeneration following injury. TGF-β is associated with various skin disorders – such as hypertrophic scarring and psoriasis – and malignancies. Cluster of differentiation 109 (CD109) is a glycosyl phosphatidyl inositol-anchored cell surface antigen and a member of the α2-macroglobulin/C3, C4, C5 family. CD109 inhibits transforming-growth-factor signaling over TGF-β receptors. TGF-β1 is a potent inhibitor of growth in most epithelial cells including keratinocytes.Citation11 This process is thought to play a role in the development of human cancers, particularly SCC. Hagikura et al have examined the relationship between CD109 expression and urothelial carcinoma stage and grade,Citation12 while Ohshima et al have suggested that CD109 is highly expressed in skin and cutaneous cancers.Citation13
The goal of the study reported here was to investigate the correlation between CD109 expression and tumor diameter, invasion depth, tumor grade, presence of lymph-node metastasis, and overall survival in patients with vulvar SCC, the most common type of VC.
Materials and methods
Twenty-six patients with squamous cell VC who underwent operations for their cancer between 1999 and 2009 in the Department of Obstetrics and Gynecology of Atatürk Training and Research Hospital, İzmir, the largest tertiary gynecological oncology center in Turkey, were included in this study. Patient age, tumor size, tumor grade and stage, invasion depth, presence of lymph-node metastasis, and survival data were obtained from patient records held in the department’s obstetrics and gynecology clinic as well as through face-to-face or phone interview. With the exception of five patients, all underwent radical vulvectomy and inguinofemoral lymph-node dissection in accordance with International Federation of Gynecology and Obstetrics (FIGO) recommendations. The remaining five patients, who were of advanced age and deemed to be at high risk of surgical complications, only underwent simple vulvectomy to minimize perioperative complications. The FIGO staging system was used in postoperative staging.
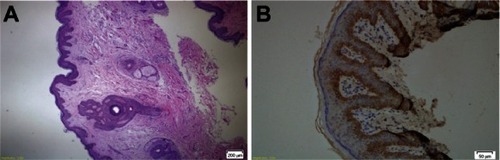
Immunohistochemical staining was performed on formalin-fixed and paraffin-embedded tissue using the Dako EnVision™ FLEX system (Glostrup, Denmark). Tissue sections were immersed into the Epitope Target Retrieval Solution, High pH (50×) in a Dako PT Link pretreatment system and incubated at 97°C for 25 minutes. Tissue sections were finally prepared for the antibody application. Anti-CD 109 antibody (rabbit monoclonal antibody, catalog no SAB1408699, Sigma-Aldrich, St Louis, MO, USA) was used as the primary antibody. The primary antibody was diluted 1:50 with Large Volume UltrAb Diluent (Thermo Fisher Scientific, Waltham, MA, USA). Sections were rinsed for 5 minutes with Dako Peroxidase Blocking Agent at a volume of 100 μL per slide, then washed with Tris-buffered saline solution. Sections were then incubated with diluted primary antibody (1:50) for 60 minutes at room temperature. Following this, the samples were incubated for 10 minutes with a solution made up of 1 drop (32μl) of 3,3′-Diaminobenzidine (DAB) Chromogen per 1.0 ml of DAB Substrate Buffer, washed with distilled water, and stained with EnVision FLEX Hematoxylin (Dako) for 5 minutes. Tissue samples were dehydrated through three changes of alcohol (80%, 96%, 99%) and purified with xylene. Normal prostate tissue was used as the positive control.Citation14 Only basal keratinocytes were stained with CD109 antibody in normal vulvar tissue ().
Figure 1 (A) Normal vulvar tissue stained with hematoxylin and eosin and (B) basal keratinocytes stained with CD109 antibody in normal vulvar tissue (both at ×20 magnification).
Evaluation of immunohistochemical staining
All sections were evaluated under light microscope by a pathologist experienced in gynecological pathology. Cytoplasmic staining in the lesion/tumor tissue was taken into consideration. All tumor fields were examined under light microscopy at low magnification (×10) and the proportion of all stained cells to tumor cells, staining percentage, and subjective assessment of the staining intensity of tumor cells were evaluated at high magnification (×20). The percentages of tumor stained were scored in four groups using a 0–3 scale in accordance with Hagiwara and colleagues’ study.Citation15 Accordingly, the percentage of expression was ranked as: 0%–10% = 0, 11%–30% = 1, 31%–60% = 2, and > 60% = 3.Citation15 The intensity of cytoplasmic staining was ranked in three groups: weak = 1, moderate = 2, and strong = 3.
Combined score was derived from the sum of the percentage of tumor tissue stained and the intensity of the cytoplasmic staining. Total score was ranked as:
combined score 0 = negative (regardless of staining intensity, if no staining or < 10% staining)
combined score 1 = weak staining (total score 2)
combined score 2 = moderate staining (total score 3–4)
combined score 3 = strong staining (total score 5–6).
Statistical analysis
Frequencies, proportions, means, and standard deviations were used as the descriptive statistics. The distribution of the variables was evaluated using the Kolmogorov–Smirnov test. The independent-sample t-test was used in the analysis of quantitative data. Qualitative data were analyzed using the Chi-square test or, when conditions for the Chi-square test were not met, Fisher’s exact test. Pearson and Spearman correlation tests were used in the correlation analysis. SPSS (v 20.0; IBM, Armonk, NY, USA) software was used for statistical analyses. P values < 0.05 were considered statistically significant.
Results
The age of patients with squamous vulvar carcinoma included in this study ranged between 47 and 85 years. Mean age was 69.7 ± 8.0 years. When patients were evaluated according to tumor grade, 13 (50%) had well-differentiated, 9 (34.61%), had moderately differentiated, and four (15.38%) had poorly differentiated tumors. Of 26 patients, 21 underwent radical vulvectomy and inguinofemoral lymph-node dissection. The remaining five patients were of advanced age and considered to be at high risk of surgical complications, so only underwent simple vulvectomy. When patients were evaluated using the FIGO surgical staging system, one patient (4.3%) had Stage IA, 15 patients (65.21%) had Stage IB, two patients (8.6%) had Stage II, and four patients (17.39%) had Stage IIIB disease. No patient was found with Stage IIIA or Stage IV disease. In the examination of resected material, four patients (18.81%) appeared to have lymph-node involvement whereas 18 patients (81.19%) did not.
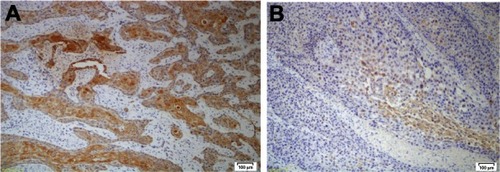
Anti-CD109 staining was positive in 15 cases and negative in the other eleven cases. Nine of 13 patients (69.23%) with well-differentiated, and six of nine patients (66.66%) with moderately differentiated squamous vulvar carcinoma showed positive staining for CD109 expression. None of the four patients (0%) with poorly differentiated tumor showed positive staining for CD109. In the well-differentiated tumor group, eight patients had a combined score of 3, one patient had a combined score of 2, one patient had a combined score of 1, and four patients had a combined score of 0 (). Of the nine patients in the moderately differentiated tumor group, one had a combined score of 3, two had a combined score of 2, three had a combined score of 1, and three had a combined score of 0 (). All four patients in the poorly differentiated group had a combined score of 0.
Figure 2 (A) CD109 expression in well-differentiated tumor and (B) in moderately differentiated tumor (both at ×20 magnification).
Tumor diameter was not found to be significantly associated with the percentage of CD109 expression, intensity of cytoplasmic expression, or combined score (P = 0.325, P = 0.169, P = 0.352, respectively). Invasion depth was also not significantly correlated with the percentage of CD109 expression (P = 0.324) or the combined score (P = 0.174). There was negative correlation between invasion depth and the intensity of cytoplasmic expression (P = 0.042). Further, there was no significant correlation between tumor stage and the percentage of CD109 expression, the intensity of cytoplasmic expression, or the combined score (P = 0.574, P = 0.389, P = 0.605, respectively). A significant positive correlation was observed between tumor grade and the percentage of CD109 expression, the intensity of cytoplasmic expression, and the combined score (P = 0.003, P = 0.018, P = 0.008, respectively) ().
Table 1 The association of CD109 expression pattern with tumor diameter, invasion depth, tumor stage, and tumor grade
No significant difference was found between the percentages of CD109 expression in patients with positive (48%) and negative (11%) lymph nodes (P = 0.058) (). However, a definitive statistical evaluation could not be made for the intensity of cytoplasmic CD109 expression and combined score due to the insufficient number of cases. The percentage of CD109 expression did not significantly differ in relation to overall survival (P = 0.483). Again, however, due to the insufficient number of patients, a definitive statistical evaluation could not be made for the intensity of cytoplasmic CD109 expression and combined score ().
Table 2 The association of CD109 expression pattern with lymph-node metastasis
Table 3 The association of CD109 expression pattern with overall survival
Discussion
Due to the rare occurrence of VC, there have been no large randomized controlled trials examining this disease. Most of the treatment guidelines are based on small retrospective studies. Further, vulvar carcinoma is rarely studied in or reported from developing countries, where most patients present at advanced stages.Citation16 VC grows slowly and tends to remain localized for a long period, evolving into invasive cancer within 8 years.Citation17 Older patients are more likely to present with disease that is further advanced. Most patients in developing countries present at – and are thus diagnosed at – an advanced stage as a result of cultural behaviors and traditional conservative attitudes.Citation18,Citation19 There are two different etiopathogenic pathways for the development of VC – one associated with HPV infection and the second independent of HPV infection.Citation20 HPV-associated premalignant lesions and carcinomas show diffuse immunostaining for p16INK4a and p14arf, and are negative for p53.Citation21,Citation22 In this regard, it has been suggested that the identification of HPV DNA sequences may not be sufficient to accurately differentiate between the HPV-associated and HPV-independent types of vulvar lesions. In contrast to the relatively large amount of data available on HPV-associated tumors, the HPV-independent VC pathway has been much less well studied.Citation20 TP53 mutations frequently correlate with immunohistochemical overexpression of the p53 protein, a frequent finding in HPV-independent VC. A strong correlation between high p53 expression and DNA aneuploidy has been observed.Citation23–Citation25 However, not all HPV-independent VCs follow the p53 pathway, and the mechanisms for tumor initiation and progression in cancers without TP53 mutation are unknown.Citation20
The new 2009 FIGO VC staging system was validated by clearly demonstrating distinct groups with differing rates of survival.Citation26 The main parameters are tumor diameter, invasion depth and lymph-node metastases for this new staging system of VC. The traditional TNM Classification of Malignant Tumors (TNM) staging system for VC is described in FIGO’s 25th annual report on gynecological cancerCitation27 and has also been used by some physicians. Tumor diameter and invasion depth are two of the main determinants of VC according to the traditional TNM staging system.Citation28 Fuh and Berek have reported the factors related to prognosis: tumor size, depth of invasion, lymph-node involvement, and presence of distant metastases.Citation29 Inguinofemoral lymph-node metastasis is considered the most important predictor of overall prognosis.Citation29
Analysis of DNA content, cell-cycle regulatory proteins; apoptosis-related proteins; epidermal growth factor receptor; and proteins that are involved in tumor invasiveness, metastasis, and angiogenesis are still being investigated for potential use as prognostic indicators of VC.Citation31 Thus, in the study reported here, we investigated the expression and expression pattern of CD109, which has been previously studied as a marker in SCCs,Citation15 pelvic organ malignancies,Citation31 and urothelial cancersCitation12 for its association with tumor diameter, invasion depth, tumor stage and grade, lymph-node involvement, and overall survival.
CD109 is a component of the TGF-β receptor. Previous studies have reported CD109 expression in a subset of fetal and adult CD34+ bone marrow mononuclear cells, but it was not detected in mature blood cells. Because almost all myeloid-erythroid and megakaryoblastic progenitors arise from the CD34+/CD109+ lineage rather than the CD34+/CD109– lineage, this suggested that the most primitive hematopoietic stem cells may be present within the CD109 subset, although its physiological functions remain largely unknown.Citation32,Citation33 CD109 is a surface glycoprotein and negative modulator of TGF-β signal in keratinocytes.Citation11 High CD109 expression has been found in SCC, glioblastoma, and some adenocarcinomas and sarcomas.Citation14 CD109 has been particularly shown to play a role in tumor growth and cell proliferation in SCC.Citation15 Higher CD109 expression has been found in squamous cell lung cancers than in cancers of other histological types (ie, adenocarcinomas, small-cell carcinomas, large-cell carcinomas).Citation34 Zhang et al found that its expression was significantly higher in cervical SCCs than in endometrial adenocarcinomas, suggesting that CD109 expression is upregulated in SCCs without regard to tissue origin.Citation31 In our study, which employed immunohistochemical methods, only basal keratinocytes were positively stained for CD109 expression. We comprehensively evaluated the pattern of CD109 expression in tumor tissue using percentage of CD109 staining (expression), intensity of cytoplasmic staining, and total score combining the two parameters.
As highlighted, the primary size and invasion depth of the vulvar tumor are the key components of both the most recent version of the FIGO staging system and traditional TNM staging system. In our study, tumor diameter was not found to be significantly associated with the percentage of expression, intensity of cytoplasmic expression, or the combined score of these (P = 0.325, P = 0.169, P = 0.352, respectively). FIGO staging of carcinoma of the cervix is similar to FIGO staging of VC. Tumor diameter and depth of invasion are some of the main parameters. While Zhang et al reported that CD109 expression in samples from squamous tumors of the cervix was not correlated with the clinical stage of the tumor, they did not give details about the relationship between CD109 expression and depth of invasion or tumor size.Citation31 In our study, we found that invasion depth was also not significantly correlated with the percentage of expression (P = 0.324) or the combined score (P = 0.174). Hagiwara et al assessed the significance of CD109 expression in tumor development and cell proliferation using human oral tumor tissues and cancer cell lines and found that CD109 overexpression accelerates cell proliferation.Citation15 In contrast, there was a negative correlation between invasion depth and the intensity of cytoplasmic expression of CD109 (P = 0.042) in our study.
Hagiwara et al conducted their study on malignant tumors of the oral cavity and found that CD109 expression was inversely correlated with tumor grade.Citation15 In another study, CD109 expression was found to be higher in low-grade urothelial carcinomas than in high-grade tumors.Citation12 In our study, a significant positive correlation was observed between tumor grade and the percentage of staining (CD109 expression), intensity of cytoplasmic expression, and the combined score of these (P = 0.003, P = 0.018, P = 0.008, respectively). Nine out of 13 patients (69.23%) with well-differentiated tumors showed positive expression for CD109 but none of the four patients (0%) with poorly differentiated tumors showed positive expression for CD109. No grade 3 tumor stained positively for CD109. Our results concerning tumor grade and CD109 expression are consistent with those of similar studies.Citation12,Citation15
Zhang et alCitation31 did not find an association between clinical stage, lymph-node involvement, or CD109 expression in SCC of the cervix. None of the patients with advanced-stage disease with lymph-node metastasis showed CD109 expression.Citation31 We found no significant difference between the percentage of CD109 staining (expression) in patients with positive (48%) and negative (11%) lymph nodes (P = 0.058) and there was no significant correlation between tumor stage and the percentage of CD109 expression, intensity of cytoplasmic expression, and the combined score of these (P = 0.574, P = 0.389, P = 0.605, respectively). CD109 is known to inhibit TGF-β/SMAD signaling. In the early stages of tumorigenesis, CD109 expression is upregulated, whereas TGF-β/SMAD signal is downregulated. This is followed by the expansion of tumor tissue. However, in advanced-stage malignancies, Bizet et al have shown that CD109 expression is downregulated. TGF-β released from tumor cells supports tumor growth in advanced stages of the disease.Citation35 Our results are consistent with both Zhang et al’s and Bizet et al’s studies.Citation31,Citation35
The percentage of CD109 staining did not significantly differ in relation to overall survival (P = 0.483). However, a definitive statistical evaluation could not be made for the intensity of cytoplasmic CD109 staining and combined score due to the insufficient number of patients. In 2012, Ramanah et al claimed that node positivity (hazard ratio, 3.12 [95% confidence interval, 2.30–4.24]) and surgery (hazard ratio, 0.41 [95% confidence interval, 0.24–0.69]) were found to be the two most predictive variables for cancer mortality, followed by age and tumor size.Citation36 The relationship between the overall survival of patients with VC and molecular markers is under investigation. In immunohistochemical investigations, de Melo Maia et al found c-KIT protein in 70.5% of the cases and this c-KIT protein positivity was associated with a higher global survival (P = 0.007).Citation37 However, variations in the levels of ZNF652 – a novel zinc finger protein – have not been found to be related to patient survival.Citation38
In a recent study that included a series of HPV-positive and HPV-negative groups that were similar in terms of FIGO stage distribution, percentage of involvement of surgical margins, ulceration, and tumor size or invasion depth at diagnosis, no significant differences were observed in survival between patients with HPV-positive and HPV-negative VC.Citation39 However, due to technical drawbacks in our study, we were not able to perform HPV identification and genotyping in the patients with VC showing CD109 expression. Zhang et al have previously reported that, in addition to SCC cell lines, CD109 expression was also upregulated in some human adenocarcinoma and sarcoma cell lines, suggesting that upregulation of CD109 expression may be induced as a result of accumulation of genetic alterations in tumor cell lines.Citation31 Their results suggested that a particular type of HPV infection may not correlate to CD109 upregulation in these cell lines.Citation31
Conclusion
Hagiwara et alCitation15 and Hagikura et alCitation12 found that CD109 expression was inversely correlated with tumor grade; our results concerning tumor grade and CD109 expression are consistent with these studies. Zhang et al did not find an association between clinical stage, lymph-node involvement, or CD109 expression in SCC of the cervix.Citation31 We found no significant difference between the percentages of CD109 (expression) in patients with positive lymph nodes or patients with negative lymph nodes. Bizet et al showed that CD109 expression is downregulated in advanced-stage malignancies.Citation35 There was no significant correlation between tumor stage and the percentage of CD109 expression, the intensity of cytoplasmic staining, or the combined score of these in our study. However, our results for CD109 expression and VC were consistent with most other studies of CD109 expression. Large and comprehensive research into this rare malignancy is needed.
Disclosure
The authors declare no conflicts of interest in this work. All authors read and approved the final version of this paper for publication.
References
- JemalATiwaniRCMurrayTCancer statistics, 2004CA Cancer J Clin200454182914974761
- FiggeDCGaudenzRInvasive carcinoma of the vulvaAm J Obstet Gynecol197411933823954827379
- TurgutAÖzlerASakMERetrospective analysis of the patients with gynecological cancer: 11-year experienceJ Clin Exp Invest201232209213
- UçarTBekarM[Gynecologic cancers in Turkey and worldwide]Türk Jinekolojik Onkoloji Dergisi20101335560 Turkish [with English abstract]
- AyhanAVelipasaogluMSalmanMCGuvenSGultekinMBayraktarOPrognostic factors for recurrence and survival in primary vulvar squamous cell cancerActa Obstet Gynecol Scand200887111143114918949585
- BastRCJrKufeDWPollockREHolland-Frei Cancer Medicine5th edHamilton (ON)BC Decker2000
- De VuystHCliffordGMNascimentoMCMadeleineMMFranceschiSPrevalence and type distribution of human papillomavirus in carcinoma and intraepithelial neoplasia of the vulva, vagina and anus: a meta-analysisInt J Cancer200812471626163619115209
- MuñozNKjaerSKSigurdssonKImpact of human papillomavirus (HPV)-6/11/16/18 vaccine on all HPV-associated genital diseases in young womenJ Natl Cancer Inst2010102532533920139221
- OrdiJAlejoMFustéVHPV-negative vulvar intraepithelial neoplasia (VIN) with basaloid histologic pattern: an unrecognized variant of simplex (differentiated) VINAm J Surg Pathol200933111659166519730361
- Centers for Disease Control and Prevention (CDC)Human papillomavirus-associated cancers – United States, 2004–2008MMWR Morb Mortal Wkly Rep20126125826122513527
- FinnsonKWTamBYYLiuKIdentification of CD109 as part of the TGF-beta receptor system in human keratinocytesFASEB J20062091525152716754747
- HagikuraMMurakumoYHasegawaMCorrelation of pathological grade and tumor stage of urothelial carcinomas with CD109 expressionPathol Int2010601173574320946523
- OhshimaYYajimaIKumasakaMYCD109 expression levels in malignant melanomaJ Dermatol Sci201057214014220034764
- HashimotoMIchiharaMWatanabeTExpression of CD109 in human cancerOncogene200423203716372015116102
- HagiwaraSMurakumoYSatoTUp-regulation of CD109 expression is associated with carcinogenesis of the squamous epithelium of the oral cavityCancer Sci200899101916192319016750
- SharmaDNRathGKKumarSBhatlaNJulkaPKSahaiPTreatment outcome of patients with carcinoma of vulva: experience from a tertiary cancer center of IndiaJ Cancer Res Ther20106450350721358089
- GotliebWHThe assessment and surgical management of early-stage vulvar cancerBest Pract Res Clin Obstet Gynaecol200317455756912965132
- CanavanTPCohenDVulvar cancerAm Fam Physician20026671269127412387439
- StehmanFBLookKYCarcinoma of the vulvaObstet Gynecol2006107371973316507947
- Del PinoMRodriguez-CarunchioLOrdiJPathways of vulvar intraepithelial neoplasia and squamous cell carcinomaHistopathology201362116117523190170
- van der AvoortIShirangoHHoevenaarsBMVulvar squamous cell carcinoma is a multifactorial disease following two separate and independent pathwaysInt J Gynecol Pathol2006251222916306780
- SantosMMontagutCMelladoBImmunohistochemical staining for p16 and p53 in premalignant and malignant epithelial lesions of the vulvaInt J Gynecol Pathol200423320621415213596
- YangBHartWRVulvar intraepithelial neoplasia of the simplex (differentiated) type: a clinicopathologic study including analysis of HPV and p53 expressionAm J Surg Pathol200024342944110716158
- ChoschzickMHantaredjaWTennstedtPGiesekingFWölberLSimonRRole of TP53 mutations in vulvar carcinomasInt J Gynecol Pathol201130549750421804386
- van der AvoortIAvan de NieuwenhofHPOtte-HöllerIHigh levels of p53 expression correlate with DNA aneuploidy in (pre) malignancies of the vulvaHum Pathol201041101475148520656324
- TanJChettyNKondalsamy-ChennakesavanSValidation of the FIGO 2009 staging system for carcinoma of the vulvaInt J Gynecol Cancer201222349850222367324
- BellerUMaisonneuvePBenedetJLCarcinoma of the vulva: FIGO 25th annual report on the results of treatment of gynecological cancerInt J Gynecol Obstet200383726
- BellerUMaisonneuvePBenedetJLCarcinoma of the vulva: FIGO 25th annual report on the results of treatment of gynecological cancerInt J Gynecol Obstet200383726
- FuhKCBerekJSCurrent management of vulvar cancerHematol Oncol Clin North Am2012261456222244661
- GadducciATanaRBarsottiCGuerrieriMEGenazzaniARClinico-pathological and biological prognostic variables in squamous cell carcinoma of the vulvaCrit Rev Oncol Hematol2012831718322015047
- ZhangJMHashimotoMKawaiKCD109 expression in squamous cell carcinoma of the uterine cervixPathol Int200555416516915826242
- SchuhACWatkinsNANguyenQA tyrosine703 serine polymorphism of CD109 defines the Gov platelet alloantigensBlood20029951692169811861285
- RappoldIZieglerBLKöhlerIFunctional and phenotypic characterization of cord blood and bone marrow subsets expressing FLT3 (CD135) receptor tyrosine kinaseBlood19979011111259207445
- SatoTMurakumoYHagiwaraSHigh-level expression of CD109 is frequently detected in lung squamous cell carcinomasPathol Int2007571171972417922683
- BizetAATran-KhanhNSaksenaALiuKBuschmannMDPhilipACD109-mediated degradation of TGF-β receptors and inhibition of TGF-β responses involve regulation of SMAD7 and Smurf2 localization and functionJ Cell Biochem2012113123824621898545
- RamanahRLesieurBBallesterMDaraiERouzierRTrends in of late-stage squamous cell vulvar carcinomas: analysis of the surveillance, epidemiology, and end results (SEER) databaseInt J Gynecol Cancer201222585485922426405
- de Melo MaiaBLavorato-RochaAMRodriguesISPrognostic significance of c-KIT in vulvar cancer: bringing this molecular marker from bench to bedsideJ Transl Med20121015022839358
- HolmRKnoppSKumarRExpression of ZNF652, a novel zinc finger protein, in vulvar carcinomas and its relation to prognosisJ Clin Pathol2008611596317468294
- AlonsoIFustéVdel PinoMDoes human papillomavirus infection imply a different prognosis in vulvar squamous cell carcinoma?Gynecol Oncol2011122350951421652058